Abstract
This paper concerns the role of apoptosis during the onset of bone histogenesis. Previous investigations by us performed on intramembranous ossification revealed the existence of two types of osteogenesis: static (SBF) and dynamic bone formation (DBF). During SBF, the first to occur, stationary osteoblasts transform into osteocytes in the same location where they differentiated, forming the primary spongiosa. DBF takes place later, when movable osteoblastic laminae differentiate along the surface of the primary trabeculae. The main distinctive feature between SBF and DBF is that the latter involves the invasion of pre-existing adjacent tissue, whereas the former does not. To ascertain whether programmed cell death during the invasive DBF process determines the fate of surrounding pre-existing mesenchyme differently from that occurring during the non-invasive SBF process, we studied apoptosis in ossification centres of tibial diaphysis in chick embryos and newborn rabbits with TUNEL and TEM. It emerged that, in both SBF and DBF, apoptosis affects mesenchymal cells located between the forming trabeculae and capillaries. However, apoptotic cells were observed more frequently during DBF than during SBF. This suggests that, during bone histogenesis, apoptosis, which is mostly associated with the invasive process of DBF, is probably dedicated to making space for advancing bone growth.
Keywords: apoptosis, dynamic osteogenesis, intramembranous ossification, osteoblasts, static osteogenesis
Introduction
In a recent investigation into intramembranous ossification, occurring around the cartilaginous bud of long bones, we pointed out the existence of two different and successive types of osteogenesis, which we termed static bone formation (SBF) and dynamic bone formation (DBF) (Ferretti et al. 2002).
During the formation of bone, as well as in the formation of many other tissues, apoptosis plays an important role. This phenomenon is known to occur in parallel to many morphogenetic processes during animal development (Clark & Clark, 1996; Ross, 1996; Jacobson et al. 1997; Tanimoto et al. 1998; Vilovic et al. 2001) by the activation of a specific programme of self-destruction, or ‘programmed cell death’, involving stereotyped morphological and biochemical cellular changes (Kerr et al. 1972; Wyllie et al. 1980; Arends & Wyllie, 1991). It is well known that this type of programmed cell death starts with cleavage of nuclear DNA into fragments and ends with the formation of apoptotic bodies that undergo rapid removal.
The importance of apoptosis in skeletal tissues and its functional relationship with bone turnover have been recently investigated by various authors (Noble et al. 1997; Jilka et al. 1998; Noble & Reeve, 2000) in order to understand whether and, if so, how the mechanism of programmed cell death interferes with bone modelling and remodelling in situations with different bone turnover, such as in adult and paediatric normal bone or in pathological bone. The cells of osteogenic lineage (mostly osteoblasts and osteocytes) (Bronckers et al. 1996; Noble et al. 1997) as well as those of osteoclastic lineage (Hughes et al. 1995; Hughes & Boyce, 1997) have been shown to be affected by apoptosis; moreover, recently, various substances (hormones, cytochines and factors) have been shown to have an influence on the amount of cell apoptosis in bone (Hughes et al. 1995; Kawakami et al. 1997; Mansukhani et al. 2000). Nevertheless, the potential role of apoptosis in the control of cell populations that form or destroy matrix in the skeleton is still to be established.
In the present study we investigated the possible role of apoptosis during the two types of osteogenesis (SBF and DBF) occurring in intramembranous ossification and which we have shown to have different characterstics. SBF, which is the first to occur, is characterized by differentiation around periosteal vessels of cords made up of 2–3 layers of stationary osteoblasts that transform into osteocytes in the same location where they differentiated, thus involving the deposition of the core of primary bony trabeculae. DBF takes place at a later stage, when typical monostratified laminae of movable osteoblasts differentiate along the surface of the primary SBF-trabeculae and increase their thickness, thus forming primary haversian systems that invade the mesenchyme filling the primary haversian spaces (Ferretti et al. 2002). From these observations it emerged that the main distinguishing feature between SBF and DBF is that the latter process involves the invasion of the pre-existing adjacent tissues, whereas the former does not.
In order to ascertain whether and, if so, how apoptosis is involved in the fate of the pre-existing condensed mesenchyme of ossification centres during static bone formation and/or dynamic bone formation, i.e. during a non-invasive as distinct from an invasive process, we studied the perichondral tissue surrounding the mid-shaft level of long bones of newborn rabbits and chick embryos by microscopic (TUNEL) and ultramicroscopic (TEM) observations.
Materials and methods
The observations were performed on the intramembranous perichondral centre of ossification surrounding the mid-shaft level of various long bones, particularly the tibiae, of five newborn rabbits and six White Leghorn chick embryos aged 8–16 days.
In situ end-labelling analysis (TUNEL)
Samples were fixed in 4% buffered paraformaldehyde (0.1 m phosphate buffer, pH 7.2) overnight at 4 °C, embedded in paraffin via a standard method, sectioned on a microtome (5 µm thick) and mounted on silanized slides. After being deparaffinated and rehydrated, the sections were treated in a humidified chamber with proteinase K (Boehringer, Mannheim, Germany) at 20 µg mL−1 for 15 min at room temperature, washed in bidistilled water, treated with 2% H2O2 in methanol for 10 min at room temperature and then washed in bidistilled water.
The slides were pre-incubated with terminal deoxynucleotidyl transferase (TdT) buffer and 1 mm CoCl2 for 5 min at room temperature and then incubated for 60 min in a humidified chamber at 37 °C with 50 µL TdT and biotinylated deoxyuridine triphosphate (Bio dUTP) (Boehringer) (TdT 0.3 U µL−1, Bio dUTP 8 µm in TdT buffer and CoCl2 1 mm). The sections were then washed four times in bidistilled apyrogen water (for 2 min each), twice in phosphate-buffered saline (PBS) (5 min each), in human serum albumin 2% (5 min) and in PBS (5 min) then covered with streptavidin–biotinylated peroxidase complex (Boehringer) diluted 1 : 100 in a humidified chamber for 45 min at room temperature, washed in PBS and stained with diaminobenzidine 50 mm (0.05%). The slides were then washed in water and counterstained, some with 0.5% methyl green for 10 min, and some with 1% toluidine blue for 5 min.
Positive and negative controls were included in each experiment. For the positive controls, sections were treated with DNase I (1 µg mL−1) (Boehringer) in DNase buffer for 10 min at room temperature before exposure to biotinylated dUTP and TdT. For negative controls, the sections were incubated without the TdT enzyme.
Histological (LM and TEM) analysis
Cross-sections (2 mm) were fixed for 2 h with 4% paraformaldehyde in 0.13 m phosphate buffer, pH 7.4, post-fixed for 1 h with 1% osmium tetroxide in 0.13 m phosphate buffer, pH 7.4, dehydrated in graded ethanol and embedded in epoxy resin (Durcupan ACM), and sectioned with a diamond knife mounted in an Ultracut-Reichert Microtome. The perichondral centres of ossification were cross-sectioned perpendicular to the longitudinal axis of the shaft. Thin sections (1 µm) were stained with toluidine blue and examined under an Axiophot-Zeiss light microscope (LM). Ultrathin sections (70–80 µm) were mounted on Formvar- and carbon-coated copper grids, stained with 1% uranyl acetate and lead citrate, and examined under a Zeiss EM109 transmission electron microscope (TEM).
Statistics
Twenty-two TUNEL-sections (two from each animal) were considered for quantitative analysis. Data concerning apoptotic cells recorded during SBF and DBF in each TUNEL-section were statistically evaluated by means of paired and unpaired Student's t-test.
Results
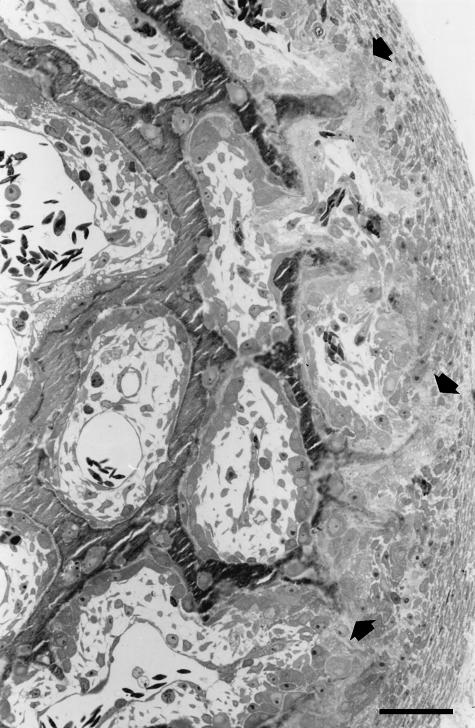
TUNEL and TEM observations were performed on cross-sections of primary spongiosa around the growing shaft of long bones of both chick embryos and newborn rabbits in which cords of stationary osteoblasts, along the periosteal surface, coexist with typical laminae of movable osteoblasts lining the surface of primitive haversian spaces (Fig. 1).
Fig. 1.
LM micrograph showing an example of a microscopic field in which TUNEL and TEM observations were performed. Cross-section of primary spongiosa around the growing tibial shaft of a 16-day-old chick embryo; the direction of bone growth is from left to right. The arrows point to the sites of differentiation of cords of stationary osteoblasts along the periosteal surface. The bony trabeculae laid down by static osteogenesis appear to be carpeted by typical movable osteoblastic laminae. Scale bar = 35 µm.
The TUNEL method, which labels the cells with DNA fragmentations with a brown colour, reveals the presence of apoptotic phenomena affecting mesenchymal cells adjacent to the sites of bone formation during the onset of osteogenesis, in both SBF and DBF. During the early stages (i.e. SBF), apoptotic cells appear between the cords of stationary osteoblasts forming the core of bony trabeculae and the capillaries (Fig. 2); during DBF, which invades the mesenchyme filling the primary haversian systems, apoptosis affects the cells located between the movable osteoblastic laminae and the vessels inside the haversian canal (Fig. 3). Of all the apoptotic cells collected in the present paper, about 72% are located behind the laminae of movable osteoblasts lining the surface of primary haversian spaces (i.e. during DBF); the remaining 28% are located adjacent to the cords of stationary osteoblasts (i.e. during SBF) (Table 1).
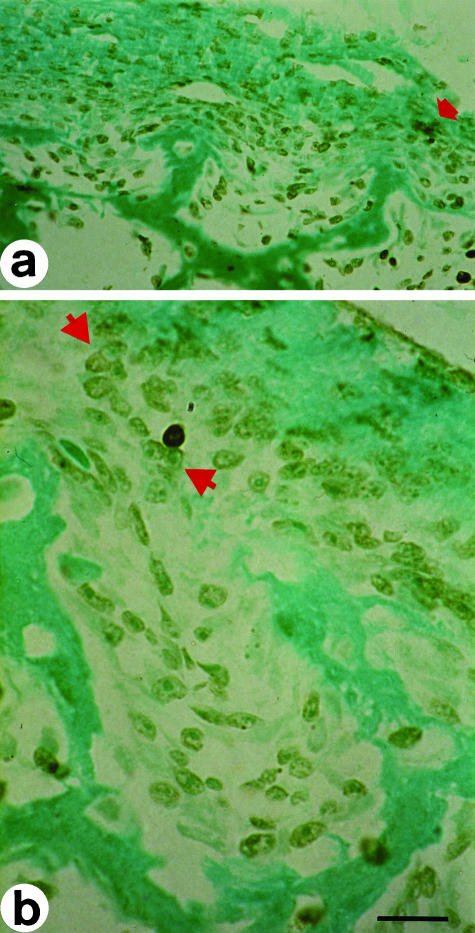
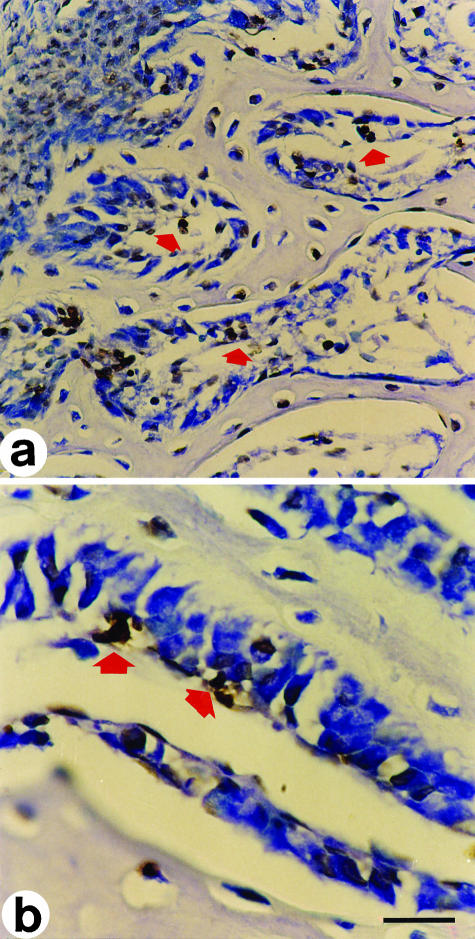
Fig. 2.
In situ end-labelling on cross-sections at the mid-diphyseal level of a newborn rabbit tibia. Labelled cells exhibit a brownish colour. (a) The arrow points to cells in apoptosis adjacent to a cord of stationary osteoblasts during SBF. (b) Cell in apoptosis behind a cord of stationary osteoblasts (between arrowsheads). LM micrographs; counterstain methyl green. Scale bars (a) = 36 µm, (b) = 15 µm.
Fig. 3.
In situ end-labelling on cross-sections at the mid-diphyseal level of a 14-day-old chick embryo tibia. Labelled cells exhibit a brownish colour. (a) The arrows point to cells in apoptosis inside primitive haversian spaces, located between the movable osteoblastic laminae and the vessels, during DBF. (b) Primary haversian space lined by a moveable osteoblastic lamina; the arrows point to cells in apoptosis behind the osteoblasts. LM micrographs; counterstain toluidine blue. Scale bars (a) = 36 µm, (b) = 15 µm.
Table 1.
Apoptotic cells recorded from 22 TUNEL-sections during SBF (apo-SBF) and DBF (apo-DBF)
| Section no. | apo-SBF* | apo-DBF* |
|---|---|---|
| 1 | 5 | 10 |
| 2 | 4 | 12 |
| 3 | 4 | 11 |
| 4 | 9 | 22 |
| 5 | 5 | 23 |
| 6 | 5 | 10 |
| 7 | 4 | 13 |
| 8 | 4 | 12 |
| 9 | 4 | 9 |
| 10 | 8 | 21 |
| 11 | 7 | 13 |
| 12 | 3 | 7 |
| 13 | 5 | 11 |
| 14 | 5 | 12 |
| 15 | 5 | 11 |
| 16 | 4 | 12 |
| 17 | 4 | 8 |
| 18 | 5 | 13 |
| 19 | 4 | 12 |
| 20 | 4 | 12 |
| 21 | 5 | 13 |
| 22 | 5 | 12 |
| mean ± SD | 4.9 ± 1.4 | 12.7 ± 4.1 |
P < 0.0001 between apo-SBF and apo-DBF by means of paired Student's t-test.
Total number of apoptotic cells recorded: 387; total number of SBF-apoptotic cells: 108; total number of DBF-apoptotic cells: 279; percentage of SBF-apoptotic cells: 27.98%; percent of DBF-apoptotic cells: 72.02%; unpaired Student's t-test: P < 0.0001.
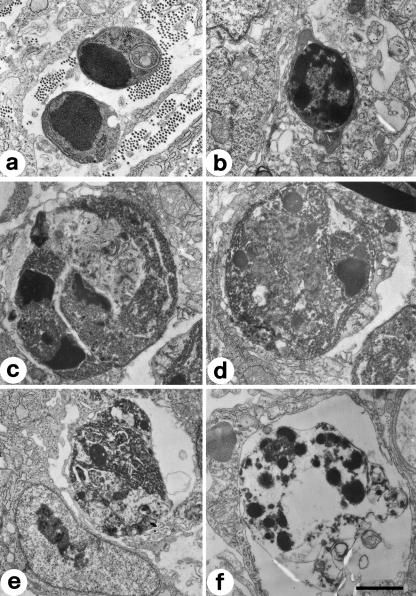
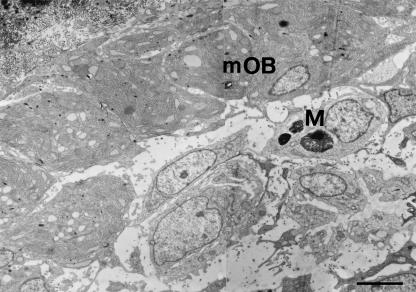
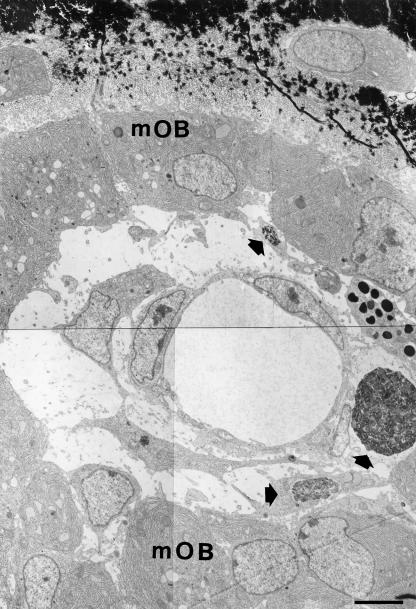
TEM observations of the same microscopic fields showed the presence of cells in apoptosis both in SBF and, more frequently, in DBF (Figs 4–6). The ultrastructure of these cells is very variable, because it is possible to observe different and progressive degrees of chromatin condensation and fragmentation. As with the cytoplasm, in some cells we observed a good preservation of the organelles, whereas in others we observed a large degree of alteration with considerable vacuolization. As regards the nucleus, the morphology of the chromatin masses is variable: it is possible to go from a low degree of condensation and fragmentation to a much higher one. The numerous observations collected under TEM allowed us to recognize the different progressive stages of cellular apoptosis, from early chromatin condensation and cell shrinkage (Fig. 7a,b) to the formation of apoptotic bodies (Fig. 7f). The latter were often observed to fill macrophages located near blood vessels, both in SBF and in DBF (Fig. 8).
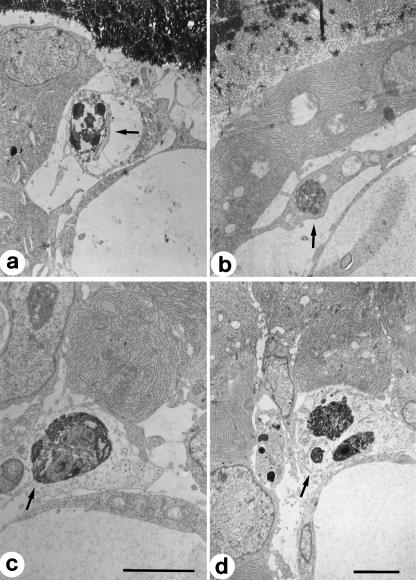
Fig. 4.
TEM micrograph showing a bony trabecular core (asterisks) laid down by stationary osteoblasts (sOB) during SBF in a 15-day-old chick embryo tibia. Top left: a blood capillary (spot). The squared area encloses an apoptotic cell. Scale bar = 3 µm.
Fig. 6.
TEM micrographs showing cells in apoptosis during DBF in newborn rabbit tibiae.Apoptotic cells (arrows) are located between movable osteoblasts and vessels.Scale bars:(a,d) = 3 µm; (b,c) = 2 µm.
Fig. 7.
TEM micrographs showing cells at different progressive stages, from (a) to (f), of apoptosis. Scale bar = 1 µm.
Fig. 8.
TEM micrograph showing a primitive haversian space in which are visible apoptotic bodies inside a macrophage (M) located behind the lamina of movable osteoblasts (mOB). Scale bar = 3 µm.
Discussion
SBF and DBF represent two processes we have shown to occur in sequence in the early phases of bone histogenesis in physiological conditions, i.e. during skeletal groth, when the formation of flat bones of the cranial volt and of diaphysis of long bones occurs (Ferretti et al. 2002), and that probably also occur in pathological conditions, such as during the formation of bone callus in bone repair. During these early phases of bone formation, as well as at the onset of formation of all other tissues and organs, the most important events that occur consist of cellular multiplication and differentiation, together with programmed cell death (i.e. apoptosis). Apoptosis, once considered to be involved mostly in the turnover of organs and tissues (Oppenheim, 1985; Haanen & Vermes, 1996; Chow et al. 1997) as well as in pathogenesis of tumour processes (Lee et al. 1998; Kulig et al. 1999; Weaver & Bissel, 1999) and in the evolution of many diseases (Orrenius, 1995; Geng, 1997; Agostini et al. 1998), has also been recognized as playing a pivotal role in tissue and organ formation during fetal development (Ross, 1996; Clark & Clark, 1996; Jacobson et al. 1997; Tanimoto et al. 1998). With regard to bone, apoptosis is thought to play an important role in regulating different steps of skeletal development: terminally differentiated chondrocytes undergo apoptosis before the replacement by osteogenic cells during the course of endochondral bone formation (Horton et al. 1998); apoptosis of osteoclasts limits the lifespan of these cells during bone remodelling (Hughes & Boyce, 1997); factors, such as RANK ligand, that promote osteoclastogenesis also serve to inhibit the apoptosis of mature osteoclasts, whereas the anti-resorptive agents Calcitonin and Bisphosphonates are pro-apoptotic (Manolagas, 2000). Relatively less is known, however, about the occurrence of apoptosis in the cells of osteogenic lineage: in vivo studies have demonstrated that osteoblasts and osteocytes undergo apoptosis relatively frequently in developing bone, but rarely in mature bone (Bronckers et al. 1996; Noble et al. 1997). Although apoptosis is observed to occur mostly at sites of active bone remodelling, the present investigation concerns apoptosis during bone modelling and, in showing that such apoptosis affects mesenchymal cells adjacent to secreting osteoblasts, agrees with the role of apoptotic phenomena in early morphogenetic processes. More precisely, the fact that a higher number of cells was observed in apoptosis during DBF (deemed an invasive process in that it involves the invasion of mesenchyme filling the primary haversian spaces, as opposed to SBF, which proceeds following a non-invasive pattern) suggests that apoptosis during intramembranous ossification is mainly dedicated to making space for advancing bone osteogenesis. This hypothesis is in line with observations on osteoblast apoptosis by Jilka et al. (1998), according to whom the rate of bone formation should be regulated by both the rate of osteogenesis and the rate of apoptosis of bone cells pertaining to the osteogenic lineage. The lower number of apoptotic cells observed in SBF can, however, be considered in the light of tissue turnover, depending on the balance between cellular differentiation and apoptotis.
Another point for discussion is the presence of apoptotic bodies in association both with blood capillaries and with macrophages. In the former, it might be inferred that, because apoptosis (whose final stages are characterized by the formation of apototic bodies) is a metabolic process with high energy demands (Cotter et al. 1990; Richter et al. 1996; Garland & Halestrap, 1997; Formigli et al. 2002), it seems likely to occur preferentially near the capillaries. With regard to macrophages, apoptotic bodies are not only frequently observed in the vicinity of the macrophages but they are also often found occupying them. It is the fate of apoptotic bodies to be phagocytosed not solely by macrophages that have probably migrated from the surrounding vessels; they can also be phagocytosed by the neighbouring cells directly (Duvall et al. 1985; Williams & Smith, 1993). However, in our study we did not observe apoptotic bodies in the surrounding mesenchymal cells or osteoblasts and stromal cells.
In conclusion, the observations reported here are in line with the general view that apoptosis has a key role in many crucial biological processes, especially those related to the development and turnover of tissues and organs. In particular, we have demonstrated that apoptosis occurring during the onset of bone histogenesis is mostly linked to invasive DBF process and could fulfil the important role of contributing to skeletal development by providing the space necessary for advancing bone growth.
Fig. 5.
TEM micrograph showing a typical lamina of movable osteoblasts (mOB) lining the surface of a primitive haversian space in a 14-day-old chick embryo tibia. Note three apoptotic cells (arrows) located between the osteoblasts and the blood capillary. Scale bar = 3 µm.
Acknowledgments
C. Palumbo and M. Ferretti dedicate the present study to their guide and mentor, Professor Gastone Marotti, on the occasion of his 68th birthday. His incomparable style both in developing research programmes and in interpersonal relationships will remain as a constant inspiration. This study was supported by 2002 FAR (ex 60%).
References
- Agostini C, Perin A, Semenzato G. Cell apoptosis and granulomatous lung diseases. Curr. Opin. Pulm. Med. 1998;4:261–266. doi: 10.1097/00063198-199809000-00004. [DOI] [PubMed] [Google Scholar]
- Arends MJ, Wyllie AH. Apoptosis: mechanism and role in pathology. Int. Rev. Exp. Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Goei W, Luo G, Karsenty G, D’Souza RN, Lyaruu DM, et al. DNA fragmentation during bone formation in neonatal rodent assessed by transferase-mediated end labeling. J. Bone Miner. Res. 1996;11:1281–1291. doi: 10.1002/jbmr.5650110913. [DOI] [PubMed] [Google Scholar]
- Chow SC, Kass GE, Orrenius S. Purines and their roles in apoptosis. Neuropharmacology. 1997;36:1149–1156. doi: 10.1016/s0028-3908(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Clarke PGH, Clarke S. Nineteenth century research on naturally occurring cell death and related phenomena. Anat. Embryol. 1996;193:81–99. doi: 10.1007/BF00214700. [DOI] [PubMed] [Google Scholar]
- Cotter TG, Lennon SV, Glynn JG, Martin SJ. Cell death via apoptosis and its relationships to growth development and differentiation of both tumor and normal cells. Anticancer Res. 1990;10:1153–1159. [PubMed] [Google Scholar]
- Duvall E, Wyllie AH, Morris RG. Macrophage recognition of cells undergoing programmed cell death (apoptosis) Immunology. 1985;56:351–358. [PMC free article] [PubMed] [Google Scholar]
- Ferretti M, Palumbo C, Contri M, Marotti G. Static and dynamic osteogenesis: two different types of bone formation. Anat. Embryol. 2002;206:21–29. doi: 10.1007/s00429-002-0265-6. [DOI] [PubMed] [Google Scholar]
- Formigli L, Zecchi-Orlandini S, Capaccioli S, Poupon MF, Bani D. Energy-dependent types of cell death in MCF-7 breast cancer cell tumours implanted into nude mice. Cells Tissues Organs. 2002;170:99–110. doi: 10.1159/000046184. [DOI] [PubMed] [Google Scholar]
- Garland JM, Halestrap A. Energy metabolism during apoptosis. BCL-2 promotes survival in hematopoietic cells induced to apoptose by growth factor withdrawal by stabilizing a form of metabolic arrest. J. Biol. Chem. 1997;272:4680–4688. doi: 10.1074/jbc.272.8.4680. [DOI] [PubMed] [Google Scholar]
- Geng YJ. Regulation of programmed cell death or apoptosis in aterosclerosis. Heart Vessels. 1997;12(Suppl.):76–80. [PubMed] [Google Scholar]
- Haanen C, Vermes I. Apoptosis: programmed cell death in fetal development. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996;64:129–133. doi: 10.1016/0301-2115(95)02261-9. [DOI] [PubMed] [Google Scholar]
- Horton WE, Jr, Feng L, Adams C. Chondrocyte apoptosis in development, aging and disease. Matrix Biol. 1998;17:107–115. doi: 10.1016/s0945-053x(98)90024-5. [DOI] [PubMed] [Google Scholar]
- Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, et al. Bisphosphonate promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Miner. Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- Hughes DE, Boyce BF. Apoptosis in bone physiology and disease. Mol. Pathol. 1997;50:132–137. doi: 10.1136/mp.50.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MD, Well M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytochines. J. Bone Miner. Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Educhi K, Matsuoka N, Tsuboi M, Koji T, Urayama S, et al. Fas and Fas ligand interaction is necessary for human osteoblast apoptosis. J. Bone Miner. Res. 1997;12:1637–1646. doi: 10.1359/jbmr.1997.12.10.1637. [DOI] [PubMed] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulig E, Jin L, Qian X, Horvath E, Kovacs K, Stefaneanu L, et al. Apoptosis in nontumorous and neoplastic human pituitaries: expression of the Bcl-2 family of proteins. Am. J. Pathol. 1999;154:767–774. doi: 10.1016/S0002-9440(10)65323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kim SJ, Lee JY, Shin MS, Dong SM, Na EY, et al. Detection of soluble Fas mRNA using in situ reverse transcription-polymerase chain reaction. Lab. Invest. 1998;78:453–459. [PubMed] [Google Scholar]
- Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- Mansukhani A, Bellosta P, Sahni M, Basilico C. Signaling by fibroblast growth factors (FGF) and fibroblast growth factors receptor 2 (FGFR2) -activating mutations blocks mineralization and induces apoptosis of osteoblasts. J. Cell Biol. 2000;149:1297–1308. doi: 10.1083/jcb.149.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble BS, Stevens H, Loveridge N, Reeve J. Identification of apoptotic changes in osteocytes in normal and pathological human bone. Bone. 1997;20:273–282. doi: 10.1016/s8756-3282(96)00365-1. [DOI] [PubMed] [Google Scholar]
- Noble BS, Reeve J. Osteocyte function, osteocyte death and bone fracture resistance. Mol. Cell. Endocrinol. 2000;159:7–13. doi: 10.1016/s0303-7207(99)00174-4. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Naturally occurring cell death during neural development. Trends Neurosci. 1985;8:487–493. [Google Scholar]
- Orrenius S. Apoptosis: molecular mechanisms and implications for human disease. J. Intern. Med. 1995;237:529–536. doi: 10.1111/j.1365-2796.1995.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Richter C, Schweizer M, Cossarizza A, Franceschi C. Control of apoptosis by the cellular ATP level. FEBS Lett. 1996;378:107–110. doi: 10.1016/0014-5793(95)01431-4. [DOI] [PubMed] [Google Scholar]
- Ross ME. Cell division and the nervous system: regulating the cycle from neural differentiation to death. Trends Neurosci. 1996;19:62–68. doi: 10.1016/0166-2236(96)89622-6. [DOI] [PubMed] [Google Scholar]
- Tanimoto T, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, et al. Growth patterns in various macroscopic types of noninvasive intramucosal colorectal carcinoma with special preference to apoptosis and cell proliferation. Dis. Colon Rectum. 1998;41:1376–1384. doi: 10.1007/BF02237053. [DOI] [PubMed] [Google Scholar]
- Vilovic K, Sapunar D, Ilijic E, Mimica MD, England MA, Saraga-Babic M. Morphological characteristics of dying cells in axial structures of developing human embryos. Cells Tissues Organs. 2001;169:347–354. doi: 10.1159/000047901. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Bissel MJ. Functional culture models to study mechanisms governing apoptosis in normal and malignant mammary epithelial cells. J. Mammary Gland Biol. Neoplasia. 1999;4:193–201. doi: 10.1023/a:1018781325716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GT, Smith CA. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993;74:777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. Int. Rev. Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]