Abstract
The therapeutic efficacy of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a novel superoxide dismutase mimetic which scavenges peroxynitrite, was investigated in rats subjected to shock induced by peritoneal injection of zymosan.
Our data show that MnTBAP (given at 1, 3 and 10 mg kg−1 intraperitoneally, 1 and 6 h after zymosan injection) significantly reduce in dose dependent manner the development of peritonitis (peritoneal exudation, high nitrate/nitrite and peroxynitrite plasma levels, leukocyte infiltration and histological examination).
Furthermore, our data suggest that there is a reduction in the lung, small intestine and liver myeloperoxidase (MPO) activity and lipid peroxidation activity from MnTBAP-treated rats.
MnTBAP also reduced the appearance of nitrotyrosine immunoreactivity in the inflamed tissues.
Furthermore, a significant reduction of suppression of mitochondrial respiration, DNA strand breakage and reduction of cellular levels of NAD+ was observed in ex vivo macrophages harvested from the peritoneal cavity of zymosan-treated rat.
In vivo treatment with MnTBAP significantly reduced in a dose-dependent manner peroxynitrite formation and prevented the appearance of DNA damage, the decrease in mitochondrial respiration and the loss of cellular levels of NAD+.
In conclusion our results showed that MnTBAP was effective in preventing the development of zymosan-induced shock.
Keywords: MnTBAP, peroxynitrite, zymosan-induced shock, multiple organ failure, nitric oxide, inflammation, myeloperoxidase, nitrotyrosine, SOD mimetic, cell injury
Introduction
Zymosan, a product consisting of purified baker's yeast ghost cells, has been reported to elicit an acute peritonitis and multiple organ failure in experimental animals characterized by functional and structural changes in liver, intestine, lung and kidneys (Deitch et al., 1990; Demling et al., 1994; Goris et al., 1991; Cuzzocrea et al., 1997a,1997b). The organ dysfunction in zymosan treated animals may be, in part, dependent on bacterial translocation (Demling et al., 1994; Goris et al., 1991; Mainous et al., 1991). The role of the production of pro-inflammatory cytokines, such as tumour necrosis factor (Von Asmuth et al., 1990) and interleukin-6 (IL-6) (Jansen et al., 1996), and pro-inflammatory lipid mediators, such as platelet-activating factor (Damas et al., 1993), and of prostaglandin metabolites (Cuzzocrea et al., 1997b; Mainous et al., 1991) is well established in the pathophysiology of zymosan-induced shock. The role of oxygen-derived free radicals (Demling et al., 1994; Mainous et al., 1991) and, more recently, the role of NO (Cuzzocrea et al., 1997a,1997b) or a related species such as peroxynitrite have also been demonstrated in various models of zymosan-induced shock and inflammation (Cuzzocrea et al., 1998a, 1999a). Recently we showed that the onset of peritonitis and the maximal end-organ damage occurred at 18 h after zymosan administration, i.e. at the same time of the onset of inflammatory response in the peritoneal cavity and was associated with systemic hypotension, high peritoneal and plasma levels of NO, maximal cellular infiltration, exudate formation and cyclooxygenase activity (Cuzzocrea et al., 1997a,1997b).
Recent data have demonstrated the protective effects of NOS inhibitors in zymosan-induced shock, suggesting the involvement of NO, or related species, such as peroxynitrite, in the zymosan-induced local or systemic inflammatory process (Cuzzocrea et al., 1997a,1997b; Teixeira et al., 1993).
Recent data have demonstrated that Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a novel, stable and cell-permeable superoxide dismutase mimetic and related compounds may also inhibit the oxidation of dihydrorhodamine 123 elicited by authentic peroxynitrite (Zingarelli et al., 1997; Faulkner et al., 1994; Day et al., 1995; Szabó et al., 1996). However MnTBAP is not a scavenger of nitric oxide (Szabó et al., 1996). In the present study we investigated whether MnTBAP provides a protective effect on shock induced by zymosan administration.
Methods
Animals
Male Sprague-Dawley rats (300–350 g; Charles River; Milan, Italy) were housed in a controlled environment and provided with standard rodent chow and water. Animal care was in compliance with Italian regulation (D.M. 116192) on protection of animals used for experimental and other scientific purpose, as well as with the EEC regulations (O.J. of E.C.L 358/1 18/12/1986).
Experimental groups
Animals were randomly divided into eight groups (n=10 per group). The first group was intraperitoneally (i.p.) treated with 0.9% NaCl and served as sham group. The second group was treated with zymosan (500 mg kg−1, suspended in saline solution, i.p.). In the third and fourth groups, rats received MnTBAP (1 mg kg−1, i.p. 1 and 6 h after zymosan or saline administration). In the fifth and sixth groups rats received MnTBAP (3 mg kg−1, i.p. 1 and 6 h after zymosan or saline administration). In the seventh and eighth groups rats received MnTBAP (10 mg kg−1, i.p. 1 and 6 h after zymosan or saline administration). After zymosan or saline injection animals were monitored for evaluation of mortality for 72 h.
Acute peritonitis assessment
In a second group of experiments at 18 h after zymosan injection, animals were sacrificed under ether anaesthesia in order to evaluate the development of acute inflammation in the peritoneum. Through an incision in the linea alba, 10 ml of phosphate buffer ((mM) saline NaCl 137; KCl 2.7; NaH2PO4 1.4; Na2HPO4 4.3; pH 7.4) was injected into the abdominal cavity. Washing buffer was removed by a plastic pipette and was transferred into a 10 ml centrifuge tube. The total volume of peritoneal exudate was evaluated removing 10 ml of the buffer. Peritoneal exudate was centrifuged at 7000×g for 10 min at room temperature, and the supernatant was utilized for NO2−+NO3− (NOx) assay. Cells were suspended in phosphate buffered saline and counted with optical microscope by Burker's chamber after vital Trypan Blue stain.
Nitrate and nitrite levels in the supernatant
Nitrite/nitrate production, an indicator of NO synthesis, was measured in supernatant samples as previously described (Cuzzocrea et al., 1998a). First, nitrate in the samples were reduced to nitrite by incubation with nitrate reductase (670 mU ml−1) and NADPH (160 μM) at room temperature for 3 h. After 3 h, the nitrite concentration in the samples was measured by Griess reaction. Briefly, 100 μl of Griess reagent (0.1% naphthalethylenediamine dihydrochloride in H2O and 1% sulphanilamide in 5% concentrated H2PO4; vol. 1 : 1) were added to 100 μl of samples. The optical density at 550 nm (OD550) was measured using a ELISA microplate reader (SLT-Labinstruments Salzburg, Austria). Nitrate concentrations were calculated by comparison to standard solutions of sodium nitrate.
Measurement of peroxynitrite production
The formation of peroxynitrite was measured by the peroxynitrite-dependent oxidation of dihydrorhodamine 123 to rhodamine 123, as previously described (Cuzzocrea et al., 1998a). Briefly, cells were rinsed with PBS and then replaced with PBS containing 5 μM dihydrorhodamine 123. After a 60 min incubation at 37°C, the fluorescence of rhodamine 123 was measured using a fluorimeter at an excitation wavelength of 500 nm, emission wavelength of 536 nm (slit widths 2.5 and 3.0 nm, respectively). The formation of peroxynitrite was also estimated in plasma samples at 18 h after zymosan administration. In separate groups of experiment, animals were injected with dihydrorhodamine 123 (2 μmol kg−1 in 0.3 ml saline i.v.). Twenty minutes later rats were sacrificed and plasma samples taken for rhodamine fluorescence evaluation using a fluorimeter at an excitation wavelength of 500 nm, emission wavelength of 536 nm (slit widths 2.5 and 3.0 nm, respectively). The rate of rhodamine formation, an index of peroxynitrite production, was calculated using a standard curve obtained with authentic rhodamine (1–30 nM) prepared in plasma obtained from untreated rats. Background plasma fluorescence was subtracted from all samples.
Immunohistochemical localization of nitrotyrosine
Tyrosine nitration was detected as previously described (Cuzzocrea et al., 1997c) in lung and small intestine sections by immunohistochemistry. At 18 h after saline or zymosan injection, tissues were fixed in 10% buffered formalin and 8 μm sections were prepared from paraffin embedded tissues. After deparaffinization, endogenous peroxidase was quenched with 0.3% H2O2 in 60% methanol for 30 min. The sections were permeabilized with 0.1% Triton X-100 in phosphate buffered saline for 20 min. Non-specific adsorption was minimized by incubating the section in 2% normal goat serum in phosphate buffered saline for 20 min. Endogenous biotin or avidin binding sites were blocked by sequential incubation for 15 min with avidin and biotin (DBA, Milan, Italy). The sections were then incubated overnight with 1 : 1000 dilution of primary anti-nitrotyrosine antibody (DBA, Milan, Italy) or with control solutions. Controls included buffer alone or non specific purified rabbit IgG. Specific labelling was detected with a biotin-conjugated goat anti-rabbit IgG and avidin-biotin peroxidase complex (DBA, Milan, Italy).
Myeloperoxidase activity
The usefulness of measuring myeloperoxidase (MPO) activity to assess neutrophil infiltration has been previously reported (Mullane et al., 1988). Briefly, after weighing, segments of various organs were suspended in 0.5% hexadecyl-trimethyl-ammonium bromide (pH 6.5, 50 mg of tissue per ml) and were then homogenized. After freezing and thawing the homogenate three times, the tissue levels of MPO were determined by utilizing 0.0005% hydrogen peroxide as a substrate for the enzyme. One unit of MPO activity is defined as that degrading 1 μmol of hydrogen peroxide per minute at 25°C and is expressed in units per gram weight (Ug−1) of wet tissue.
Malonaldehyde measurement
Levels of malonaldehyde (MDA) in the lung, intestinal and liver tissues were determined as an index of lipid peroxidation, as described by Ohkawa et al. (1979). Lung, intestine and liver tissues were homogenized in 1.15% KCl solution. An aliquot (100 μl) of the homogenate was added to a reaction mixture containing 200 μl of 8.1% SDS, 1500 μl of 20% acetic acid (pH 3.5), 1500 μl of 0.8% thiobarbituric acid and 700 μl of distilled water. Samples were then boiled for 1 h at 95°C and centrifuged at 3000×g for 10 min. The absorbance of the supernatant was measured by spectrophotometry at 650 nm.
Light microscopy
Lung and small intestine samples were taken 18 h after saline or zymosan injection. The tissue slices were fixed in Dietric solution (14.2% ethanol, 1.8% formaldehyde, 1% acetic acid) for 1 week at room temperature, dehydrated by graded ethanol and embedded in Paraplast (Sherwood Medical, Mahwah, NJ, U.S.A.). Section (thickness 7 μm) were deparaffinized with xylene, stained with trichromic Van Gieson and observed in Dialux 22 Leitz microscope.
Metabolic changes
Plasma samples were taken at 18 h after saline or zymosan injection and levels of bilirubin, lactate dehydrogenase, alkaline phosphatase and alanine aminotransferase were determined by a clinical laboratory.
Cell culture
Peritoneal macrophages from rats were harvested by peritoneum lavage with DMEM medium, supplemented with L-glutamine (3.5 mM), penicillin (50 U ml−1), streptomycin (50 μg ml−1) and heparin sodium (10 U ml−1). The cells were collected 18 h after the zymosan injection from rats treated with or without MnTBAP. The cells were plated on 12-well plates at 1 million cells ml−1 and incubated for 2 h at 37°C in a humidified 5% CO2 incubator. After incubation, supernatant was collected for the measurement of nitrite and nitrate. Nonadherent cells were removed by rinsing the plates three times with 5% dextrose water. After removing nonadherent cells, adherent macrophages were scraped for the measurement of DNA strand breaks and cellular NAD+. Mitochondrial respiration and peroxynitrite formation were measured in the adherent cells in the subsequent 1-h period.
Measurement of cellular energetic status
Cell respiration was assessed by the mitochondrial-dependent reduction of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to formazan (Cuzzocrea et al., 1998a). Cells in 96-well plates were incubated at 37°C with MTT (0.2 mg ml−1) for 1 h. Culture medium was removed by aspiration and the cells were solubilized in DMSO (100 μl). The extent of reduction of MTT to formazan within cells was quantitated by the measurement of OD550. As previously discussed (Darley-Usmar & Halliwell, 1996), the measurement of reduction of MTT appears to be mainly by the mitochondrial complexes I and II, it also may involve NADH- and NADPH-dependent energetic processes that occur outside the mitochondrial inner membrane. Thus, this method cannot be used to separate the effect of free radicals, oxidants or other factors on the individual enzymes in the mitochondrial respiratory chain, but is useful to monitor changes in the general energetic status of the cells (Darley-Usmar & Halliwell, 1996).
Determination of DNA single-strand breaks
The formation of DNA strand breaks in double-stranded DNA was determined by the alkaline unwinding methods as previously described (Cuzzocrea et al., 1998a). Cells in 12-well plates were scraped into 0.2 ml of solution A buffer (mM): myoinositol 250; NaH2PO3 10; MgCl2 1; pH 7.2). The cell lysate was then transferred into plastic tubes designated T (maximum fluorescence), P (fluorescence in sample used to estimate extent of DNA unwinding), or B (background fluorescence). To each tube, 0.2 ml of solution B (alkaline lysis solution: (mM): NaOH 10; urea 9 M; ethylenediaminetetraacetic acid 2.5; sodium dodecyl sulphate 0.1%) was added and incubated at 4°C for 10 min to allow cell lysis and chromatin disruption. 0.1 ml each of solutions C (0.45 volume solution B in 0.2 N NaOH) and D (0.4 volume solution B in 0.2 N NaOH) were then added to the P and B tubes. 0.1 ml of solution E (neutralizing solution: glucose 1 M, mercaptoethanol 14 mM) was added to the T tubes before solutions C and D were added. From this point incubations were carried out in the dark. A 30-min incubation period at 0°C was then allowed during which the alkali diffused into the viscous lysate. Since the neutralizing solution, solution E, was added to the T tubes before addition of the alkaline solutions C and D, the DNA in the T tubes was never exposed to a denaturing pH. At the end of the 30 min incubation, the contents of the B tubes were sonicated for 30 s to ensure rapid denaturation of DNA in the alkaline solution. All tubes were then incubated at 15°C for 10 min. Denaturation was stopped by chilling to 0°C and adding 0.4 ml of solution E to the P and B tubes. 1.5 ml of solution F (ethidium bromide 6.7 μg ml−1 in 13.3 mM NaOH) was added to all the tubes and fluorescence (excitation: 520 nm, emission: 590 nm) was measured by a fluorimeter. Under the conditions used, in which ethidium bromide binds preferentially to double stranded DNA, the percentage of double stranded DNA (D) may be determined using the equation: % D=100 X [F(P) - F(B)]/[F(T) - F(B)]; where F(P) is the fluorescence of the sample, F(B) the background fluorescence, i.e. fluorescence due to all cell components other than double stranded DNA, and F(T) the maximum fluorescence.
Measurement of cellular NAD+ levels
Cells in 12-well plates were extracted in 0.25 ml of 0.5 N HClO4 scraped, neutralized with 3 M KOH, and centrifuged for 2 min at 10,000×g. The supernatant was assayed for NAD+ using a modification of the colorimetric method (Heller et al., 1995) in which NADH produced by enzymatic cycling with alcohol dehydrogenase, reduces MTT to formazan through the intermediation of phenazine methasulfate. The rate of MTT reduction is proportional to the concentration of the co-enzyme. The reaction mixture contained 10 μl of a solution of 2.5 mg ml MTT, 20 μl of a solution of 4 mg ml−1 phenazine methosulfate, 10 μl of a solution of 0.6 mg ml alcohol dehydrogenase (300 U mg−1), and 190 μl of 0.065 M glycyl-glycine buffer, pH 7.4, that contained 0.1 M nicotinamide and 0.5 M ethanol. The mixture was warmed to 37°C for 10 min, and the reaction was started by the addition of 20 μl of the sample. The rate of increase in absorbance was read immediately after the addition of NAD+ samples and after 10- and 20-min incubation at 37°C against blank at 560 nm in the ELISA microplate reader (SLT-Labinstruments Salzburg, Austria).
Reagents
MnTBAP was obtained from Calbiochem. Zymosan A from Saccharomyces cerevisiae, and all the other reagents and compounds used for the biochemical assays were obtained from Sigma Chemical (Milan, Italy). Biotin blocking kit, biotin-conjugated goat anti-rabbit IgG, avidin-biotin peroxidase complex and primary anti-nitrotyrosine antibody were obtained from DBA (Milan, Italy).
Data analysis
Data are expressed as means±s.e.mean analysis of variance test was used to compare means followed by Bonferroni's test for multiple comparison. Statistical analysis for survival data was calculated by Fisher's exact probability test. Differences were considered significant when the P value was less than 0.05.
Results
MnTBAP treatment reduces the development of zymosan-induced shock model
All zymosan-injected rats developed an acute peritonitis, as indicated by the production of turbid exudate (Figure 1A). Trypan blue stains revealed a significant increase in the polymorphonuclear leukocytes in comparison to sham rats (Figure 1B). Sham animals demonstrated no abnormalities in the peritoneal cavity or fluid. The degree of peritoneal exudation and polymorphonuclear migration were significantly reduced in dose dependent manner in rats treated with MnTBAP (1, 3 and 10 mg kg−1, i.p. 1 and 6 h after zymosan) (Figure 1A,B). In sham rats MnTBAP treatment did not cause significant changes in these parameters (Figure 1A,B).
Figure 1.
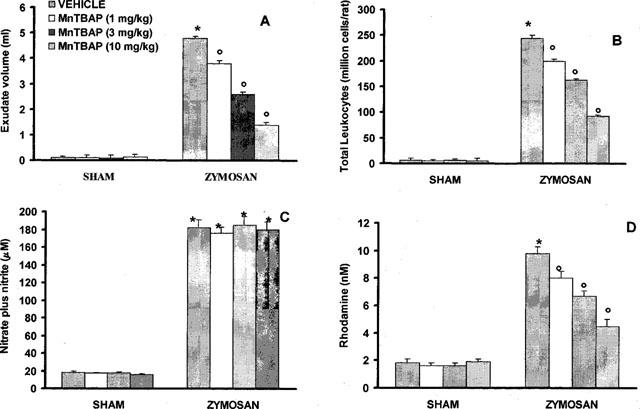
Data represent values at 18 h after vehicle or zymosan administration (500 mg kg−1 i.p.). (A) Exudate volumes; (B) leukocytes accumulation; (C) plasma NOx levels; (D) plasma peroxynitrite production. MnTBAP (1, 3 and 10 mg kg−1, i.p. 1 and 6 h after zymosan) treatment induced in a dose dependent manner a significant decrease of peritoneal exudate, leukocyte migration and NOx and peroxynitrite levels. Data are means±s.e.mean of 10 rats for each group. *P<0.01 versus sham. °P<0.01 versus zymosan.
The biochemical and inflammatory changes observed in the peritoneal cavity of zymosan-treated rats were associated with a significant elevation of plasma NOx and peroxynitrite levels. Nitrate/nitrite levels were significantly elevated in zymosan treated rats (182±22 μM) in comparison to sham rats (18±2 μM, P<0.01) (Figure 1C). MnTBAP (1, 3 and 10 mg kg−1, i.p. 1 and 6 h after zymosan) treatment did not affect nitrite/nitrate levels (Figure 1C).
Plasma levels of peroxynitrite, as assessed by oxidation of dihydrorhodamine 123 to rhodamine 123, were also significantly increased in the zymosan-treated rats in comparison to sham animals (Figure 1D). MnTBAP (1, 3 and 10 mg kg−1, i.p. 1 and 6 h after zymosan) treatment reduced in dose dependent manner the peroxynitrite formation (Figure 1D). At 18 h lung, and small intestine were also analysed for the presence of nitrotyrosine, revealing a positive staining in lung and small intestine from zymosan-treated rats, (Figure 2A–C and 3A). A marked reduction of nitrotyrosine staining was found in the lung and small intestine of the zymosan-treated rats treated with MnTBAP (10 mg kg−1 i.p. 1 and 6 h after zymosan) (Figure 2D and 3B). Staining was absent in tissue from sham rats (data not shown).
Figure 2.
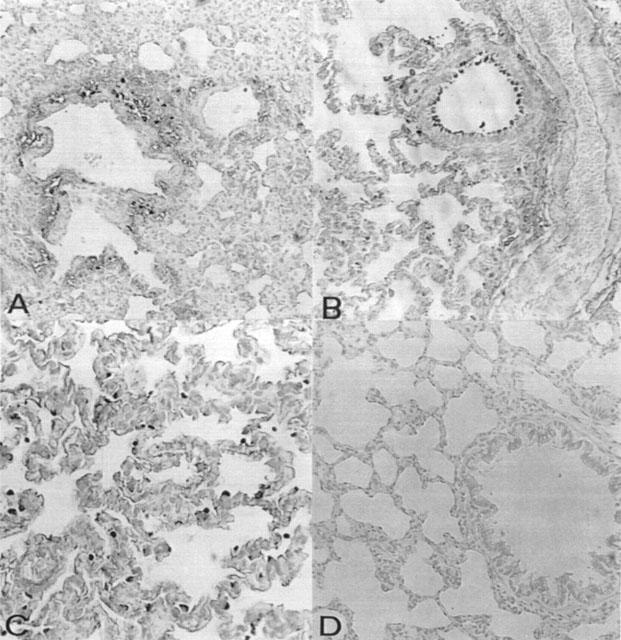
Immunohistochemical localization of nitrotyrosine in the rat lung. Eighteen following zymosan injection, nitrotyrosine immunoreactivity was localized mainly in vascular wall (A), bronchial (B) and macrophages (C). There was no detectable immunostaining in the lungs of zymosan-treated rats when rats were treated with MnTBAP (D). Original magnification: ×100.
Figure 3.
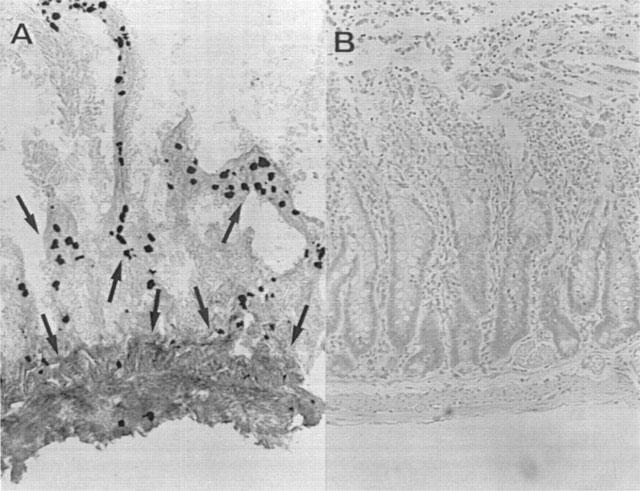
Immunohistochemical localization of nitrotyrosine in the rat small intestine. Positive staining was found in the intestine from zymosan-treated rats (A). There was no detectable immunostaining in the intestine of zymosan-treated rats when they were treated with MnTBAP (10 mg kg−1, i.p. 1 and 6 h after zymosan) (B). Original magnification: A-B 100×; C-D 200×.
At 18 h after zymosan administration, lung, small intestine and liver were investigated for MDA levels in order to estimate lipid peroxidation and MPO activity, indicative of neutrophil infiltration. As shown in Figure 4A,B, MPO activity and MDA levels were significantly increased in the examined organs (P<0.01) at 18 h after zymosan injection. Histological examinations of lung and small intestine (see representative sections at Figures 5 and 6) revealed pathologic changes. In particular, lung biopsy examination revealed inflammatory infiltration by neutrophil, macrophage and plasma cells (Figure 5A). The histological examination of ileum section showed inflammatory infiltration by neutrophil, lymphocytes and plasma cells extending through the wall and concentrated below the epithelial layer; occasionally focal ulceration, sometimes extending through the muscularis mucosa were observed (Figure 6A). However, MPO activity and MDA levels were significantly (P<0.01) reduced in dose dependent manner by MnTBAP (3 and 10 mg kg−1, i.p. 1 and 6 h after zymosan) treatment (Figure 4A,B). In addition, treatment with MnTBAP (10 mg kg−1 i.p. 1 and 6 h after zymosan) markedly reduced the zymosan-induced organ injury (Figures 5B and 6B).
Figure 4.
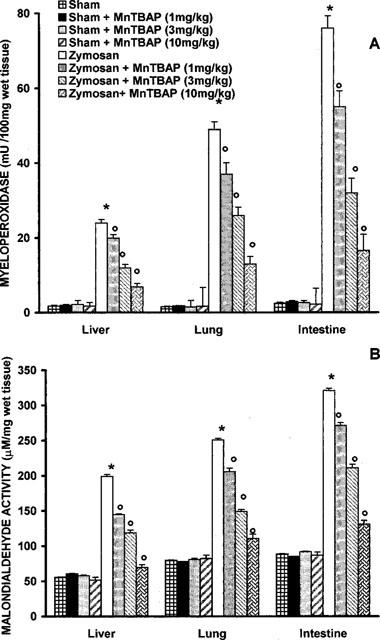
Myeloperoxidase (MPO) activity (A) and malondialdehyde (MDA) (B) in the lungs, small intestine and liver of zymosan-treated rats. MPO activity and MDA levels were significantly increased in the lung, small intestine and liver of the zymosan-treated rats in comparison to sham ras MnTBAP (1, 3 and 10 mg kg−1, i.p. 1 and 6 h after zymosan) treatment significantly reduce in dose dependent manner the zymosan-induced increase in MPO activity and MDA levels. Each value is the mean±s.e.mean for n=10. *P<.01 versus sham; °P<.01 versus zymosan.
Figure 5.
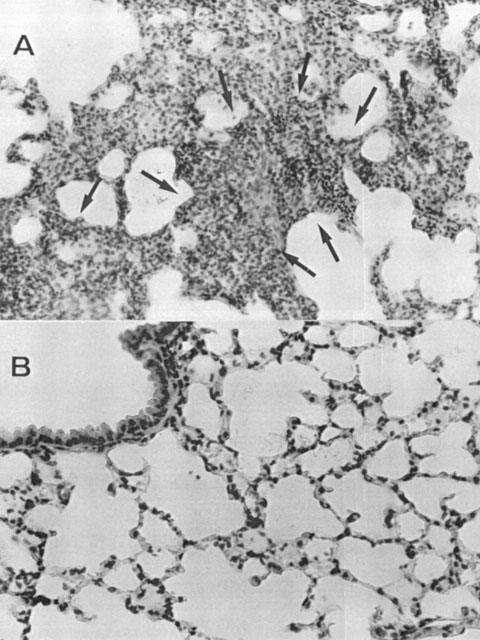
Lung sections from a zymosan-treated rat revealed inflammatory infiltration of neutrophil, macrophage and plasma cells (A). Lung sections from zymosan-treated rat that received MnTBAP (10 mg kg−1, i.p. 1 and 6 h after zymosan) demonstrate reduced inflammatory infiltration (B). Sections are representative of n=4 rats examined for each group. Original magnification: ×100.
Figure 6.
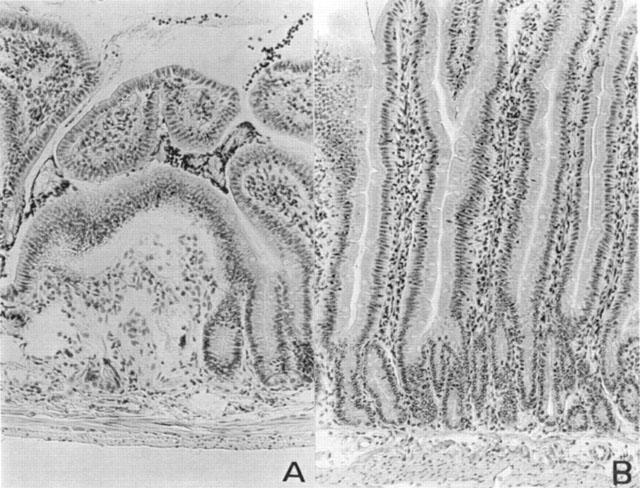
Distal ileum section from a zymosan-treated rat showed inflammatory infiltration of neutrophil, lymphocytes and plasma cells extending through the wall and concentrated below the epithelial layer (A). Distal ileum from zymosan-treated rat that received MnTBAP (10 mg kg−1, i.p. 1 and 6 h after zymosan) showed reduced zymosan-induced inflammatory infiltration (B). Original magnification: ×100.
Metabolic changes paralleled the organ morphological alterations. For example, blood levels of alanine aminotransferase increased from basal levels of 46±2.6 U l−1 to 241±12 U l−1 at 18 h after zymosan administration (P<0.01). Zymosan-treated rats exhibited also a marked hyperbilirubinemia from basal levels of 0.15±0.04 mg dl−1 to 1.6±0.13 mg dl−1 (Figure 7A,B). A significant increase of lactade dehydrogenase and alkaline phosphatase was also found at 18 h after zymosan injection (Figure 7C,D). Treatment with MnTBAP (10 mg kg−1, i.p. 1 and 6 h after zymosan) lowered the increase in alanine aminotransferase, bilirubin, lactade dehydrogenase and alkaline phosphatase blood levels in the zymosan-treated rats (Figure 7). In a separate set of experiment animals were observed for 72 h. At the end of observation period (72 h), 53% of zymosan-treated rats were dead (Figure 8). Sham animals injected only with saline appeared healthy and active through the observation period. MnTBAP (10 mg kg−1; i.p. 1 and 6 h after zymosan) reduced the mortality (Figure 8).
Figure 7.
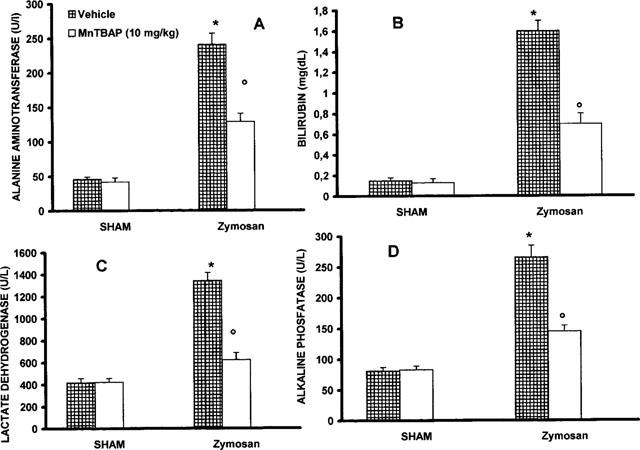
Plasma level of alanine aminotransferase (A), bilirubin (B), lactade dehydrogenase (C) and alkaline phosphatase (D). Zymosan-treated rats exhibited a dysmetabolic change. Administration of MnTBAP (10 mg kg−1, i.p. 1 and 6 h after zymosan) prevented the metabolic modification. Each value is the mean±s.e.mean for n=10. *P<.01 versus sham; °P<.01 versus zymosan.
Figure 8.
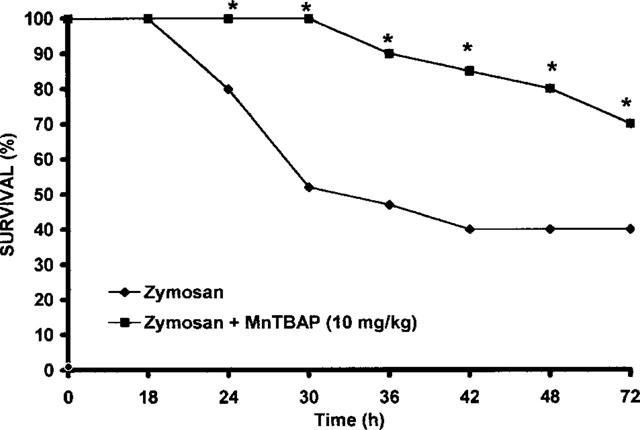
Effect of MnTBAP treatment on zymosan-induced mortality. n=10 rats for each group. *P<0.01 versus zymosan.
MnTBAP protects against the cellular energetic failure
In peritoneal macrophages obtained 18 h after zymosan injection, a significant nitrate/nitrite production was detectable (52±3.3 μM; n=4; Figure 9A). A rapid and sustained production of peroxynitrite (66±2.4 pmoles/min/million cells) in comparison to sham (2.8±0.8 pmoles/min/million cells) was also observed after zymosan-induced shock (n=4; Figure 9B). A marked increase in DNA strand breakage was also observed after zymosan-induced shock (Figure 9C). Zymosan-mediated disruption of cellular energetic pool was evidenced by a significant decrease in energetic status and intracellular concentration of NAD+ (Figure 9D,E). The in vivo treatment of the animals with MnTBAP significantly inhibited in a dose dependent manner dihydrorhodamine 123 oxidation, prevented the -induced DNA single strand breakage (Figure 9B,C), significantly inhibited the decrease in cellular energetic status and partially restored the depletion of intracellular levels of NAD+ (Figure 9D,E). Production of nitrite/nitrate was unaffected by MnTBAP treatment (Figure 9A).
Figure 9.
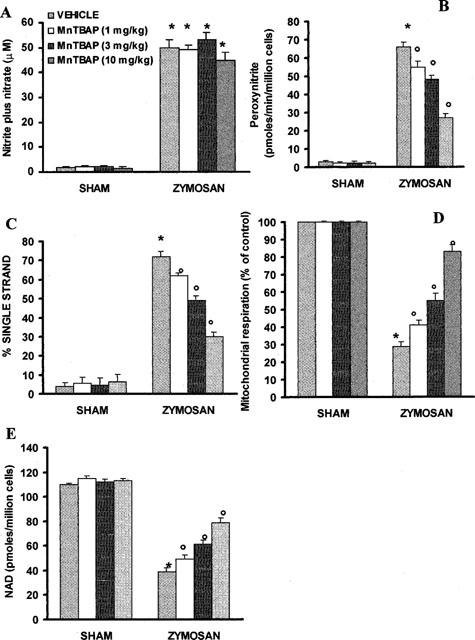
Nitrate/nitrite production (A), peroxynitrite production (B), DNA single strand breakage (C), reduction of mitochondrial respiration (D) and cellular levels of NAD+ (E) in peritoneal macrophages harvested 18 h after zymosan administration. *P<0.01 versus macrophages from control rats; °P<0.01 represents protective effects of MnTBAP.
Discussion
The cellular and molecular mechanism of the zymosan-induced shock has been well characterized. In fact several pro-inflammatory cytokines, such as tumor necrosis factor (Von Asmuth et al., 1990), and pro-inflammatory lipid mediators, such as platelet-activating factor (Damas et al., 1993), and cyclo-oxygenase metabolites (Mainous et al., 1991; Cuzzocrea et al., 1997b; Doherty et al., 1990; Rao et al., 1993) have been proposed as responsible for the zymosan-induced shock. The role of oxygen-derived free radicals (Demling et al., 1994; Van Bebber et al., 1989; Mainous et al., 1995) and, more recently, the role of NO (Cuzzocrea et al., 1997a,1997b) has also been demonstrated in zymosan-induced shock and inflammation. It is well established that the treatment of plasma with zymosan activates the complement system and generates C5a, which is a potent chemotactic factor for neutrophils (Fernandez et al., 1978; Snyderman et al., 1970). There is evidence that zymosan triggers the production of oxygen-derived free radicals in various cell types (Murohara et al., 1993; Mehta et al., 1991), and it also induces peroxynitrite generation (Cuzzocrea et al., 1997c). In fact, potent novel, potent, cell-permeable superoxide dismutase mimetics prevent peroxynitrite formation and protect against the cellular injury in various models of inflammation (Zingarelli et al., 1997; Szabó et al., 1996).
The main findings of the current study are the following: MnTBAP reduces (1) the development of zymosan-induced peritonitis; (2) morphological injury and neutrophil infiltration; (3) ONOO− production and (4) MnTBAP reduces nitrotyrosine staining in the lung and small intestine from zymosan-treated rats.
The therapeutic efficacy of SOD itself in animals with systemic inflammation, haemorrhage and shock is controversial. The following reasons may explain the lack of effect of SOD against the tissue injury associated with local or systemic inflammation: (1) SOD scavenges superoxide, but without efficient removal of the hydrogen peroxide which is produced, levels of hydroxyl radicals may increase (Goode & Webster, 1983). Indeed, SOD may function as a pro-oxidant by catalysing the conversion of hydrogen peroxide to hydroxyl radicals (Yim et al., 1990). (2) Neither SOD nor superoxide anions easily cross biological membranes. Thus, an increase in the amounts of extracellular SOD does not attenuate the effects of superoxide anions generated by intracellular sources (Fridovich, 1995). In contrast to SOD, spin trapping nitrones, such as phenyl N-tert-butyl nitrone (PBN), consistently improve outcome in rat models of endotoxic (McKechnie et al., 1986) and traumatic shock (Novelli et al., 1992)
What, then, is the mechanism by which MnTBAP protects the MOF injury associated with this model of shock. MnTBAP (Mn(III)tetrakis (4-benzoic acid) porphyrin) is a novel, stable and cell-permeable superoxide dismutase mimetic which permeates biological membranes and scavenges superoxide anions and peroxynitrite in vitro (Zingarelli et al., 1997); Faulkner et al., 1994; Day et al., 1995; Szabó et al., 1996). Thus, MnTBAP scavenges intracellular superoxide anions and prevents the formation of hydroxyl radicals. Unlike recombinant SOD, which is not able to cross biological membranes, hence, MnTBAP crosses biological membranes and functions as an intracellular scavenger of superoxide anions. In addition, we demonstrate that MnTBAP attenuates the nitrosylation of proteins in the lung and intestine of rats treated with zymosan. Nitrotyrosine formation, along with its detection by immunostaining, was initially proposed as a relatively specific marker for the detection of the endogenous formation ‘footprint' of peroxynitrite (Beckman, 1996). There is, however, recent evidence that certain other reactions can also induce tyrosine nitration; e.g., the reaction of nitrite with hypoclorous acid and the reaction of myeloperoxidase with hydrogen peroxide can lead to the formation of nitrotyrosine (Eiserich et al., 1998). Increased nitrotyrosine staining is considered, therefore, as an indication of ‘increased nitrosative stress' rather than a specific marker of the generation of peroxynitrite. Thus, it is possible that the inflammatory effects observed in response to zymosan represent the sum of a complex interaction between various oxygen- and nitrogen-derived radicals and oxidants. It is logical to assume that the pharmacological effects of MnTBAP arise from the combination of the following action: (1) by inactivating superoxide, and thus preventing the formation of peroxynitrite, with consequent protection against the development of peroxynitrite-induced cellular energetic failure, (2) by scavenging peroxynitrite, direct inhibition of peroxynitrite-induced oxidative process and (3) by reducing neutrophil recruitment into the inflammatory site. At present we cannot see a suitable pharmacological approach to separate the above factors. The reduced neutrophil infiltration into the inflamed tissue is not clear at present. It may be related to a prevention by MnTBAP of endothelial oxidant injury and to a preservation of endothelial barrier function. Clearly, the understanding of the relative contribution of MnTBAP's multiple mechanism of action to the anti-inflammatory effects observed in the current study requires further investigations. Recently a novel pathway of inflammation, governed by the nuclear enzyme poly (ADP-ribose) synthetase (PARS) has been proposed in relation to hydroxyl radical- and peroxynitrite-induced DNA single strand breakage (Szabó et al., 1997a,1997b; Cochrane 1991; Cuzzocrea et al., 1998b). This pathway plays an important role in various forms of shock and reperfusion injury (Cuzzocrea et al., 1997d; Thiemermann et al., 1997). It is clear that MnTBAP has potent protective effects in experimental systems where the injury is likely to be mediated by superoxide alone (Zingarelli et al. 1997). Therefore, recent studies have demonstrated that peroxynitrite potently triggers DNA single strand breakage in different cell types, including thymocytes, macrophages and vascular smooth muscle cells by PARS activation with consequent reduction of mitochondrial respiration (Szabó et al., 1997a; Cuzzocrea et al., 1998b).
Zingarelli et al. (1997) have recently demonstrated that this mesoporphyrin compound protects against the suppression of mitochondrial respiration induced by authentic peroxynitrite in vascular smooth muscle cells and on the cellular energetic failure in a rodents model of endotoxin shock.
Therefore the protection by MnTBAP against the development of DNA single strand breakage and the partially restore of the depletion of intracellular levels of NAD+ (as shown in Figure 7C), may be related to either superoxide and peroxynitrite and thus prevent the activation of PARS in inflammation (Cuzzocrea et al., 1999b).
Taken together, the results of the present study, coupled with recent data by ours and several other groups support the view that MnTBAP can exert potent anti-inflammatory effects. Based on the current results, we propose that the mode of MnTBAP' s protective action is, at least in part, related to: (1) reduction of superoxide formation and (2) scavenging of peroxynitrire. Therefore the relative contribution of the two effects of MnTBAP during zymosan induced non-septic shock is considerable and worthy of further investigation.
Acknowledgments
This study was supported by grant from Centro Nazionale delle Ricerche. The authors would like to Fabio Giuffrè and Carmelo La Spada for the excellent technical assistance during this study, Mrs Caterina Cutrona for secretarial assistance and Miss Valentina Malvagni for editorial assistance with the manuscript.
Abbreviations
- iNOS
inducible nitric oxide synthase
- MDA
malonaldehyde
- MnTBAP
Mn(III)tetrakis (4-benzoic acid) porphyrin
- MPO
myeloperoxidase
- NO
nitric oxide
- PBS
phosphate-buffered saline
- PMN
polymorphonuclear leukocyte
References
- BECKMAN J.S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- COCHRANE C.G. Mechanism of oxidant injury of cells. Mol. Aspects Med. 1991;12:137–147. doi: 10.1016/0098-2997(91)90009-b. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., CAPUTI A.P., ZINGARELLI B. Peroxynitrite-mediated DNA strand breakage activates Poly (ADP-ribose) synthetase and causes cellular energy depletion in carrageenan-induced pleurisy. Immunology. 1998b;93:96–101. doi: 10.1046/j.1365-2567.1998.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUZZOCREA S., FILIPPELLI A., ZINGARELLI B., FALCIANI M., CAPUTI A.P., ROSSI F. Role of nitric oxide in a non-septic shock model induced by zymosan in the rat. Shock. 1997a;7:351–358. doi: 10.1097/00024382-199705000-00007. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., CAPUTI A.P. Peroxynitrite-mediated DNA strand breakage activates Poly (ADP-ribose) synthetase and causes cellular energy depletion in a nonseptic shock model induced by zymosan in the rat. Shock. 1998a;9:336–340. doi: 10.1097/00024382-199805000-00004. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., COSTANTINO G., CAPUTI A.P. Beneficial effects of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in carrageenan-pleurisy. Free Radic. Biol. Med. 1999b;26:25–33. doi: 10.1016/s0891-5849(98)00142-7. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., COSTANTINO G., SOTTILE A., TETI D., CAPUTI A.P. Protective effect of poly (ADP-Ribose) synthetase inhibition on multiple organ failure following zymosan-induced peritonitis. Crit. Care Med. 1999a;27:1517–1523. doi: 10.1097/00003246-199908000-00020. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., COSTANTINO G., SZABÓ A., SALZMAN A.L., CAPUTI A.P., SZABÓ C. Beneficial effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase in a rat model of splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 1997d;121:1065–1074. doi: 10.1038/sj.bjp.0701234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., O'CONNOR M., SALZMAN A.L., CAPUTI A.P., SZABÓ C. Role of peroxynitrite and activation of Poly (ADP Ribose) Synthetase in the vascular failure induced by zymosan-activated plasma. Br. J. Pharmacol. 1997c;122:493–503. doi: 10.1038/sj.bjp.0701387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., SAUTEBIN L., RIZZO A., CRISAFULLI C., CAMPO G.M., COSTANTINO G., CALAPAI G., NAVA F., DI ROSA M., CAPUTI A.P. Multiple organ failure following zymosan-induced peritonitis is mediated by nitric oxide. Shock. 1997b;4:268–275. doi: 10.1097/00024382-199710000-00006. [DOI] [PubMed] [Google Scholar]
- DAMAS J., REMACLE-VOLON G., BOURDON V. Platelet-activating factor and vascular effects of zymosan in rats. Eur. J. Pharmacol. 1993;231:231–236. doi: 10.1016/0014-2999(93)90454-p. [DOI] [PubMed] [Google Scholar]
- DARLEY-USMAR V., HALLIWELL B. Blood radicals. Reactive nitrogen species, reactive oxygen species, transition metal ions, and the vascular system. Pharmaceut. Res. 1996;13:649–655. doi: 10.1023/a:1016079012214. [DOI] [PubMed] [Google Scholar]
- DAY B.J., SHAVEN S., LIOCHEV S.I., CRAPO J.D. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced endothelial injury in vitro. J. Pharmacol. Exper. Ther. 1995;275:1227–1232. [PubMed] [Google Scholar]
- DEITCH E.A., MA L., BERG R.D., SPECIANR R.D. Protein malnutrition predisposes to inflammatory-induced gut-origin septic states. Ann. Surg. 1990;221:560–567. doi: 10.1097/00000658-199005000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMLING R., NAYAKMU I., IKEGAMI K.C., LALODE C. Comparison between lung and liver peroxidation and mortality after zymosan peritonitis in the rats. Shock. 1994;2:222–227. doi: 10.1097/00024382-199409000-00011. [DOI] [PubMed] [Google Scholar]
- DOHERTY N.S., BEAVER T.H., CHAN K.Y., DINERSTEIN R.J., DIEKEMA K.A. The antinociceptive activity of paracetamol in zymosan-induced peritonitis in mice: the role of prostacyclin and reactive oxygen species. Br. J. Pharmacol. 1990;101:869–874. doi: 10.1111/j.1476-5381.1990.tb14173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISERICH J.P., HRISTOVA M., CROSS C.E., JONES A.D., FREEMAN B.A., HALLIWELL B., VAN DER VLIET A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- FAULKNER K.M., LIOCHEV S.I., FRIDOVICH I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J. Biol. Chem. 1994;269:23471–23476. [PubMed] [Google Scholar]
- FERNANDEZ P.M., HENSEN A., OTANI & HUGLI T.E. Chemotactic response to human C3a and C5a anaphylatoxins: Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo condition. J. Immunol. 1978;120:109–115. [PubMed] [Google Scholar]
- FRIDOVICH I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- GOODE H.F., WEBSTER N.R. Free radicals and antioxidants in sepsis. Crit. Care Med. 1993;21:1770–1776. doi: 10.1097/00003246-199311000-00029. [DOI] [PubMed] [Google Scholar]
- GORIS R.J.A., VAN BEBBER I.P.T., MOLLEN R.M.H., KOOPMAN J.P. Dose selective decontamination of the gastrointestinal tract prevent multiple organ failure. Arch. Surg. 1991;126:561–570. doi: 10.1001/archsurg.1991.01410290033006. [DOI] [PubMed] [Google Scholar]
- HELLER B., WANG Z.Q., WAGNER E.F., RADONS J., BURKLE A., FEHSEL K., BURKART V., KOLB H. Inactivation of the poly(ADP-ribose) polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells. J. Biol. Chem. 1995;270:11176. doi: 10.1074/jbc.270.19.11176. [DOI] [PubMed] [Google Scholar]
- JANSEN M.J., HENDRIKS T., VOGELS M.T.E., VAN DER MEER J.W.M., GORIS R.J.A. Inflammatory cytokines in an experimental model for the multiple organ dysfunction syndrome. Crit. Care Med. 1996;24:1196–1204. doi: 10.1097/00003246-199607000-00022. [DOI] [PubMed] [Google Scholar]
- MAINOUS M.R., TAO P., BERG R.D., DEITCH E.A. Studies of the route, magnitude, and time course of bacterial translocation in a model of systemic inflammation. Arch. Surg. 1991;126:33–37. doi: 10.1001/archsurg.1991.01410250037005. [DOI] [PubMed] [Google Scholar]
- MCKECHNIE K., FURMAN B.L., PARRATT J.R. Modification by oxygen free radical scavengers of the metabolic and cardiovascular effects of endotoxin infusion in conscious rats. Circ. Shock. 1986;19:429–439. [PubMed] [Google Scholar]
- MEHTA J.L., LAWSON D.L., NICOLINI F.A., ROSS M.H., PLAYER D.W. Effects of activated polimorphonuclear leukocytes on vascular smooth muscle tone. Am. J. Physiol. 1991;261:H327–H334. doi: 10.1152/ajpheart.1991.261.2.H327. [DOI] [PubMed] [Google Scholar]
- MULLANE K.M., WESTLIN W., KRAEMER R. Activated neutrophils release mediators that may contribute to myocardial injury and dysfunction associated with ischemia and reperfusion. Ann. N.Y. Acad. Sci. 1988;524:103–121. doi: 10.1111/j.1749-6632.1988.tb38534.x. [DOI] [PubMed] [Google Scholar]
- MUROHARA T., KUGYYAMA K., SUGIYAMA S., OHGUSHI M., YASUE H. Activated human polymorphonuclear leukocytes elicit endothelium-dependent contraction in isolated pig coronary arteries. J. Card. Pharm. 1993;21:760–766. doi: 10.1097/00005344-199305000-00011. [DOI] [PubMed] [Google Scholar]
- NOVELLI G.P., ANGIOLINI P., TANI R., CONSALES G., BORDI L. Phenyl-T-butyl-nitrone is active against traumatic shock in rats. Free Radic. Res. Commun. 1992;1:321–327. doi: 10.3109/10715768609080971. [DOI] [PubMed] [Google Scholar]
- OHKAWA H., OHISHI N., YAGI K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- RAO T.S., CURRIE J.L., SHAFFER A.F., ISAKSON P.C. In vivo characterization of zymosan-induced mouse peritoneal inflammation. J. Pharmacol. Exp. Therap. 1993;269:917–925. [PubMed] [Google Scholar]
- SNYDERMAN J., PHILLIPS F., MERGENHAGEN S.E. Polymorphonuclear leukocyte chemotactic activity in rabbit serum and guinea pig serum treated with immune complexes. Evidence for C5a as the major chemotactic factor. Infect. Immun. 1970;1:521–525. doi: 10.1128/iai.1.6.521-525.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABÓ C., CUZZOCREA S., ZINGARELLI B., O'CONNOR M., SALZMAN A.L. Endothelial dysfunction in endotoxic shock: importance of the activation of poly (ADP ribose synthetase (PARS) by peroxynitrite. J. Clin. Invest. 1997a;100:723–735. doi: 10.1172/JCI119585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABÓ C., DAY B.J., SALZMAN A.L. Evaluation of the relative contribution of nitric oxide and peroxynitritre to the suppression of mithochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and peroxynitrite scavenger. FEBS Lett. 1996;381:82–86. doi: 10.1016/0014-5793(96)00087-7. [DOI] [PubMed] [Google Scholar]
- SZABÓ C., LIM L.H.K., CUZZOCREA S., GETTING S.J., ZINGARELLI B., FLOWER R.J., SALZMAN A.L., PERRETTI M. Inhibition of poly (ADP-ribose) synthetase exerts anti-inflammatory effects and inhibits neutrophil recruitment. J. Exp. Med. 1997b;186:1041–1049. doi: 10.1084/jem.186.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA T.J., WILLIAMS P.G., HELLEWELL P.G. Role of prostaglandins and nitric oxide in acute inflammatory reactions in guinea-pig skin. Br. J. Pharmacol. 1993;110:416–422. doi: 10.1111/j.1476-5381.1993.tb13994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIEMERMANN C., BOWES J., MYNNY F.P., VANE J.R. Inhibition of the activity of poly(ADP ribose) synthase reduces ischaemia-reperfusion injury in the heart and skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 1997;94:679–683. doi: 10.1073/pnas.94.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN BEBBER I.P., LIENERS C.F.J., KOLDEWIJN E.L., REDL H., GORIS R.J.A. Superoxide dismutase and catalase in a experimental model of muliple organ failure. J. Surg. Res. 1989;52:265–270. doi: 10.1016/0022-4804(92)90084-d. [DOI] [PubMed] [Google Scholar]
- VON ASMUTH E.J.U., MAESSEN I.G., VAN DER LINDEN C.J., BUURMAN W.A. Tumor necrosis factor alpha and interleukin 6 in zymosan-induced shock model. Scand. J. Immunol. 1990;32:313–319. doi: 10.1111/j.1365-3083.1990.tb02925.x. [DOI] [PubMed] [Google Scholar]
- YIM M.B., CHOCK P.B., STADTMAN E.R. Copper, zinc superoxide dismutase catalyses hydroxyl radical production from hydrogen peroxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINGARELLI B., DAY B.J., CRAPO J., SALZMAN A.L., SZABÓ C. The potential involvement of peroxynitrite in the pathogenesis of endotoxic shock. Br. J. Pharmacol. 1997;120:259–264. doi: 10.1038/sj.bjp.0700872. [DOI] [PMC free article] [PubMed] [Google Scholar]


