Abstract
The anti-spasmogenic potential of SK&F 94120 (PDE3-selective), rolipram (PDE4-selective), zaprinast (PDE5-selective), zardaverine (dual PDE3/4 inhibitor) and theophylline (non-selective) was evaluated in guinea-pig trachealis.
SK&F 94120 or rolipram (10 and 100 μM) antagonized histamine-induced tension generation in a concentration-dependent and non-competitive manner whereas ACh-induced contractions were unaffected. Similarly, SK&F 94120 and rolipram in combination were anti-spasmogenic with respect to both contractile agonists to an extent that was greater than the effect of either drug alone. Identical results were obtained with zardaverine (1, 10 and 100 μM) and theophylline (100 μM and 1 mM).
Zaprinast protected guinea-pig trachealis against histamine-, but not ACh-induced contractile responses in a manner that was indistinguishable from the results obtained with SK&F 94120. However, in contrast to the interaction between SK&F 94120 and rolipram, no further antagonism was seen when zaprinast and rolipram were used in combination.
Pre-treatment of tissues with SNP (10 and 100 μM) antagonized histamine-induced tension generation in a concentration-dependent and non-competitive manner. However, no further antagonism was produced when SNP and rolipram were used concurrently. Likewise, the protection afforded by a combination SNP and SK&F 94120 was no greater than that produced by SNP alone.
These results demonstrate that an inhibitor of PDE3 enhances the anti-spasmogenic activity of rolipram but not drugs that elevate cyclic GMP mass. Moreover, the ability of SNP and zaprinast to protect guinea-pig trachealis against histamine-induced contractions apparently is not due to the inhibition of PDE3.
Keywords: Guinea-pig trachealis, phosphodiesterase inhibitors–anti-spasmogenic activity, SK&F 94120–anti-spasmogenic activity, rolipram, zardaverine, zaprinast, asthma–treatment with phosphodiesterase inhibitors
Introduction
Cyclic nucleotide phosphodiesterases (PDEs) represent an expanding group of proteins whose sole function is to hydrolyse, and thereby inactive, the second messenger purine nucleotides, cyclic AMP and cyclic GMP (Beavo, 1987; 1995). Ten families, denoted PDE1 to 10, comprising a multitude of immunologically distinct proteins have been described thus far, which are categorized according to specific criteria including substrate specificity, inhibitor sensitivity and primary amino acid sequence (Beavo et al., 1994; Conti et al., 1995; Fisher et al., 1998a,1998b; Giembycz & Kelly, 1994; Loughney & Ferguson, 1994; 1996; Soderling et al., 1998; 1999; Fujishige et al., 1999; Loughney et al., 1999).
From a therapeutic perspective, the last decade has seen a renaissance in PDE inhibitors as novel therapeutic agents. In particular, drugs that suppress selectively the cyclic AMP-specific, or PDE4, isoenzyme family, whose primary function is to degrade cyclic AMP, are currently undergoing clinical trials for a number of inflammatory disorders such as asthma (Dent & Giembycz, 1995; Giembycz, 1992; Giembycz & Souness, 1996; Torphy, 1998; Torphy & Undem, 1991; Torphy, et al., 1994), chronic obstructive pulmonary disease (Barnes, 1998; Rogers & Giembycz, 1998), arthritis (Souness & Foster, 1998) and atopic dermatitis (Hanifin et al., 1996). Small molecule inhibitors that block both PDE4 and PDE3, another cyclic AMP PDE that is inhibited by cyclic GMP, similarly have been investigated since they may be effective at concentrations lower than selective PDE4 inhibitors and might possess fewer or less severe side-effects (Hatzelmann et al., 1996).
The rationale for developing isoenzyme-selective PDE inhibitors has stemmed from the realization that the distribution of PDE proteins is differentially expressed between tissues (Nicholson et al., 1991). Indeed, in essentially all pro-inflammatory and immune cells implicated in asthma pathogenesis, PDE4 is the principal enzyme responsible for degrading cyclic AMP. PDE3 also is present in certain cells including T-lymphocytes, alveolar macrophages, basophils and platelets (Giembycz, 1992; Giembycz et al., 1997; Torphy, 1998; Torphy & Undem, 1991) which has lead to the view that the development of compounds which selectively inhibit PDE4, or PDE3 and PDE4 might be associated with reduced side-effect profiles when compared to non-selective drugs such as theophylline.
Although PDE4 and hybrid PDE3/4 inhibitors are being developed primarily as a new class of steroid-sparing anti-inflammatory agents, they also have the potential to relax airways smooth muscle. In the trachealis of many species including the guinea-pig, at least one representative of the PDE1, 2, 3, 4 and 5 isoenzyme family is expressed (Miyamoto et al., 1994) implying that the regulation of cyclic nucleotide homeostasis in this tissue is highly co-ordinated and more complex than in pro-inflammatory and immunocompetent cells (Giembycz et al., 1997). However, while the spasmolytic activity of these drugs has been studied in some detail (see Souness & Giembycz, 1994), those data may be considered academic since if PDE4 inhibitors are ultimately shown to be active clinically in asthma, they will be administered prophylactically. It is clear, therefore, that under those conditions any additional beneficial effects would be to protect the airways against mediators that elicit bronchoconstriction.
Relatively little is known of the anti-spasmogenic activity of PDE inhibitors when compared to their ability to promote bronchodilatation. Moreover, the limited investigations thus far conducted have provided inconsistent findings that may relate to differences in species, airway level and/or the nature of spasmogen examined. For example, in bovine trachealis benafentrine, a mixed inhibitor of the PDE3 and PDE4 isoenzyme families, protects against methacholine-induced tension generation when low concentrations of the cholinomimetic are used (Giembycz & Barnes, 1991). Conversely, selective inhibitors of both PDE3 (siguazodan) and PDE4 (rolipram) fail to antagonize histamine and leukotriene D4-induced tension generation in guinea-pig trachea (Underwood et al., 1994). Given the paucity of information on the anti-spasmogenic activity of PDE inhibitors, we have performed a systematic study designed to assess the potential anti-spasmogenic activity of selective PDE inhibitors in guinea-pig tracheal smooth muscle with emphasis on those agents that target the PDE3 and/or PDE4 isoenzyme families.
Methods
Preparation of guinea-pig trachea
Male Dunkin-Hartley guinea-pigs (Harlan-Olac Ltd, Bicester, Oxfordshire), ranging in weight from 300–500 g, were killed by cervical dislocation. The tracheae were rapidly excised, placed in oxygenated (95% O2, 5% CO2) Krebs-Henseleit (KH) solution (composition in mM: NaCl 118, KCl 5.9, NaHCO3 25.5, MgSO4 1.2, CaCl2 2.5, NaH2PO4 1.2 and glucose 5.6) at room temperature and trimmed free of adherent fat and connective tissue. Indomethacin (10 μM) was present in the KH solution to prevent the formation of broncho-active prostanoids. Each trachea was cut into eight rings containing three to four adjacent cartilage plates and denuded of epithelium by gentle rubbing the mucosal surface with a cotton wool-coated probe. The rings were suspended by steel hooks in 5 ml organ baths containing KH solution at 37°C and connected via silk threads to Grass FT-03 force-displacement transducers (Quincy, MA, U.S.A.) to detect changes in isometric tension which were recorded on a Grass 7D polygraph. The tissues were placed under a resting force of 10 mN (optimal for determining changes in tension) and allowed to equilibrate for 60 min during which time tissues were washed frequently.
Protocol
Tracheal rings were challenged with a maximally effective concentration of acetylcholine (ACh; 10 mM). When the contractions had reached a plateau, the tissues were washed extensively until resting tone was re-established. After equilibration of tissues for a further 30 min, PDE inhibitors, sodium nitroprusside (SNP) or their respective vehicles were added to the baths and cumulative concentrations-response curves were constructed for histamine and ACh 20 min later according to the method of Van Rossum (1963). One concentration-response curve was generated per tissue. Agonist-induced changes in force are expressed as a percentage of the maximum contraction elicited by ACh (10 mM).
Drugs and analytical reagents
Indomethacin, histamine diphosphate, ACh chloride, SNP, EHNA (erythro-9-(2-hydroxy-3-nonyl) adenine hydrochloride), vinpocetine and theophylline were purchased from the Sigma Chemical Company (Poole, Dorset). SK&F 94120, rolipram, zaprinast and zardaverine were kindly donated by SmithKline-Beecham (Welwyn, Hertfordshire), Schering AG (Berlin, Germany), Rhone-Poulenc Rorer (Dagenham, Essex) and Byk Gulden (Konstanz, Germany) respectively.
Stock solutions of drugs were made at a concentration of 10 mM in ethanol (rolipram), DMSO (zardaverine, vinpocetine), 0.1 M NaOH (zaprinast, SK&F 94120, theophylline) or distilled water (histamine, ACh, SNP, ENHA) and subsequently diluted to the desired working concentration in KH solution. Indomethacin was prepared in alkaline phosphate buffer (KH2PO4 20 mM, Na2HPO4 120 mM; pH 7.8) at 1 mg ml−1.
Analysis of data
Data are expressed as mean±s.e.mean of n independent observations. Concentration-response curves were analysed by least squares, non-linear iterative regression with the ‘PRISM' curve-fitting program (GraphPad software, San Diego, CA, U.S.A.) and pD2 values were subsequently interpolated from curves of best fit. Statistical analysis was conducted by Mann Whitney's two-sample test. The null hypothesis was rejected when P<0.05.
Results
Influence of a PDE3 and a PDE4 inhibitor on histamine- and ACh-induced tension development
Histamine and ACh produced a concentration-dependent contraction of guinea-pig tracheal rings with pD2 values of 5.88±0.02 (n=36) and 5.48±0.04 (n=21) respectively; both agonists generated approximately the same force (18.9±8 mN, n=36; 20.6±9 mN, n=21 respectively) at their maximally effective concentrations. To examine whether PDE3 and PDE4 can regulate the spasmogenic activity of the aforementioned agonists, SK&F 94120 and rolipram were employed, which are selective inhibitors of these isoenzyme families respectively. Previous studies, in cell-free systems, have established that SK&F 94120 and rolipram retain isoenzyme selectivity at concentrations up to 30 μM and display only weak cross-reactivity (IC50>>100 μM at 1 μM cyclic AMP) at 100 μM (Torphy & Cieslinski, 1990; Murray et al., 1992).
Pre-treatment (20 min) of tracheal rings with SK&F 94120 (10 and 100 μM), had a negligible effect on baseline tone, but antagonized histamine-induced tension generation in a concentration-dependent manner. The nature of the antagonism resembled classical non-competitive behaviour; there was a parallel shift of the concentration-response curve to the right and an associated reduction in the maximal force generated (Figure 1a; Table 1). In contrast, SK&F 94120 did not significantly affect ACh-induced contractions at either concentration examined (Figure 1a, Tables 1 and 2).
Figure 1.
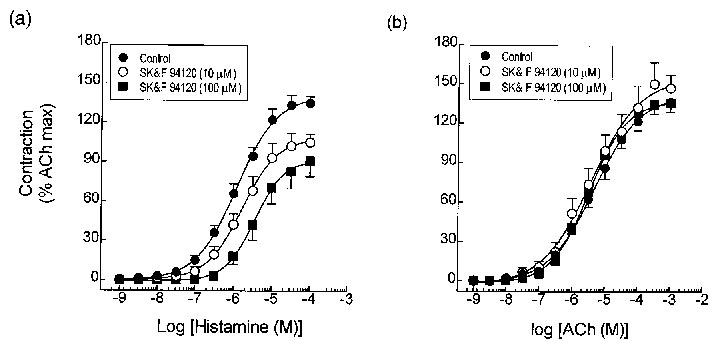
Effect of the PDE3 inhibitor, SK&F 94120, on histamine- and ACh-induced contractile responses in guinea-pig trachea. Rings of tracheal smooth muscle were pre-treated for 20 min with 10 or 100 μM SK&F 94120, or its vehicle. Cumulative concentration-response curves were then constructed to histamine (a) and ACh (b) and EC50 values and the maximum tension generated relative to that effected by 10 mM ACh were then interpolated from curves of best fit. Data represent the mean±s.e.mean of 6–8 determinations. See Methods for further details.
Table 1.
Effect of isoenzyme-selective PDE inhibitors and SNP on the contractile potency of histamine in guinea-pig tracheal rings

Table 2.
Effect of isoenzyme-selective PDE inhibitors and SNP on the contractile potency of ACh in guinea-pig tracheal rings
A different pattern of activity was seen in tissues pre-treated (20 min) with the selective PDE4 inhibitor, rolipram (10 and 100 μM). Thus, at the low concentration, rolipram failed to effect baseline tone or the concentration-response curves that described histamine-induced tension generation. However, increasing the concentration of rolipram to 100 μM produced a parallel, rightwards displacement of the histamine concentration-response curves without reducing the maximum response (Figure 2a and Table 1). This effect of rolipram was modest (∼2 fold shift at the EC50) but, nevertheless, achieved statistical significance (P<0.05). ACh-induced responses were unaffected by rolipram at either concentration examined (Figure 2b and Table 2).
Figure 2.

Effect of the PDE4 inhibitor, rolipram, on contractile responses evoked by histamine (a) and ACh (b) in guinea-pig trachea. Data represent the mean±s.e.mean of 6–8 determinations. See legend to Figure 1 and Methods for further details.
Influence of a PDE3 and a PDE4 inhibitor in combination, a mixed PDE3/PDE4 inhibitor and a non-selective PDE inhibitor on histamine- and ACh-induced tension development
In many cells and tissues PDE3 and PDE4 inhibitors interact synergistically both at the biochemical and functional level (e.g. Turner et al., 1994; Giembycz et al., 1996; Palmer et al., 1998). To establish if this phenomenon also applied to their anti-spasmogenic effect in guinea-pig trachealis, SK&F 94120 and rolipram were used in combination and compared with the mixed PDE3/PDE4 inhibitor, zardaverine (Schudt et al., 1991), and theophylline, which indiscriminately suppresses the activity of all PDE isoenzymes that have been studied.
Pre-treatment (20 min) of tracheal rings with SK&F 94120 and rolipram antagonized histamine-induced tension generation in a concentration-dependent manner. Again, the antagonism was non-competitive and of greater magnitude than that produced by either drug alone (Figure 3a and Table 1). In the presence of a concentration of SK&F 94120 (100 μM) known to abolish PDE3 activity in various cells and tissues, 100 μM rolipram effected greater antagonism of histamine-induced tension generation than when a lower, isoenzyme-selective concentration (10 μM), was employed (Figure 3a and Table 1). Thus, in intact guinea-pig tracheal strips a high concentration of rolipram apparently is required to fully inhibit PDE4.
Figure 3.

Effect of the PDE5 inhibitor, zaprinast, on contractile responses evoked by histamine (a) and ACh (b) in guinea-pig trachea. Data represent the mean±s.e.mean of 6–8 determinations. See legend to Figure 1 and Methods for further details.
Rolipram (10 μM) and SK&F 94120 (10 μM) similarly displaced ACh concentration-response curves to the right (∼2 fold at the EC50) without reducing the maximum response (Figure 3b). A much greater antagonism was seen when 100 μM of each inhibitor was examined although in this case, it was not possible to ascertain whether this was associated with a suppression of the asymptote as maximal responses were not attained (Figure 3b and Table 2). Identical results were found with tracheal smooth muscle rings pre-treated (20 min) with zardaverine (1, 10 and 100 μM) and theophylline (100 μM and 1 mM). Thus, contractions evoked by histamine and ACh were inhibited in a concentration-dependent and non-competitive manner with histamine-induced tone being more sensitive to the PDE inhibitors (Figures 4 and 5 and Tables 1 and 2).
Figure 4.
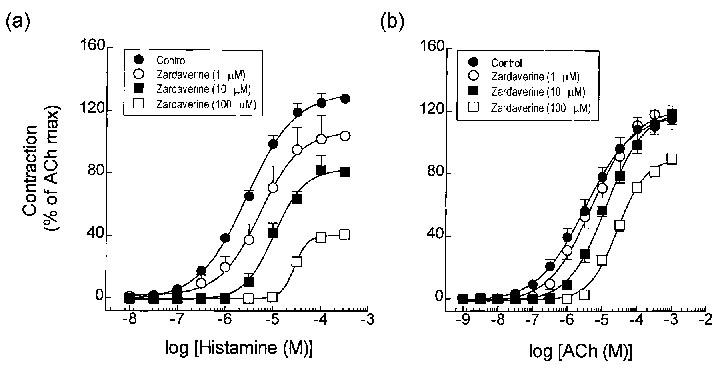
Effect of the zardaverine, an inhibitor of PDE3 and PDE4, on contractile responses evoked by histamine (a) and ACh (b) in guinea-pig trachea. Data represent the mean±s.e.mean of 6–8 determinations. See legend to Figure 1 and Methods for further details.
Figure 5.

Effect of theophylline on contractile responses evoked by histamine (a) and ACh (b) in guinea-pig trachea. Data represent the mean±s.e.mean of 6–8 determinations. See legend to Figure 1 and Methods for further details.
Influence of a PDE5 inhibitor, alone and in combination with rolipram, on histamine- and ACh-induced tension development
One characteristic of PDE3 is that cyclic GMP binds to the catalytic site with high affinity but is hydrolysed with a Vmax that is only approximately 10% of that achieved when cyclic AMP is substrate (see Degerman et al., 1997 for review). As such, cyclic GMP behaves as a competitive antagonist and can increase cyclic AMP mass in intact cells and tissues (Degerman et al., 1997). The ability of SK&F 94120 to protect the airways against the contractile effect of histamine and to act synergistically with rolipram in that respect, suggests that drugs which elevate cyclic GMP could behave similarly through the inhibition of PDE3. To test this hypothesis, the effect of the PDE5 inhibitor, zaprinast (10 and 100 μM), alone and in the presence of rolipram (10 and 100 μM), on histamine- and ACh-induced tension development was examined. As illustrated in Figure 6a,b, zaprinast protected airways smooth muscle against histamine-, but not ACh-induced tone in a manner that was indistinguishable from the results obtained with SK&F 94120 (c.f. Figure 1 and Tables 1 and 2). However, in contrast to the ability of the PDE3 and PDE4 inhibitor to act synergistically, no further antagonism of histamine-induced tone was seen when zaprinast and rolipram were used in combination (Figure 6c and Table 1). Similarly, there was no statistical difference between the pD2 of ACh in vehicle- and zaprinast/rolipram-treated tissues (Figure 6d and Table 2).
Figure 6.
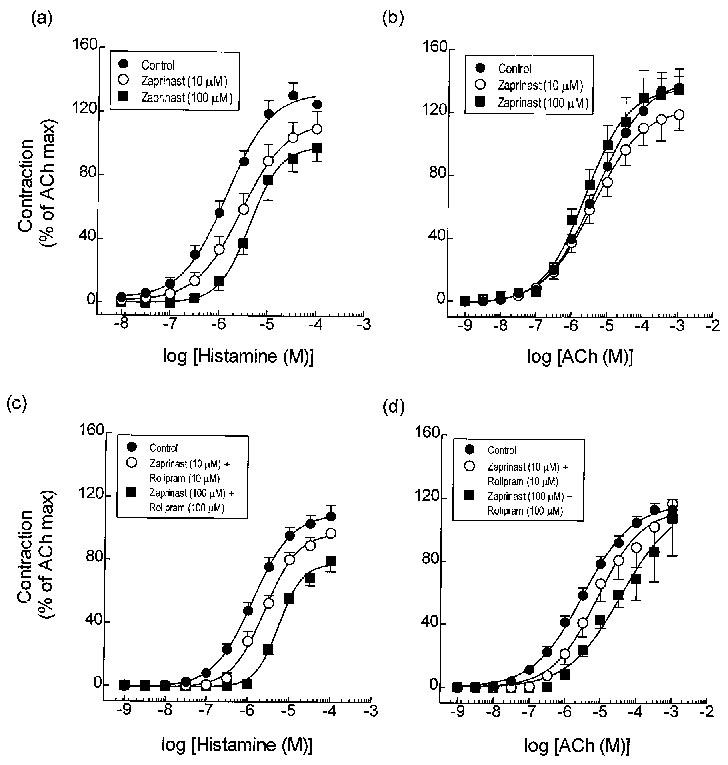
Effect of zaprinast, and zaprinast and rolipram in combination on contractile responses evoked by histamine (a) and (c) and ACh (b) and (d) in guinea-pig trachea. Data represent the mean±s.e.mean of 6–8 determinations. See legend to Figure 1 and Methods for further details.
Influence of SNP, alone and in combination with rolipram or SK&F 94120, on histamine-induced tension development
To explore further the relationship between cyclic GMP-elevating drugs and PDE3 in regulating guinea-pig tracheal smooth muscle tone, the potential anti-spasmogenic effect of SNP, an activator of soluble guanylyl cyclase, was examined on histamine-induced contractile responses alone and in combination with rolipram and SK&F 94120. Concordant with the results obtained with zaprinast and the PDE3 inhibitor, SK&F 94120, pre-treatment of tracheal rings with SNP (10 and 100 μM), antagonized histamine-induced tension generation in a concentration-dependent and non-competitive manner (Figure 7a and Table 1). No further antagonism was afforded when SNP and rolipram (10 and 100 μM respectively) were used in combination (Figure 7b,c and Table 1). Likewise, the protection afforded by a combination of SNP (100 μM) and SK&F 94120 (10 μM), and SNP (100 μM), SK&F 94120 (10 μM) and rolipram (10 (M) was not statistically greater than that produced by SNP alone (Figure 7d,e and Figure 1).
Figure 7.
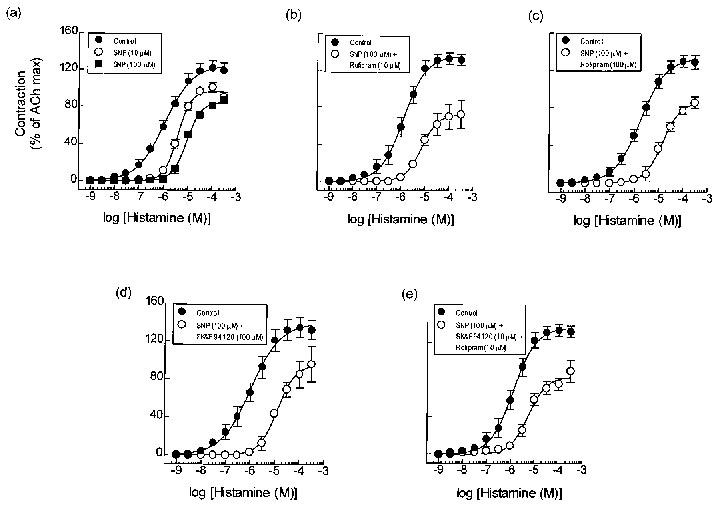
Effect of SNP in the absence (a) and presence of rolipram (10 μM) (b), SK&F 94120 (100 μM) (c), rolipram (100 μM) (d) and SK&F 94120 (10 μM) and rolipram (10 μM in combination (e) on contractile responses evoked by histamine in guinea-pig trachea. Data represent the mean±s.e.mean of 5–8 determinations. See legend to Figure 1 and Methods for further details.
Lack of effect of vinpocetine and ENHA on histamine-induced tension development
Pre-treatment (20 min) of tracheal rings with 10 and 100 μM of the putative PDE1 and PDE2 inhibitors vinpocetine (Hagiwara et al., 1984) and EHNA (Podzuweit et al., 1995) respectively, did not antagonize histamine- or ACh-induced tension generation (Tables 1 and 2).
Discussion
There is considerable optimism that PDE4 and/or hybrid PDE3/4 inhibitors ultimately may constitute a new generation of steroid-sparing, anti-inflammatory agents with therapeutic utility in the treatment of a number of diseases including asthma (Dent & Giembycz, 1995; Giembycz, 1992; Giembycz et al., 1997; Torphy, 1998; Torphy et al., 1994; Torphy & Undem, 1991) and chronic obstructive pulmonary disease (Barnes, 1998; Rogers & Giembycz, 1998). Although PDE inhibitors are designed to exert their beneficial effects by suppressing the activity of pro-inflammatory and immune cells, an additional anti-spasmogenic action at the level of airways smooth muscle might also be predicted given that they will be administered prophylactically. Surprisingly, however, while there is a wealth of literature describing the ability of selective PDE inhibitors to promote relaxation of airways smooth muscle in a number of species including the dog, guinea-pig, cow and humans (see Souness & Giembycz, 1994), few reports have formally determined if these drugs can also protect the airways against stimuli that elicit contraction. Thus, in the present study we have assessed if representative inhibitors of the PDE1, 2, 3, 4 and 5 isoenzyme families protect guinea-pig tracheal smooth muscle against contractions evoked by two mediators, ACh and histamine. These spasmogens were selected since they are recognized mediators of bronchoconstriction in subjects with nocturnal asthma and contribute to the acute bronchoconstriction that develops in asthmatic individuals in response to allergen provocation respectively.
Pre-treatment of guinea-pig trachea with vinpocetine and EHNA, drugs purported to selectively inhibit PDE1 (Hagiwara et al., 1994) and PDE2 (Podzuweit et al., 1995) respectively, failed to affect the histamine concentration-response relationship. These data are entirely consistent with the proposal that these isoenzyme families have no role in regulating airways contractility despite the presence of appreciable PDE1 and PDE2 activity in airways smooth muscle from all species studied (Giembycz & Barnes, 1991; Miyamoto et al., 1994; Souness & Giembycz, 1994; Torphy et al., 1991; 1993). In contrast, pre-treatment of guinea-pig tracheal smooth muscle with SK&F 94120, a selective inhibitor of PDE3, antagonized the ability of histamine to evoke contraction. In contrast, the PDE4 inhibitor, rolipram, was weakly active under identical experimental conditions. In fact, given that guinea-pig airways smooth muscle contains, principally, a conformer of PDE4 for which rolipram has nanomolar affinity (Harris et al., 1989; see Torphy, 1998), these data suggest that PDE4 does not exert a major anti-spasmogenic effect in this species. Taken together, these results are reminiscent of the spasmolytic action of PDE inhibitors (Torphy et al., 1991; 1993) and are equally difficult to interpret given that tracheal smooth muscle from many species expresses PDE3 and PDE4 (Giembycz & Barnes, 1991; Miyamoto et al., 1994; see Souness & Giembycz, 1994; Torphy & Cieslinski, 1990; Torphy et al., 1993). One possibility is that PDE3 is, functionally, the most important cyclic AMP-metabolising enzyme in these tissues and that PDE4 plays, at most, a supportive role. Support for this contention was the finding that at isoenzyme-selective concentrations rolipram enhanced the anti-spasmogenic activity of SK&F 94120 in guinea-pig trachea in a manner that was similar to the protection afforded by the dual PDE3/4 inhibitor, zardaverine, and the non-selective PDE inhibitor, theophylline. Consistent with these results, Turner et al. (1994) reported synergy between rolipram and the PDE3 inhibitor, siguazodan, in elevating the cyclic AMP content in guinea-pig trachea although it is inappropriate to ascribe the elevation in cyclic AMP solely to airway smooth muscle cells due to the heterogeneity of the preparation. Thus, the data described in the present study confirm and extend the results reported by others (Turner et al., 1994) and indicate an important functional role for PDE3 and possibly PDE4 in regulating airways smooth muscle contractility in the guinea-pig.
The archetypal PDE5 inhibitor, zaprinast, which selectively hydrolyses cyclic GMP, and SNP, an activator of soluble guanylyl cyclase, also protected guinea-pig tracheal smooth muscle against the contractile action of histamine. Classically, activation of the cyclic GMP/cyclic GMP-dependent protein kinase cascade is believed to mediate the effects of agents such as nitrovasodilators, and evidence that this mechanism operates in airways smooth muscle is available (Zhou & Torphy, 1991; Turner et al., 1994). Equally, however, the increase in cyclic GMP effected by zaprinast and SNP subsequently could lead to the activation of cyclic AMP-dependent protein kinase through the inhibition PDE3 (Maurice & Haslam, 1990; Turner et al., 1994). It was reasoned that if the latter hypothesis is correct then rolipram should potentiate the anti-spasmogenic effect of zaprinast, SNP and SK&F 94120 to the same or a similar extent. Indeed, high concentrations of SNP have been shown to increase cyclic AMP levels in canine trachealis (Torphy et al., 1985), and Maurice & Haslam (1990) reported that SNP enhanced isoprenaline-induced cyclic AMP accumulation in vascular smooth muscle at concentrations that elevated cyclic GMP mass. However, in the present study neither the pD2 of histamine nor the maximum tension generated in zaprinast- or SNP-treated tissues was significantly affected by rolipram whereas the anti-spasmogenic activity of SK&F 94120 was significantly potentiated. Thus, based on these data, it is logical to conclude that PDE3 does not play a major role in the anti-spasmogenic action of the cyclic GMP-elevating agents studied. However, SK&F 94120 at a concentration (100 μM) that should abolish PDE3 (and possibly suppress PDE4 to a modest extent) in intact tracheal rings failed to enhance the antagonistic activity of SNP. This lack of effect is contrary to what would be predicted if a cyclic AMP-independent mechanism mediates the effect of SNP. The possibility that SNP exerted the maximum anti-spasmogenic effect attainable in the tissue was excluded on the basis that a greater inhibitory effect was produced when rolipram and SK&F 94120 were used in combination. An alternative explanation is that SNP (100 μM), by increasing cyclic GMP, abolished PDE3 activity such that SK&F 94120 was unable to exert any further inhibition of the functional response. Again, this interpretation is inconsistent with the data since if this is correct then SNP/rolipram should have antagonized histamine-induced tension generation to the same extent as SK&F 94120/rolipram. Thus, at the present time it is unclear why SK&F 94120 and SNP do not act additively.
The apparent PDE3-independent mechanism of action of SNP and zaprinast is contrary to what is established for the relaxant action of cyclic GMP-elevating drugs in guinea-pig trachea where a marked synergy is seen between SNP and rolipram (Turner et al., 1994). The reason(s) for this discrepancy is currently unclear. One possibility is that the maximum rate of cyclic GMP hydrolysis by PDE3 in airways smooth muscle is uncharacteristically high (Torphy et al., 1993) which prevents it acting as a competitive inhibitor of cyclic AMP degradation. However, this explanation cannot be reconciled with the data reported by Turner and colleagues (1994) who provided good evidence that cyclic GMP does, indeed, block PDE3 in guinea-pig trachealis. A more likely explanation of this paradox derives from the knowledge that the mechanisms which govern tension generation differ from those which maintain force and that cyclic nucleotides differentially affect these processes (see Giembycz & Raeburn, 1991). Thus, in its most simplistic form the spatial distribution of soluble guanylyl cyclases and the various PDE isoforms within a guinea-pig tracheal smooth muscle cell might dictate whether nitric oxide donors and isoenzyme-selective PDE inhibitors can exert a spasmolytic and/or an anti-spasmogenic effect (see Houslay & Milligan, 1997).
A consistent finding in this study was that SNP and the PDE inhibitors were considerably less able to prevent ACh-induced contractions than equivalent responses effected by histamine. Although the reason for this difference was beyond the objectives of this study, similar observations have been reported for guinea-pig and bovine airways smooth muscle and evidence has been provided that part of this effect may relate to differences in the magnitude of histamine- and ACh-induced inositol (1,4,5) trisphosphate formation and the ability of cyclic AMP to suppress this effect (Van Amsterdam et al., 1989; Challiss et al., 1998).
In conclusion, the results of this study demonstrate that an inhibitor of PDE3 significantly enhances the modest anti-spasmogenic activity of rolipram in guinea-pig tracheal rings but not drugs that elevate cyclic GMP. Furthermore, the ability of SNP and zaprinast to protect airways smooth muscle against histamine-induced contractions does not seem to be due to the indirect inhibition of PDE3. If the results presented herein are predictive of the effect of PDE inhibitors on human airways smooth muscle, significant anti-spasmogenic activity might be expected of dual PDE3/PDE inhibitors. However, selective PDE4 inhibitors are likely to be less effective in this respect unless they are able to synergize with endogenous activators of adenylyl cyclase such as prostacyclin, E-series prostaglandins and catecholamines.
Acknowledgments
The authors acknowledge the Medical Research Council, the National Asthma Campaign and the British Lung Foundation (BLF) for financial support.
Abbreviations
- ACh
acetylcholine
- cyclic AMP
adenosine-3′,5′-cyclic monophosphate
- cyclic GMP
guanosine-3′,5′-cyclic monophosphate
- EHNA
erythro-9-(2-hydroxy-3-nonyl) adenine hydrochloride
- KH
Krebs-Henseleit
- PDE
phosphodiesterase
- SNP
sodium nitroprusside
References
- BARNES P.J. Chronic obstructive pulmonary disease: new opportunities for drug development. Trends. Pharmacol. Sci. 1998;19:415–423. doi: 10.1016/s0165-6147(98)01245-0. [DOI] [PubMed] [Google Scholar]
- BEAVO J. Multiple isozymes of cyclic nucleotide phosphodi-esterase. Adv. Second Messenger. Phosphoprotein.Res. 1987;22:1–38. [PubMed] [Google Scholar]
- BEAVO J. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol. Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- BEAVO J., CONTI M., HEASLIP R.J. Multiple cyclic nucleotide phosphodiesterases. Mol. Pharmacol. 1994;46:399–405. [PubMed] [Google Scholar]
- CHALLISS R.A., ADAMS D., MISTRY R., NICHOLSON C.D. Modulation of spasmogen-stimulated Ins(1,4,5)P3 generation and functional responses by selective inhibitors of types 3 and 4 phosphodiesterase in airways smooth muscle. Br. J. Pharmacol. 1998;124:47–54. doi: 10.1038/sj.bjp.0701792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTI M., NEMOZ G., SETTE C., VINCINI E. Recent progress in understanding the hormonal regulation of phosphodiesterases. Endocrine. Rev. 1995;16:370–389. doi: 10.1210/edrv-16-3-370. [DOI] [PubMed] [Google Scholar]
- DEGERMAN E., BELFRAGE P., MANGANIELLO V.C. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3) J. Biol. Chem. 1997;272:6823–6826. doi: 10.1074/jbc.272.11.6823. [DOI] [PubMed] [Google Scholar]
- DENT G., GIEMBYCZ M.A. Selective phosphodiesterase inhibitors in the therapy of asthma. Clin. Immunother. 1995;3:423–437. [Google Scholar]
- FISHER D.A., SMITH J.F., PILLAR J.S., STDENIS S.H., CHENG J.B. Isolation and characterization of PDE8A, a novel human cAMP-specific phosphodiesterase. Biochem. Biophys. Res. Commun. 1998a;246:570–577. doi: 10.1006/bbrc.1998.8684. [DOI] [PubMed] [Google Scholar]
- FISHER D.A., SMITH J.F., PILLAR J.S., STDENIS S.H., CHENG J.B. Isolation and characterization of PDE9A, a novel human cGMP-specific phosphodiesterase. J. Biol. Chem. 1998b;273:15559–15564. doi: 10.1074/jbc.273.25.15559. [DOI] [PubMed] [Google Scholar]
- FUJISHIGE K., KOTERA J., MICHIBATA H., YUASA K., TAKEBAYASHI S.I., OKUMURA K., OMORI K. Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP. J. Biol. Chem. 1999;274:18438–18445. doi: 10.1074/jbc.274.26.18438. [DOI] [PubMed] [Google Scholar]
- GIEMBYCZ M.A. Could isoenzyme-selective phosphodiesterase inhibitors render bronchodilator therapy redundant in the treatment of asthma. Biochem. Pharmacol. 1992;43:2041–2051. doi: 10.1016/0006-2952(92)90160-k. [DOI] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., BARNES P.J. Selective inhibition of a high affinity type IV cyclic AMP phosphodiesterase in bovine trachealis by AH 21-132. Relevance to the spasmolytic and anti-spasmogenic actions of AH 21-132 in the intact tissue. Biochem. Pharmacol. 1991;42:663–677. doi: 10.1016/0006-2952(91)90330-8. [DOI] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., CORRIGAN C.J., SEYBOLD J., NEWTON R., BARNES P.J. Identification of cyclic AMP phosphodiesterases 3, 4 and 7 in human CD4+ and CD8+ T-lymphocytes: role in regulating proliferation and the biosynthesis of interleukin-2. Br. J. Pharmacol. 1996;118:1945–1958. doi: 10.1111/j.1476-5381.1996.tb15629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., DENT G., SOUNESS J.E.Theophylline and isoenzyme-selective phosphodiesterase inhibitors Allergy & Allergic Diseases 1997Blackwell Scientific: London; 531–567.In: Kay, A.B. (ed.) [Google Scholar]
- GIEMBYCZ M.A., KELLY J.J.Current status of cyclic nucleotide phosphodiesterase isoenzymes Methylxanthines and Phosphodiesterase Inhibitors and the Treatment of Respiratory Disease 1994Parthenon Publishing: London; 27–80.In: Piper, P.J. & Costello, J. (eds) [Google Scholar]
- GIEMBYCZ M.A., RAEBURN D. Putative substrates for cyclic nucleotide-dependent protein kinases and the control of airway smooth muscle tone. J. Auton. Pharmacol. 1991;11:365–398. doi: 10.1111/j.1474-8673.1991.tb00260.x. [DOI] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., SOUNESS J.E.Phosphodiesterase 4 inhibitors as potential therapeutic agents in allergic disease Immunopharmacology of Allergic Disease 1996Marcel-Dekker: New York; 523–559.In: Townley, R.G. & Agarwal, D.K. (eds) [Google Scholar]
- HAGIWARA M, , ENDO T., HIDAKA H. Effects of vinpocetine on cyclic nucleotide metabolism in vascular smooth muscle. Biochem. Pharmacol. 1984;33:453–457. doi: 10.1016/0006-2952(84)90240-5. [DOI] [PubMed] [Google Scholar]
- HANIFIN J.M., CHAN S.C., CHENG J.B., TOFTE S.J., HENDERSON W.R., KIRBY D.S., WEINER E.S. Type 4 phosphodiesterase inhibitors have clinical and in vitro anti-inflammatory effects in atopic dermatitis. J. Invest. Dermatol. 1996;107:151–156. doi: 10.1111/1523-1747.ep12297888. [DOI] [PubMed] [Google Scholar]
- HARRIS A.L., CONNELL M.J., FERGUSON E.W., WALLACE A.M., GORDON R.J., PAGANI E.D., SILVER P.J. Role of low Km cyclic AMP phosphodiesterase inhibition in tracheal relaxation and bronchodilation in the guinea pig. J. Pharmacol. Exp. Ther. 1989;251:199–206. [PubMed] [Google Scholar]
- HATZELMANN A., ENGELSTATTER R., MORLEY J., MAZZONI L.Enzymatic and functional aspects of dual-selective PDE3/4 inhibitors Phosphodiesterase Inhibitors 1996Academic Press: London; 147–160.In: Schudt, C., Dent, G. & Rabe, K.F. (eds) [Google Scholar]
- HOUSLAY M.D., MILLIGAN G. Tailoring cAMP-signalling responses through isoform multiplicity. Trends. Biochem.Sci. 1997;22:217–224. doi: 10.1016/s0968-0004(97)01050-5. [DOI] [PubMed] [Google Scholar]
- LOUGHNEY K., FERGUSON K.The human cyclic nucleotide phosphodiesterases Methylxanthines and Phosphodiesterase Inhibitors and the Treatment of Respiratory Disease 1994Parthenon Publishing: London; 81–100.In: Piper, P.J. & Costello, J. (eds) [Google Scholar]
- LOUGHNEY K., FERGUSON K.Identification and quantification of PDE isoenzymes and subtypes by molecular biological methods Phosphodiesterase Inhibitors 1996Academic Press: London; 1–19.In: Schudt, C., Dent, G. & Rabe, K.F. (eds) [Google Scholar]
- LOUGHNEY K., SNYDER P.B., UHER L., ROSMAN G.J., FERGUSON K., FLORIO V.A. Isolation and characterization of PDE10A, a novel human 3′, 5′-cyclic nucleotide phosphodiesterase. Gene. 1999;234:109–117. doi: 10.1016/s0378-1119(99)00171-7. [DOI] [PubMed] [Google Scholar]
- MAURICE D.H., HASLAM R.J. Nitroprusside enhances isoprenaline-induced increases in cAMP in rat aortic smooth muscle. Eur. J. Pharmacol. 1990;191:471–475. doi: 10.1016/0014-2999(90)94182-w. [DOI] [PubMed] [Google Scholar]
- MIYAMOTO K., KURITA M., SAKAI R., SANAE F., WAKUSAWA S, TAKAGI K. Cyclic nucleotide phosphodiesterase isoenzymes in guinea-pig tracheal muscle and bronchorelaxation by alkylxanthines. Biochem. Pharmacol. 1994;48:1219–1223. doi: 10.1016/0006-2952(94)90159-7. [DOI] [PubMed] [Google Scholar]
- MURRAY K.J., EDEN R.J., DOLAN J.S., GRIMSDITCH D.C., STUTCHBURY C.A., PATEL B., KNOWLES A., WORBY A, , LYNHAM J.A., COATES W.J. The effect of SK&F 95654, a novel phosphodiesterase inhibitor, on cardiovascular, respiratory and platelet function. Br. J. Pharmacol. 1992;107:463–470. doi: 10.1111/j.1476-5381.1992.tb12768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLSON C.D., CHALLISS R.A.J., SHAHID M. Differential modulation of tissue function and therapeutic potential of selective inhibitors of cyclic nucleotide phosphodiesterase isoenzymes. Trends. Pharmacol. Sci. 1991;12:19–27. doi: 10.1016/0165-6147(91)90484-a. [DOI] [PubMed] [Google Scholar]
- PALMER D., TSOI K., MAURICE D.H. Synergistic inhibition of vascular smooth muscle cell migration by phosphodiesterase 3 and phosphodiesterase 4 inhibitors. Circ. Res. 1998;82:852–861. doi: 10.1161/01.res.82.8.852. [DOI] [PubMed] [Google Scholar]
- PODZUWEIT T., NENNSTIEL P., MULLER A. Isozyme selective inhibition of cGMP-stimulated cyclic nucleotide phosphodiesterases by erythro-9-(2-hydroxy-3-nonyl) adenine. Cell Signal. 1995;7:733–738. doi: 10.1016/0898-6568(95)00042-n. [DOI] [PubMed] [Google Scholar]
- ROGERS D.F., GIEMBYCZ M.A. Conquering airway inflammation in the 21st century. Drug Discovery Today. 1998;3:532–535. [Google Scholar]
- SCHUDT C., WINDER S., MULLER B., UKENA D. Zardaverine as a selective inhibitor of phosphodiesterase isoenzymes. Biochem. Pharmacol. 1991;42:153–162. doi: 10.1016/0006-2952(91)90694-z. [DOI] [PubMed] [Google Scholar]
- SODERLING S.H., BAYUGA S.J., BEAVO J.A. Identification and characterization of a novel family of cyclic nucleotide phosphodiesterases. J. Biol. Chem. 1998;273:15553–15558. doi: 10.1074/jbc.273.25.15553. [DOI] [PubMed] [Google Scholar]
- SODERLING S.H., BAYUGA S.J., BEAVO J.A. Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7071–7076. doi: 10.1073/pnas.96.12.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUNESS J.E., FOSTER M. Potential of phosphodiesterase type 4 inhibitors in the treatment of rheumatoid arthritis. Curr. Res. Rheum. Arthr. 1998;2:255–268. [PubMed] [Google Scholar]
- SOUNESS J.E., GIEMBYCZ M.A.Cyclic nucleotide phosphodiesterases in airways smooth muscle Airways Smooth Muscle: Biochemical Control of Contraction and Relaxation 1994Birkhauser Verlag: Basel; 273–308.In: Raeburn, D. & Giembycz, M.A. (eds) [Google Scholar]
- TORPHY T.J. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am. J. Respir. Crit. Care. Med. 1998;157:351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J., CIESLINSKI L.B. Characterization and selective inhibition of cyclic nucleotide phosphodiesterase isozymes in canine tracheal smooth muscle. Mol. Pharmacol. 1990;37:206–214. [PubMed] [Google Scholar]
- TORPHY T.J., MURRAY K.J., ARCH J.R.S.Selective phosphodiesterase isoenzyme inhibitors Drugs and the Lung 1994Raven Press: New York; 397–477.In: Page, C.P. & Metzger, W.J. (eds) [Google Scholar]
- TORPHY T.J., UNDEM B.J. Phosphodiesterase inhibitors: new opportunities for the treatment of asthma. Thorax. 1991;46:512–523. doi: 10.1136/thx.46.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORPHY T.J., UNDEM B.J., CIESLINSKI L.B., LUTTMANN M.A., REEVES M.L., HAY D.W.P. Identification, characterization and functional role of phosphodiesterase isoenzymes in human airway smooth muscle. J. Pharmacol. Exp. Ther. 1993;265:1213–1223. [PubMed] [Google Scholar]
- TORPHY T.J., ZHENG C., PETERSON S.M., FISCUS R.R., RINARD G.A., MAYER S.E. Inhibitory effect of methacholine on drug-induced relaxation, cyclic AMP accumulation, and cyclic AMP-dependent protein kinase activation in canine tracheal smooth muscle. J. Pharmacol. Exp. Ther. 1985;233:409–417. [PubMed] [Google Scholar]
- TURNER N.C., LAMB J., WORBY A., MURRAY K.J. Relaxation of guinea-pig trachea by cyclic AMP phosphodiesterase inhibitors and their enhancement by sodium nitroprusside. Br. J. Pharmacol. 1994;111:1047–1052. doi: 10.1111/j.1476-5381.1994.tb14850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDERWOOD D.C., KOTZER C.J., BOCHNOWICZ S., OSBORN R.R., LUTTMANN M.A., HAY D.W.P., TORPHY T.J. Comparison of phosphodiesterase III, IV and dual III/IV inhibitors on bronchospasm and pulmonary eosinophil influx in guinea pigs. J. Pharmacol. Exp. Ther. 1994;270:250–259. [PubMed] [Google Scholar]
- VAN AMSTERDAM R.G.M., MEURS H., BROUWER F., POSTEMA J.B., TIMMERMANS A., ZAAGSMA J. Role of phosphoinositide metabolism in functional antagonism of airway smooth muscle contraction by β-adrenoceptor agonists. Eur. J. Pharmacol. 1989;172:175–183. doi: 10.1016/0922-4106(89)90008-4. [DOI] [PubMed] [Google Scholar]
- VAN ROSSUM J.M. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch. Int. Pharmacodyn. Ther. 1963;143:299–330. [PubMed] [Google Scholar]
- ZHOU H.L., TORPHY T.J. Relationship between cyclic guanosine monophosphate accumulation and relaxation of canine trachealis induced by nitrovasodilators. J. Pharmacol. Exp. Ther. 1991;258:972–978. [PubMed] [Google Scholar]


