Abstract
The adult mammalian central nervous system (CNS) does not repair after injury. However, we and others have shown in earlier work that the neonatal CNS is capable of repair and importantly of allowing regenerating axons to re-navigate through the same pathways as they did during development. This phase of neonatal repair is restricted by the fragility of neurons after injury and a lack of trophic factors that enable their survival. Our aim is to define better the factors that sustain neurons after injury and allow regeneration to occur. We describe some of our work using Schwann cells to promote the regeneration of neurons from young postnatal rodents. We have established rapid methods for purifying Schwann cells without the use of either anti-mitotic agents to suppress contaminating fibroblasts or mitotic stimulation to generate large numbers of Schwann cells. The rapidly purified Schwann cells have been used to generate conditioned medium that we have shown stimulates axon regeneration in cultured retinal ganglion cell neurons. We also show that the positive effects of Schwann cells are still present after pharmacological blockade of the neurotrophin receptors, suggesting that novel factors mediate these effects.
Keywords: glial cell, neurotrophin, peripheral nerve, regeneration
The failure of the adult CNS to support regeneration
The adult mammalian CNS does not support axonal regeneration. This means that damage to the CNS through disease or injury results in permanent loss of function. A major goal for neuroscience is to understand the constraints that exist in the CNS which prevent repair, and to devise strategies which allow us to overcome these constraints without damaging side-effects (Horner & Gage, 2000).
It was long thought that the adult CNS showed no signs of regenerative response after injury. However, it is now clear that those neurons that survive injury do attempt to regenerate (Richardson et al. 1980; Schwab, 2002). The regeneration of these axons is prevented by the environment of the CNS, which is inhibitory, and by the lack of positive stimulating conditions for axon growth. The glial cell-mediated inhibition is via astrocytes that form inhibitory scars (Fawcett & Asher, 1999; Logan & Berry, 2002) and oligodendrocytes that express inhibitory myelin proteins (Filbin, 2003; Woolf, 2003). One suggestion concerning the inhibitory nature of the CNS environment is that it is designed to maintain stability. The astrocytes respond to injury by sealing the damaged area from the rest of the unaffected CNS and therefore allow the maintenance of whatever function is left (Morgenstern et al. 2002; Rhodes et al. 2003). The normal function of myelin-associated inhibition may be a mechanism that is used to prevent sprouting of neurons after the period when connections have been made (Colello & Schwab, 1994; Liu et al. 2002).
It is also clear that after an injury to the CNS, uninjured neurons are able to sprout and grow new processes, which clearly demonstrates the remarkable plasticity of the CNS, even in adult life (Raineteau & Schwab, 2001; Raineteau et al. 2002). On an optimistic level, this plasticity provides hope for functional recovery, both through regeneration of damaged neurons and also through sprouting and re-wiring of spared or associated axon tracts. However, it also raises a major problem of control of the regenerative process, as aberrant sprouting and establishment of abnormal connections may have consequences that are worse than the loss of function associated with the original injury (Edgerton et al. 2001).
In contrast to the CNS, the peripheral nervous system (PNS) does regenerate and function can be restored after peripheral nerve injury (Fawcett & Keynes, 1990). The major factor enabling the successful regeneration of peripheral nerves is the presence of Schwann cells. Schwann cells are the myelinating glial cells of the PNS, but following peripheral nerve damage they de-differentiate and secrete survival- and growth-promoting factors (Fawcett & Keynes, 1990). It is the precise nature of these factors and their effects on CNS neurons that we have been interested in exploring. On one level of investigation, we can consider Schwann cells as transplants that could provide a stimulus for CNS regeneration. This has been explored extensively in the adult lesioned CNS, where there is considerable debate and controversy about how positive the effects of such transplants might be (Li & Raisman, 1994; Dezawa & Adachi-Usami, 2000; Iwashita et al. 2000). Secondly, if we can understand more clearly the crucial factors made by Schwann cells that promote CNS regeneration, we may be able to manipulate other cells to provide these effects. It is to this end that we have tried to define the nature of Schwann cell factors and their effects on regenerating neurons.
Regeneration in the neonatal system
Whereas the adult CNS shows little signs of repair, the neonatal CNS offers some hope for successful repair. In the neonate, the CNS is still in the developmental phase, so the conditions promoting the growth of axons and the formation of connections are working in favour of regeneration. It is our belief that if we can repair the nervous system at this stage, we may take advantage of the relative plasticity to optimize functional recovery. There are obvious advantages of trying to stimulate CNS regeneration at this early stage as the growth-promoting environment that exists in the developing nervous system may still be present during the neonatal period. Indeed in certain systems, such as the corticospinal tract where axon growth continues postnatally, the necessary substrates and guidance cues are still expressed in an appropriate fashion to be used by regenerating axons (Schreyer & Jones, 1988; Cohen et al. 1998).
Investigating CNS repair at this stage also has a logical advantage in allowing the factors known to impede regeneration in the adult CNS to be addressed independently of the repair process. In the neonate, the inhibitory environment of the adult CNS is not yet developed so this major limitation does not have to be overcome. The astrocytes are positive stimulators of axon growth, secreting permissive extracellular matrix. Although there are limited data available, it is not thought that astrocytes at this stage form the same inhibitory scars as are found in the adult system. As myelination proceeds, the effects of the neurite growth inhibitors NOGO and myelin-associated glycoprotein (MAG) can be assessed and manipulated to optimize repair (Filbin, 2003).
Furthermore, the target cells of most systems are within the major phase of developmentally associated plasticity. This period is associated with large-scale neuronal death and the refinement of nerve connections (O'Leary et al. 1986). Although it is clear that the dated view of the hard-wired nervous system has been revised, because even the adult system shows plasticity, for most systems the early postnatal phase is when the CNS is most easily re-wired.
One useful animal model in which CNS regeneration during the early postnatal period has been explored is the marsupial (Nicholls & Sanders, 1996; Maclaren & Taylor, 1997b). Marsupial CNS development is largely postnatal, so experimental interference can be made both during and shortly after the major period of axon growth to study both normal development and neonatal regeneration.
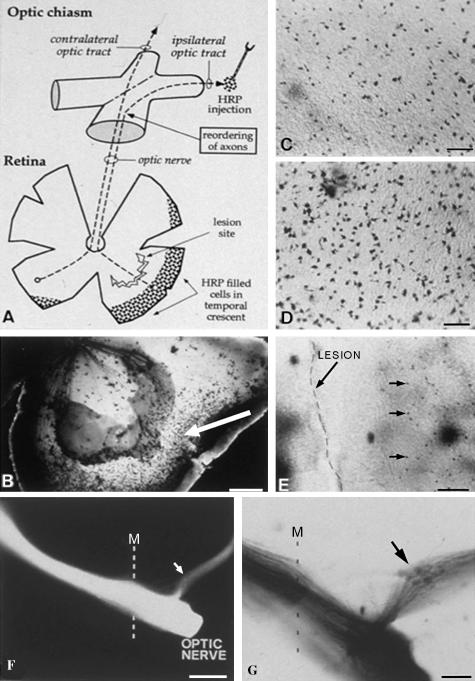
We used the short grey tailed opossum to study both retinofugal pathway development and regeneration of this pathway during the period towards the end of normal visual system development (Maclaren & Taylor, 1997a,b). Retinal ganglion cells (RGCs) were axotomized and a double labelling protocol used to detect regenerating axons. In brief, RGCs were labelled at their terminal mitosis using tritiated thymidine and these cells were then allowed to initiate their axons. We showed that the axons of these marked RGCs had reached the optic chiasm in the ventral diencephalon after 2 days. We therefore chose to lesion the retina 3 days after thymidine labelling, severing the RGC axons. Two to 3 weeks were allowed for the axons to regenerate and the cells in the retina were then labelled retrogradely with horse radish peroxidase (HRP) from the optic tract. By lesioning the temporo-ventral retina, the ability of the ipsilaterally projecting RGCs to re-navigate a correct path through the optic chiasm could also be assessed (Maclaren & Taylor, 1995). Our results showed that retinal axons could not only regenerate but also re-navigate the visual pathways and re-innervate the correct target nuclei within the CNS (Fig. 1). However, we found that there was a developmental time window after which axons failed to regenerate. The end of this neonatal regeneration period does not correlate with myelination (Maclaren, 1996a), nor with the expression of injury associated inhibitory molecules such as Chondroitin sulphate proteoglycans (CSPGs) (Maclaren, 1996b). The loss of regenerative ability does correlate with the time of innervation of the superior colliculus. In other studies this period of innervation has been shown to correspond to the peak period of susceptibility of neurons to cell death and hence an increased susceptibility to axotomy (Fagiolini et al. 1997; Ma et al. 1998).
Fig. 1.
Lesions of the marsupial (Monodelphis domestica) retina during the neonatal period result in regeneration with correct re-navigation of the visual pathways. (A) Schematic diagram showing the lesion of the temporo-ventral retina and position of HRP labelling in the ipsilateral optic tract, which was used to define the successful regeneration of RGCs with uncrossed projections. (B) The retina 10 days after lesion showing retrogradely labelled cells in the periphery behind the lesion (arrow). (C) Higher magnification view of regenerated retrogradely labelled retinal ganglion cells, which are fewer in number than those retrogradely labelled in un-lesioned control retinae (D). (E) The lesion and the regenerated RGCs. (F, G) Axons labelled with Dil (1, 1′ -dioctadecyl -3, 3, 3′, 3′ -tetramethylindocarbocyanide perchlorate) and photo-converted Dil, respectively, show the anterograde tracing of regenerating axons from behind a lesion in the temporo-ventral retina showing successful re-navigation of the optic chiasm. Scale bars in B and G, 200 µm; C and D, 25 µm; E, 50 µm; F, 500 µm.
Schwann cells as sources of regeneration stimulating factors
In the neonatal system it seemed that the major limitation on successful regeneration was not the inhibition generated by CNS glial cells or the inability of axons to grow and navigate along the correct CNS pathways, but that the cells did not survive the injury. We therefore switched our focus to trying to find factors that would allow retinal ganglion cells to both survive and then encourage the outgrowth of axons. As peripheral nervous system grafts or Schwann cells have been shown to encourage adult CNS regeneration (David & Aguayo, 1981; Li & Raisman, 1994; Cheng et al. 1996; Xu et al. 1997, 1999), including regeneration of adult retinal ganglion cells (So & Aguayo, 1985; Plant et al. 1995; Berry et al. 1996; Negishi et al. 2001), we focused on Schwann-cell-derived factors. We have isolated Schwann cells in vitro to examine the CNS regeneration-promoting factors that they produce. These effects have not been studied in neonatal neurons, in which the factors promoting regeneration may be different to those that are effective in the adult.
If the sciatic nerve is simply dissociated and grown in culture a mixture of Schwann cells and fibroblasts results. In such cultures the fibroblasts, with a greater mitotic index, rapidly dominate. To generate pure Schwann cells, fibroblasts must either be eliminated or suppressed. Many published methods use combinations of anti-mitotic agents to suppress early fibroblast division followed by mitogens to stimulate Schwann cell division once the fibroblasts have been eliminated (Raff et al. 1978; Brockes et al. 1979). We have used immunopanning to purify both rat and mouse Schwann cells taken from the sciatic nerves of neonatal animals (Barres et al. 1988; Bampton & Taylor, 2001). This technique allows pure Schwann cells to be isolated rapidly (Fig. 2A) without the use of mitotic inhibitors or stimulators commonly used in previous studies. These agents may affect the properties of the Schwann cells, particularly by changing the factors that they secrete (Yamamoto et al. 1993; Bermingham et al. 2001).
Fig. 2.
Purified rat P4 Schwann cells 3 days after immunopanning stained with S100. Retinal ganglion cells isolated from P4 rat retina, stained with carboxyfluorescein diacetate, growing in the presence of Schwann cell conditioned medium. The extensive neurite outgrowth of the neurons is regenerative. Scale bars, 50 µm.
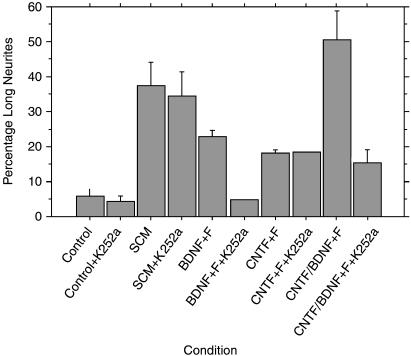
We used medium conditioned by these immunopurified Schwann cells to examine the effects on postnatal retinal ganglion cell survival and regeneration. We again used immunopanning (Barres et al. 1988) to isolate pure retinal ganglion cells. We have shown that Schwann cell conditioned medium has clear survival and neuritogenic properties (Fig. 2B). Because the axons of these RGCs would have been innervating their target cells at the time of their isolation, the promotion of neurite growth in these neurons is a clear measure of regeneration. The neuritogenic effects of Schwann cell conditioned medium were as good as a positive control medium containing a combination of brain derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF) and forskolin (Fig. 3). We also examined whether two Schwann cell lines, SCTM41 and PVGSV40T, had similar regeneration-promoting properties. We showed neither cell line has any significant effect in stimulating retinal ganglion cell regeneration at these stages.
Fig. 3.
Graphs showing the effects of different Schwann cell conditioned media (SCM) on RGC regeneration. Positive control medium containing BDNF or a combination of CNTF/BDNF and forskolin have slightly better neuritogenic effects than SCM. The effects of BDNF, but not those of CNTF (which does not act through a Trk receptor), are lost when the kinase inhibitor K252a is added. By contrast, SCM shows minimal loss of function when K252a is added. This suggests that the SCM positive regeneration-promoting factors are not acting through neurotrophin receptors.
An important question is what do Schwann cells make that underlies these effects? We know that Schwann cells produce a range of factors including neurotrophins (Funakoshi et al. 1993; Cai et al. 1999; Meier et al. 1999), but it is likely that, in addition, other factors are involved. For example, blocking neurotrophin effects by inhibiting tyrosine kinases (which include the major neurotrophin receptors of the Trk family), does not not significantly diminish the effects of Schwann cell medium on neuritogenesis. To identify these other factors we have used a radiolabelling assay, devised by Aviva Tolkovsky, in which the Schwann-cell-secreted proteins are labelled and fed to target cells (Fig. 4A; Bampton et al. 2003). By blocking protein synthesis in the target cells, any radioactive proteins must have been taken up from the conditioned medium. This assay revealed that only a very few proteins were taken up from the Schwann cell conditioned medium by the regenerating retinal ganglion cells (Fig. 4B; Bampton et al. 2003). Our efforts are now directed to isolating and purifying these proteins to test on purified RGC cultures both alone and in combination with other neurotrophic factors to try to stimulate optimal regeneration.
Fig. 4.
(A,B) A method used to radiolabel proteins made by Schwann cells, which are then fed to RGCs. The Schwann-cell-derived factors are taken up by RGCs and then isolated by fluorography. (B) Radiolabelled proteins in Schwann cell conditioned medium (lane 1) and after passing through a spin column to remove unbound radiolabel (lane 2). Lanes 3 and 4 show the residual radiolabelled SCM after incubation with retinal ganglion cells (different preparations). Lanes 5 and 6 show two different exposures of RGC lysates after 24 h incubation, showing a number of radiolabelled bands, including a clear band at 40 kDa (indicated by arrow), which has been taken up by RGCs from the Schwann cell conditioned medium.
One criticism of this work is that RGCs never encounter Schwann cells, so factors that enhance survival and regrowth may simply be a part of a more generalized response, but one that could only be used therapeutically if Schwann cells were transplanted to the retina. For obvious reasons, using the natural glia of the retina, the Müller glia, to induce positive stimulation of regeneration would be advantageous. We can purify Müller glial cells and have shown that these again produce factors that stimulate sympathetic neuronal survival and outgrowth (Reis et al. 2002). The Müller glial cell conditioned medium has also been shown to promote retinal ganglion cell survival and regeneration, to a level that is better than the defined neurotrophin-containing medium (Reis et al. 2003).
In summary, we think that the neonatal visual system offers a very useful model for studies of CNS repair. First, the factors acting against regeneration are fewer in the neonatal CNS and the major obstacle is maintaining cells after axotomy. To prevent neuronal cell death after axotomy, neurotrophins can be used (Lui et al. 1998), but we have also found that other factors may be used as alternatives, or in combination with neurotrophins. Experiments using Schwann cells, and current work on Müller glial cells, show that there are novel regeneration-promoting factors that are taken up by axotomized retinal ganglion cells. Using the neonatal visual system, in which information on the normal pathfinding cues that are used by developing axons is perhaps best described (Mason & Sretavan, 1997), we can analyse the continued or re-expression of these cues during regeneration. We have shown that within this early postnatal phase the regenerating axons do have the ability to re-navigate that provides hope that long-distance projection neurons may be stimulated to restore functional connections.
References
- Bampton ETW, Taylor JSH. RT-PCR analysis of neurotrophic factor expression in Schwann cells prepared by different methods. Soc. Neurosci. 2001;27:219.16. [Google Scholar]
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Jr, Shumas S, Whisenhunt T, Rosenfeld MG, Scherer SS. Modification of representational difference analysis applied to the isolation of forskolin-regulated genes from Schwann cells. J. Neurosci. Res. 2001;63:516–524. doi: 10.1002/jnr.1046. [DOI] [PubMed] [Google Scholar]
- Berry M, Carlile J, Hunter A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J. Neurocytol. 1996;25:147–170. doi: 10.1007/BF02284793. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Cai F, Campana WM, Tomlinson DR, Fernyhough P. Transforming growth factor-beta1 and glial growth factor 2 reduce neurotrophin-3 mRNA expression in cultured Schwann cells via a cAMP-dependent pathway. Brain Res. Mol. Brain Res. 1999;71:256–264. doi: 10.1016/s0169-328x(99)00200-4. [DOI] [PubMed] [Google Scholar]
- Cheng H, Cao Y, Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- Cho EYP, So K-F. Characterisation of the sprouting response of axon-like processes from retinal ganglion cells after axotomy in adult hamsters: a model using intravitreal implantation of a peripheral nerve. J. Neurocytol. 1992;21:589–603. doi: 10.1007/BF01187119. [DOI] [PubMed] [Google Scholar]
- Cohen NR, Taylor JSH, Scott LB, Guillery RW, Soriano P, Furley AJ. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr. Biol. 1998;8:26–33. doi: 10.1016/s0960-9822(98)70017-x. [DOI] [PubMed] [Google Scholar]
- Colello RJ, Schwab ME. A role for oligodendrocytes in the stabilization of optic axon numbers. J. Neurosci. 1994;14:6446–6452. doi: 10.1523/JNEUROSCI.14-11-06446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, So K-F, Yip HK. Major biological effects of neurotrophic factors on retinal ganglion cells in mammals. Biol. Sig. Recept. 1998;7:220–226. doi: 10.1159/000014546. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal regeneration into peripheral nervous system ‘bridges’ after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Dezawa M, Adachi-Usami E. Role of Schwann cells in retinal ganglion cell regeneration. Prog. Ret. Eye Res. 2000;19:171–204. doi: 10.1016/s1350-9462(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, et al. Retraining the injured spinal cord. J. Physiol. 2001;533:15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Caleo M, Strettoi E, Maffei L. Axonal transport blockade in the neonatal rat optic nerve induces limited retinal ganglion cell death. J. Neurosci. 1997;17:7045–7052. doi: 10.1523/JNEUROSCI.17-18-07045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu. Rev. Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res. Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev. Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge VM, et al. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J. Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Iwashita Y, Fawcett JW, Crang AJ, Franklin RJ, Blakemore WF. Schwann cells transplanted into normal and X-irradiated adult white matter do not migrate extensively and show poor long-term survival. Exp. Neurol. 2000;164:292–302. doi: 10.1006/exnr.2000.7440. [DOI] [PubMed] [Google Scholar]
- Li Y, Raisman G. Schwann cells induce sprouting in motor and sensory axons in the adult rat spinal cord. J. Neurosci. 1994;14:4050–4063. doi: 10.1523/JNEUROSCI.14-07-04050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Logan A, Berry M. Cellular and molecular determinants of glial scar formation. Adv. Exp. Med. Biol. 2002;513:115–158. doi: 10.1007/978-1-4615-0123-7_4. [DOI] [PubMed] [Google Scholar]
- Ma YT, Hsieh T, Forbes ME, Johnson JE, Frost DO. BDNF injected into the superior colliculus reduces developmental retinal ganglion cell death. J. Neurosci. 1998;18:2097–2107. doi: 10.1523/JNEUROSCI.18-06-02097.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclaren RE, Taylor JSH. A critical period for axon regrowth through a lesion in the developing mammalian retina. Eur. J. Neurosci. 1995;7:2111–2118. doi: 10.1111/j.1460-9568.1995.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Maclaren RE. Expression of myelin proteins in the opossum optic nerve: late appearance of inhibitors implicates an earlier non-myelin factor in preventing ganglion cell regeneration. J. Comp. Neurol. 1996a;372:27–36. doi: 10.1002/(SICI)1096-9861(19960812)372:1<27::AID-CNE3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Maclaren RE. Development and role of retinal glia in regeneration of ganglion cells following retinal injury. Br. J. Ophthalmol. 1996b;80:458–464. doi: 10.1136/bjo.80.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclaren RE, Taylor JSH. Chiasmatic specificity in the regenerating mammalian optic nerve. Exp. Neurol. 1997a;147:279–286. doi: 10.1006/exnr.1997.6620. [DOI] [PubMed] [Google Scholar]
- Maclaren RE, Taylor JSH. Regeneration in the developing optic nerve: correlating observations in the opossum to other mammalian systems. Prog. Neurobiol. 1997b;53:381–398. doi: 10.1016/s0301-0082(97)00041-5. [DOI] [PubMed] [Google Scholar]
- Mason CA, Sretavan DW. Glia, neurons, and axon pathfinding during optic chiasm development. Curr. Opin. Neurobiol. 1997;7:647–653. doi: 10.1016/s0959-4388(97)80084-0. [DOI] [PubMed] [Google Scholar]
- Meier C, Parmantier E, Brennan A, Mirsky R, Jessen KR. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrince circuit involving insulin-like growth factor, neurotrophin-3 and platelet-derived growth factor-BB. J. Neurosci. 1999;19:3847–3859. doi: 10.1523/JNEUROSCI.19-10-03847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Morgenstern DA, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 2002;137:313–332. doi: 10.1016/s0079-6123(02)37024-9. [DOI] [PubMed] [Google Scholar]
- Negishi H, Dezawa M, Oshitari T, Adachi-Usami E. Optic nerve regeneration within artificial Schwann cell graft in the adult rat. Brain Res. Bull. 2001;55:409–419. doi: 10.1016/s0361-9230(01)00534-2. [DOI] [PubMed] [Google Scholar]
- Nicholls J, Sanders N. Regeneration of immature mammalian spinal cord after injury. Trends Neurosci. 1996;19:229–234. doi: 10.1016/0166-2236(96)10021-7. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Fawcett JW, Cowan WM. Topographic targeting errors in the retinocollicular projection and their elimination by selective ganglion cell death. J. Neurosci. 1986;6:3692–3705. doi: 10.1523/JNEUROSCI.06-12-03692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant GW, Harvey AR, Chirila TV. Axonal growth within poly (2-hydroxyethyl methacrylate) sponges infiltrated with Schwann cells and implanted into the lesioned rat optic tract. Brain Res. 1995;671:119–130. doi: 10.1016/0006-8993(94)01312-6. [DOI] [PubMed] [Google Scholar]
- Raff MC, Hornby-Smith A, Brockes JP. Cyclic AMP as a mitogenic signal for cultured rat Schwann cells. Nature. 1978;273:672–673. doi: 10.1038/273672a0. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev. Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Fouad K, Bareyre FM, Schwab ME. Reorganization of descending motor tracts in the rat spinal cord. Eur. J. Neurosci. 2002;16:1761–1771. doi: 10.1046/j.1460-9568.2002.02243.x. [DOI] [PubMed] [Google Scholar]
- Reis RA, Cabral da Silva MC, Loureiro dos Santos NE, Bampton ETW, Taylor JSH, de Mello FG, et al. Sympathetic neuronal survival induced by retinal trophic factors. J. Neurobiol. 2002;50:13–23. doi: 10.1002/neu.10008. [DOI] [PubMed] [Google Scholar]
- Reis RAM, Cabral-da-Silva MC, de Mello FG, Linden R, Taylor JSH. Mice Müller glia factors and their role in retinal ganglion cell survival and neuritogenesis. Soc. Neurosci. 2003;29:147.19. [Google Scholar]
- Rhodes KE, Moon LD, Fawcett JW. Inhibiting cell proliferation during formation of the glial scar: effects on axon regeneration in the CNS. Neuroscience. 2003;120:41–56. doi: 10.1016/s0306-4522(03)00285-9. [DOI] [PubMed] [Google Scholar]
- Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- Schreyer DJ, Jones EH. Topographic sequence of outgrowth of corticospinal axons in the rat: a study using retrograde axonal labeling with Fast blue. Brain Res. 1988;466:89–101. doi: 10.1016/0165-3806(88)90088-0. [DOI] [PubMed] [Google Scholar]
- Schwab ME. Repairing the injured spinal cord. Science. 2002;295:1029–1031. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- So K-F, Aguayo AJ. Lengthy regrowth of cut axons from ganglion cells after peripheral nerve transplantation into the retina of adult rats. Brain Res. 1985;328:349–354. doi: 10.1016/0006-8993(85)91047-9. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. No Nogo: now where to go? Neuron. 2003;38:153–156. doi: 10.1016/s0896-6273(03)00233-2. [DOI] [PubMed] [Google Scholar]
- Xu XM, Chen A, Guenard V, Kleitman N, Bunge MB. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J. Neurocytol. 1997;26:1–16. doi: 10.1023/a:1018557923309. [DOI] [PubMed] [Google Scholar]
- Xu XM, Zhang S-X, Li H, Aebischer P, Bunge MB. Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur. J. Neurosci. 1999;11:1723–1740. doi: 10.1046/j.1460-9568.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sobue G, Li M, Arakawa Y, Mitsuma T, Kimata K. Nerve growth factor (NGF) and low-affinity nerve growth factor receptor (LNGFR) mRNA levels in cultured rat Schwann cells; differential time- and dose-dependent regulation by cAMP. Neurosci. Lett. 1993;152:37–40. doi: 10.1016/0304-3940(93)90477-3. [DOI] [PubMed] [Google Scholar]