Figure 1.
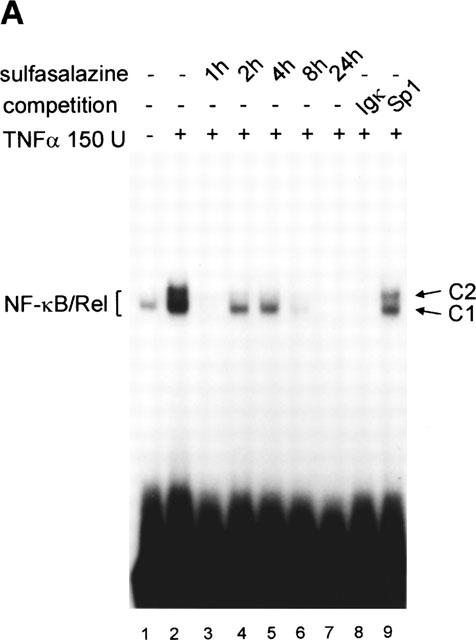
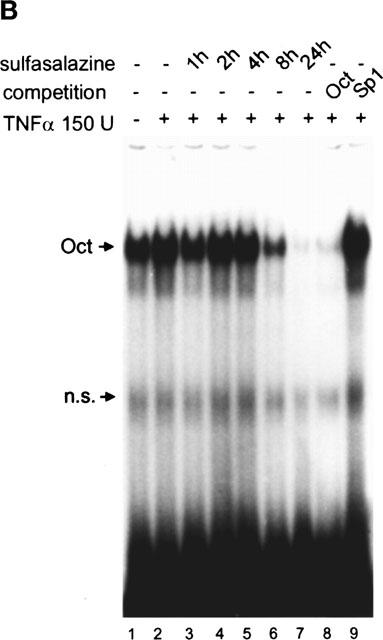
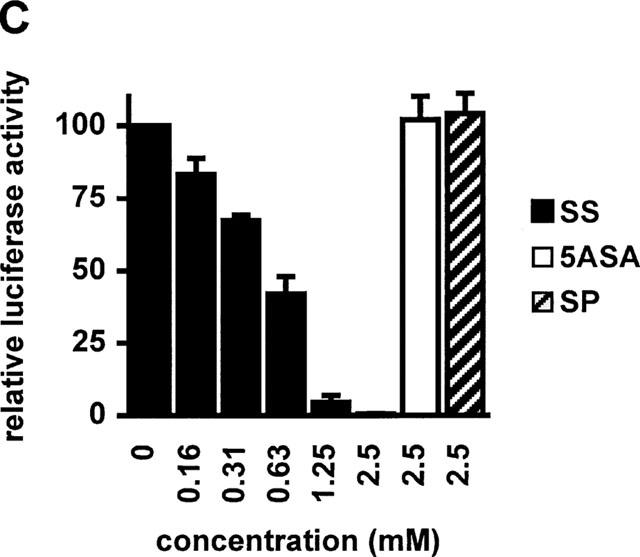
Sulfasalazine is a potent inhibitor of NF-κB activity in RBL5 T-lymphocytes. (A) Rapid inhibition of NF-κB binding activity by treatment with sulfasalazine. RBL5 cells were left untreated (lane 1), stimulated for 1 h with TNFα (lanes 2–9), and pretreated with 1.25 mM sulfasalazine for the indicated time periods (lanes 3–7). Nuclear proteins were extracted and electrophoretic mobility shift assays performed using a κB specific probe (IgκB). For competition experiments a 20 fold molar excess of unlabelled IgκB (lane 8) or Sp1 oligonucleotides (lane 9) were used. Running position of the NF-κB/Rel/DNA complexes c1 and c2 are indicated. One representative experiment is shown (n=4). (B) Sulfasalazine is a specific inhibitor of NF-κB and does not interfere with Oct-1 binding. The same nuclear extracts as in (A) were used in electrophoretic mobility shift assays using an Oct-1 probe. Running positions of the Oct-1 and a non-specific (n.s.) complex are indicated by arrows. One representative experiment is shown (n=3). (C) Sulfasalazine, but not its metabolic moieties 5ASA and sulfapyridine, is a potent inhibitor of NF-κB-dependent transcription. RBL5 cells were transiently transfected with a κB-dependent reporter plasmid (3xIgκBLuc). Cells were pretreated with medium or serial dilutions of sulfasalazine (SS) (black bars), 2.5 mM 5ASA (white bar), or 2.5 mM sulfapyridine (SP) (hatched bar) for 1 h, followed by stimulation with 150 U ml−1 TNFα for three additional hours. Luciferase activity from transfected cells stimulated with TNFα was used as reference and set to 100% luciferase activity. Experiments were done in duplicate. Mean values and standard error of the mean (s.e.mean) of four independent experiments (n=8) are shown.
