Abstract
The diversity of α2 and purinergic autoreceptor actions on action potential evoked calcium transients in single varicosities has been investigated using the calcium indicator Oregon Green 488 BAPTA-1.
During long trains of impulses (10 Hz for 30 s), the change in calcium concentration in varicosities from its resting level (Δ[Ca2+]v) increased in many varicosities during the first 3 s of stimulation before reaching a plateau.
The α2 adrenoceptor agonist clonidine (1 μM) decreased Δ[Ca2+]v by over 40% during short trains (five impulses at 5 Hz) in most varicosities, although some were unaffected. The α2 adrenoceptor antagonist idazoxan (2 μM) increased the Δ[Ca2+]v plateau following long trains in most varicosities. Hence, most varicosities possess α2 adrenoceptors which are activated when noradrenaline accumulates extracellularly.
During long trains of impulses, the P2y-purinergic receptor agonist 2-methyl-thio-ATP (100 μM) decreased Δ[Ca2+]v plateau by about 50% in most varicosities; α,β-methylene ATP (100 μM) decreased it by about 50% in a minority of varicosities; adenosine (200 μM) had no significant effect. Suramin (100 μM) increased the Δ[Ca2+]v during all stimulus protocols in most varicosities, suggesting that ambient ATP modulates Δ[Ca2+]v responses. The P2y receptor antagonist reactive blue (100 μM) affected a minority of varicosities. Given that most varicosities respond to suramin, other P2 receptor subtypes are probably present.
The ATP ectoenzyme antagonist ARL67157 (50 μM) decreased the plateau Δ[Ca2+]v during long trains in complete strings of varicosities but not in others.
The present technique indicates that varicosities have diverse autoreceptor utilization.
Keywords: Calcium, sympathetic, varicosities, vas deferens, autoinhibition
Introduction
The efficacy of transmitter release from sympathetic terminal varicosities during trains of impulses is modulated by endogenous autoinhibitory substances which include the cotransmitter noradrenaline (Starke et al., 1989; 1996). The extent to which these act by modulating the intra-terminal calcium concentration during and after a train of impulses, by changing the entry of calcium into the varicosities or modifying calcium-induced calcium release in the varicosities or the calcium sequestering processes, is now being elucidated. It has been recently shown that Δ[Ca2+]v in sympathetic varicosities following an impulse or short train of impulses is due to the influx of calcium ions into the varicosities and not to a significant contribution of calcium from intra-varicosity stores (Brain & Bennett, 1997). Furthermore, the α2 antagonist yohimbine has a profound effect on Δ[Ca2+]v amplitude following short trains of impulses in some sympathetic varicosities without affecting the sequestering processes (Brain & Bennett, 1997; Cheung et al., 1998). It is therefore likely, at least during short trains of impulses, that α2 autoreceptors operate by reducing the calcium influx, which reduces transmitter release and subsequently reduces the Δ[Ca2+]v.
Other autoreceptors exist on sympathetic varicosities. There are P1-(A1-) purinoceptors (Driessen et al., 1994; Hoyle et al., 1995), agonists of which decrease the release of the cotransmitter ATP as indicated by electrophysiological experiments (Sneddon et al., 1984). The recent discovery that soluble nucleotidases are released from sympathetic nerve terminals with the cotransmitters raises the possibility that adenosine derived from the breakdown of ATP acts on P1-autoreceptors to inhibit transmitter release (Todorov et al., 1997). However the P1-purinoceptor antagonist 8-phenyltheophylline does not affect the junctional potentials due to sympathetic nerve stimulation so that it is uncertain whether these receptors are functionally relevant (Blakeley et al., 1988). P2y-purinoceptors also exist on sympathetic varicosities in the mouse vas deferens (von Kugelgen et al., 1989a). There is good evidence that these are activated by the endogenously released ATP, producing a negative feedback control on ATP release (von Kugelgen et al., 1994). In the present work an investigation has been made into whether activation of α2, P2y and P1 autoreceptors leads to changes in Δ[Ca2+]v for all or some varicosities, either during short trains of impulses or during long trains like those typically used in pharmacological experiments in which contraction of the final effector is observed. In addition, the likelihood that autoreceptors modify Δ[Ca2+]v transients by changing calcium influx or calcium sequestration has been ascertained.
Methods
Tissue preparation
Vasa deferentia were excised from mice (strain Balb/c) aged 5–7 weeks. Animals were anaesthetized with CO2 then killed by cervical fracture. Animal ethics approval was granted by the Animal Care Ethics Committee, University of Sydney. Both vasa deferentia were removed, together with the connective tissue. The epididymal end of each was pinned to a sheet of Sylgard, and the sheath of epimysium carefully trimmed. This dissection, and the subsequent loading of the dye, was carried out in a bath which was continuously perfused with a Ringer's solution of the following composition (mM): Na+, 134.7; K+, 4.0; Ca2+, 1.0–2.0; Mg2+, 1.0; Cl−, 124.7–126.7; HCO3−, 16.3; H2PO4−, 1.7; glucose, 7.8. In order to maintain the pH the bathing solution was continually bubbled with 95% O2 and 5% CO2.
The cut prostatic end of the vas deferens was secured in a suction electrode and immersed in a solution of Oregon Green™ 488 BAPTA-1, 10 kD dextran (Oregon-BAPTA; Molecular Probes). This concentrated indicator solution was prepared by adding 3 μl of orthophosphate-free ringer's solution to 1 mg of the indicator. In each experiment, 0.2–0.5 μl of this stock solution was used. The dextran linked form of the dye allows orthograde transport of the indicator along the axons to the nerve terminals. The tissue was then left in the bath for 4–5 h to allow time for the sympathetic axons to load with the dye. While loading, the bath was covered to prevent photobleaching of the indicator. The cut end of the vas deferens was then removed from the Oregon-BAPTA solution but was kept in the bath solution for a further 0.5–3 h to reduce non-specific staining of the tissue.
Measurement of the calcium transient
The vas deferens was placed in a bath on the stage of an inverted Leica 4D laser scanning confocal microscope. The bath was continuously perfused with ringer solution, which was bubbled in a reservoir to maintain the correct pH. The base of the bath was a no. 0 coverslip to allow imaging deep within the tissue while using a relatively short working distance objective. The prostatic end (which had been exposed to the dye) was secured in a suction electrode.
The Oregon-BAPTA indicator was excited using an Ar-Kr laser at a wavelength of 488 nm. Fluorescence images were captured using the confocal microscope with an oil immersion 16×/0.5 objective, and a 515 nm long pass emission filter. The vas deferens was stimulated to initiate action potentials within the axons using an isolated stimulator (Digitimer DS2) triggered by a Master-8 programmable pulse generator (AMPI). The stimulus duration was kept constant at 0.08 ms throughout all experiments. The stimulation threshold voltage was determined by finding the smallest applied voltage amplitude which consistently produced a response to a single impulse. The vas deferens was then stimulated at approximately 30% above this threshold value. This stimulus protocol elicits calcium transients which are abolished by TTX and are hence secondary to action potentials (Trout et al., 1999). All experiments were carried out in the presence of 10 μM nifedipine and 3 μM prazosin in order to reduce the movement artefact induced by contraction. It has been previously shown by us (Brain & Bennett, 1997) that nifedipine (even at 20 μM) has no significant response on the amplitude of response to single action potentials (increased by 1±19%, n=5; P=0.95).
The scan rate of the confocal microscope during experiments (1500 lines s−1) was such that a full image (256×256 pixels) was captured every 216 ms (which includes the time for the scanner to reset between images). The time of exposure of the sample to the laser was minimized, however a certain amount of photobleaching and toxicity was unavoidable. These effects were particularly troublesome for the experiments involving short trains of impulses since these typically involved small amplitude calcium transients. In these experiments the effects of bleaching and toxicity were compensated for by subtracting the average of several control recordings from the recording of the evoked response.
Recordings were transferred to a Power Macintosh 6200/75 and processed using NIH Image 1.60. For each varicosity a region of interest was defined and the mean pixel intensity within this region determined for every image in the recording. Any variation in the position of the varicosity was accounted for by an automated procedure which tracked the varicosity between frames by locating the strongest local fluorescence signal compared with the background (Brain & Bennett, 1997). In this way a record of the variation of the mean fluorescent intensity following an action potential, within a single given varicosity, was obtained.
The change in fluorescence was converted to a change in calcium concentration via a relationship derived previously for this preparation (Brain & Bennett, 1997). Significance was evaluated with a Student's two-tailed, paired t-test. Unless otherwise stated, all errors quoted are standard errors of the mean. The recovery of Δ[Ca2+]v following stimulation was modelled by fitting the data to a double exponential function (Brain & Bennett, 1997).
To test for heterogeneity in the response of different varicosities to each drug the following method was used. The null hypothesis was that all varicosities are equally affected by each experimental protocol. In each experimental protocol the average effect of the drug was calculated. An expected response from each varicosity in the presence of the drug was calculated, based on its control response and assuming the null hypothesis. The measured response was compared with the expected response. When at least 25% of the varicosities (and at least two varicosities) had a measured response that was statistically significantly different (P<0.05) from the expected response then the null hypothesis is violated and hence the response to the experimental protocol was called heterogenous.
2-methylthioadenosine 5′-triphosphate, α,β-methyleneadenosine 5′-triphosphate, adenosine, basilen (reactive) blue E-3G, idazoxan, nifedipine, prazosin hydrochloride and suramin were obtained from Sigma Aldrich Pty. Ltd. (Sydney, Australia). Clonidine was obtained from Research Biochemicals International, distributed by Australian Laboratory Services (Sydney, Australia).
Results
The effect of long trains of impulses on Δ[Ca2+]v in different varicosities
Stimulation of the sympathetic nerve terminals with trains of impulses at 10 Hz for 30 s led, in many varicosities, to an increase in Δ[Ca2+]v over the first 3 s of stimulation until a plateau level of Δ[Ca2+]v was reached that was sustained for almost the entire period of stimulation (Figures 1a and 2a). However, in some varicosities the Δ[Ca2+]v initially increased, but then declined before the end of the train (Figure 2b,c). In other varicosities, the Δ[Ca2+]v increased throughout the period of stimulation (Figure 2d). The average Δ[Ca2+]v response within each of these four categories are shown in Figure 2e. Varicosities that gave a sustained Δ[Ca2+]v plateau during stimulation predominated (see class A in Figure 2f).
Figure 1.
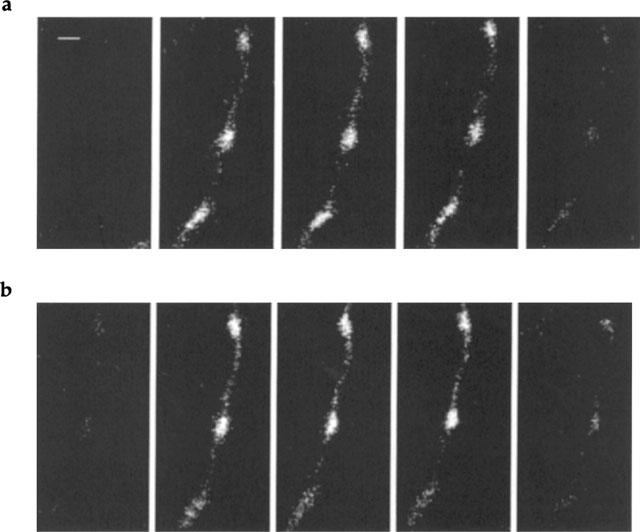
Images of Oregon Green 488 BAPTA-1 fluorescence in a string of varicosities during long trains of impulses before and after the introduction of clonidine. Images of the fluorescent signal are shown before, during and after a train of impulses at 10 Hz for 30 s for a string of varicosities under control conditions (a) and in the presence of clonidine (b; 1 μM). Stimulation commenced immediately after the first image and ended just before the last image. Successive images are separated by 8.64 s and displayed in chronological order (left to right). Calibration bar is 2 μm. The brighter parts of the image correspond to greater measured fluorescent intensity.
Figure 2.
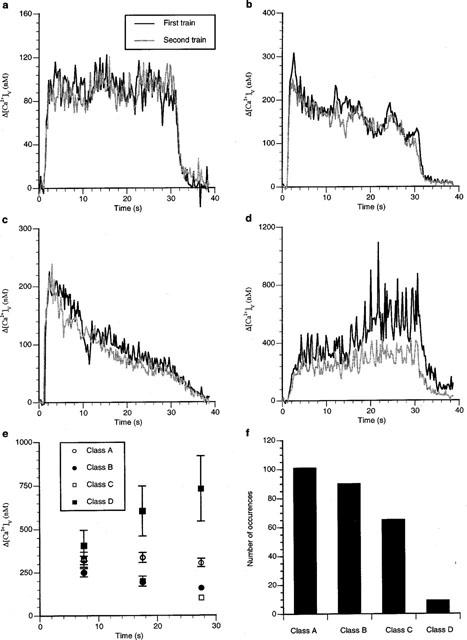
Different varicosities show different patterns of Δ[Ca2+]v during long trains of impulses. (a–d) show the average change in Δ[Ca2+]v during stimulation at 10 Hz for 30 s for four different varicosities and the changes some 15 min later in response to the same stimulus protocol. The pattern of change in Δ[Ca2+]v is reproducible in each varicosity. (e) gives the average Δ[Ca2+]v at intervals of 10 s, with t=0 being the beginning of stimulation, for all varicosities studied in the present work (nv=78) that are referred to as classes A to D, illustrated in (a–d). (f) gives the frequency distribution of varicosities with a particular class of Δ[Ca2+]v response.
The effect of different frequencies of stimulation of varicosities that showed a sustained plateau Δ[Ca2+]v (that is, showed a class A response) was determined. Increasing the frequency of stimulation between 1 and 10 Hz gave plateau values of Δ[Ca2+]v that increased with the frequency (Figure 3a–c). A plot of the average value of Δ[Ca2+]v over all seven varicosities that were tested in this way showed that whilst the plateau Δ[Ca2+]v increased with frequency, this relationship was non-linear, with the higher frequencies showing a smaller value of plateau Δ[Ca2+]v than that expected from the plateau Δ[Ca2+]v at the lower frequencies (Figure 3d). Treating this as a linear relationship gave a 25 nM increase in the plateau Δ[Ca2+]v for each 1 Hz increase in frequency.
Figure 3.
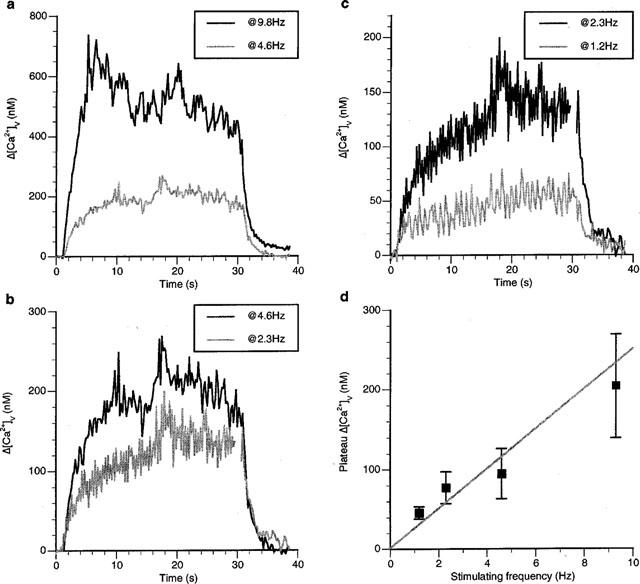
The plateau Δ[Ca2+]v in class A responses during a long train increases with stimulation frequency. (a–c) show the Δ[Ca2+]v response during different frequencies of stimulation in a single varicosity with a class A response. (d) gives the relationship between the Δ[Ca2+]v at 20 s into the stimulation train and the frequency of stimulation for seven varicosities.
The effect of an α2 receptor agonist on Δ[Ca2+]v
The α2 receptor agonist clonidine (1 μM) significantly decreased the peak Δ[Ca2+]v due to a single impulse in only a small minority of varicosities (Figure 4a). The decrease in Δ[Ca2+]v amounted to about 52% in two out of nine varicosities (nv=9) on five strings (ns=5) studied in three preparations (ne=3; Figure 4e). The two affected varicosities were on the same string which also possessed a varicosity that was not affected (open triangle in Figure 4e). Clonidine affected the peak Δ[Ca2+]v due to short trains of impulse (five impulses at 5 Hz) in over 50% of the varicosities studied, decreasing the Δ[Ca2+]v peak by about 45% (Figure 4b). One string showed no effects of clonidine (filled square, Figure 4f), another possessed only one varicosity that was affected (filled circle, Figure 4f), whereas five out of six varicosities were affected on two other strings close to each other in the same preparation (triangles, Figure 4f; nv=11, ns=4, ne=3). The rate of calcium recovery following a single impulse or short trains of impulses was unaffected by clonidine in any varicosity studied (Figure 4c,d).
Figure 4.
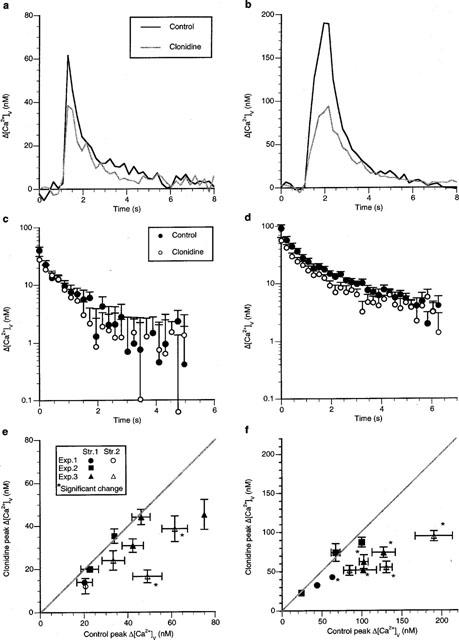
Clonidine decreases Δ[Ca2+]v in some varicosities following an impulse but in a majority of varicosities during short trains of impulses without changing the rate of sequestration of Δ[Ca2+]v. (a and b) show the typical Δ[Ca2+]v response before, during and following a single impulse and a short train of five impulses at 5 Hz, respectively, during a class A response in a single varicosity in response to clonidine (1 μM). (c and d) show the average changes in Δ[Ca2+]v from eight varicosities on log-linear coordinates following the stimulation protocols given in (a) and (b) respectively; if a double exponential curve is fitted to these results then the following amplitudes and time constants are obtained (c) control amplitudes of 31±4 and 13±3 nM with time constants of 4.0±0.7 and 0.15±0.09 s respectively; clonidine amplitudes of 26±3 and 5±2 nM with time constants of 1.7±0.2 and 0.1±0.1 s respectively. (d) control amplitudes of 76±16 and 22±6 nM with time constants of 3.2±1.1 and 0.22±0.7 s respectively; clonidine amplitudes of 51±5 and 12±6 nM with time constants of 1.7±0.4 and 0.02±0.08 s respectively). (e) gives the average peak Δ[Ca2+]v due to a single impulse in the presence of clonidine plotted against that in the control solution. (f) gives the average peak Δ[Ca2+]v amplitude due to a short train of impulses (five impulses at 5 Hz) in the presence of clonidine plotted against that for the same varicosity in control solution.
The plateau Δ[Ca2+]v during long trains of impulses (10 Hz for 30 s) was greatly decreased by clonidine in the majority of varicosities (Figure 5a). Significant decreases were observed in five varicosities (nv=9, ns=4, ne=4), with in some cases a decrease of over 75% (Figure 5b). However some strings possessed varicosities that did not show any changes at all, whilst other strings possessed a minority of varicosities which did not show significant changes (Figure 5b). It is interesting to note that the varicosities with the highest control plateaus seem to be most affected by clonidine (Figure 5b). Overall, the response to clonidine was heterogenous (as described in the Methods) following single impulses, short trains and long trains of impulses.
Figure 5.
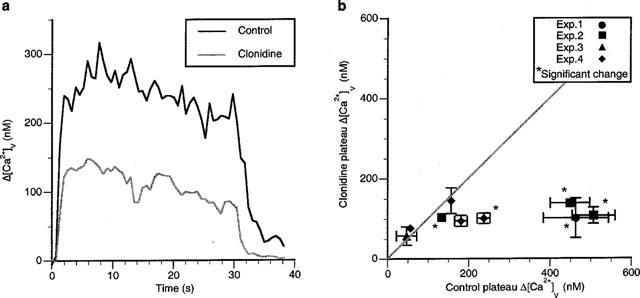
Clonidine decreases the Δ[Ca2+]v plateau in most varicosities during long trains of impulses. (a) an example of the plateau Δ[Ca2+]v reached in a varicosity during stimulation at 10 Hz for 30 s in response to clonidine. (b) gives the average changes in the plateau amplitude of Δ[Ca2+]v due to a long train of impulses in the presence of clonidine plotted against the average value of Δ[Ca2+]v for the same varicosity in the control solution.
The effect of an α2 receptor antagonist on Δ[Ca2+]v
A check was next made of the ability of endogenously released noradrenaline to affect the α2 receptors on varicosities, using the α2 receptor antagonist idazoxan (2 μM). This drug only affected the peak Δ[Ca2+]v following a single impulse by increasing it in a small minority of varicosities (Figure 6a). Only three varicosities were affected in this way, with the majority of strings of varicosities showing no significant changes in Δ[Ca2+]v (Figure 6d; nv=10, ns=5, ne=3). The peak Δ[Ca2+]v during a short train of impulses was also elevated significantly in only a small minority of varicosities (Figure 6b,e). Only three varicosities showed a significant increase in their peak Δ[Ca2+]v, and each of these was on a separate string (Figure 6e; nv=12, ns=5, ne=3). The rate of calcium sequestration, modelled as the sum of two exponentials, following single impulses or short trains of impulses was unaffected by idazoxan in any varicosity (for single impulses: control component amplitudes of 23±5 and 13±3 nM with time constants of 3.9±1.2 and 0.19±0.05 s respectively; idazoxan component amplitudes of 24±4 and 19±4 nM with time constants of 5.6±1.7 and 0.23±0.12 s respectively. For short trains of impulses: control component amplitudes of 74±21 and 74±18 nM with time constants of 1.0±0.2 and 0.7±0.2 s respectively; idazoxan component amplitudes of 70±16 and 80±19 nM with time constants of 2.5±0.8 and 0.31±0.06 s respectively).
Figure 6.
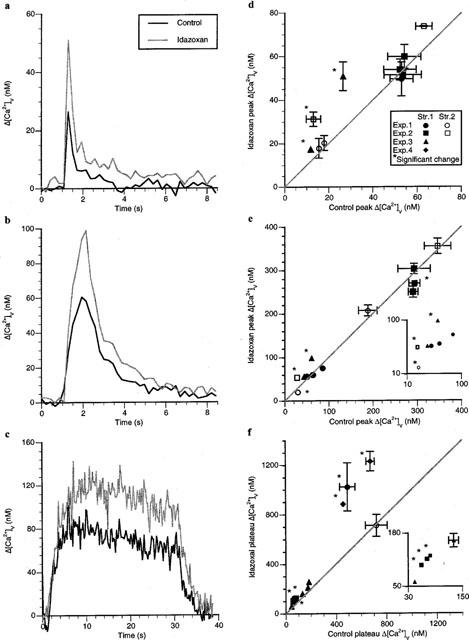
Idazoxan mainly affects Δ[Ca2+]v following long trains of impulses. Typical Δ[Ca2+]v responses during and following a single impulse (a), a short train of impulses (b; five impulses at 5 Hz) and a long train of impulses (c; 30 s of stimulation at 10 Hz) in a varicosity of response class A in response to idazoxan (2 μM). (d–f) give the changes in the Δ[Ca2+]v peak or plateau in the presence of idazoxan for a number of a number of varicosities following a single impulse, a short train of impulses and a long train of impulses respectively. Each symbol gives the average changes in Δ[Ca2+]v peak or plateau in the presence of idazoxan plotted against that for the same varicosity in the control solution.
The plateau Δ[Ca2+]v reached during long trains of impulses in the presence of idazoxan was increased in a majority of varicosities (Figure 6c,f). Significant increases of about 80% were detected in six varicosities (nv=11, ns=5, ne=4), with all three varicosities on one string being affected (filled squares, Figure 6f) and none on another (filled triangles, Figure 6f).
The response to idazoxan was heterogenous (as described in the Methods) following single impulses and long trains of impulses. These observations on the effects of clonidine and idazoxan suggest that about half of the varicosities possess α2 receptors, that many of these varicosities are on the same string, and that as the endogenous release of noradrenaline is increased by long trains of impulses most of these varicosities have their Δ[Ca2+]v modulated.
The effect of purinergic receptor agonists on Δ[Ca2+]v
The effect of different P2 and P1 receptor agonists on Δ[Ca2+]v was next investigated in order to determine if such receptors exist on varicosities and modulate Δ[Ca2+]v. The P2y receptor agonist 2-methyl-thio-ATP (100 μM) decreased the plateau Δ[Ca2+]v during stimulation with long trains of impulses for 30 s at 10 Hz (Figure 7a). This occurred in nearly all varicosities examined (Figure 7d; nv=9, ns=7, ne=4) consistent with the idea that most varicosities possess P2y autoreceptors, but the amplitude of the change was heterogenous. The P2×1/P2×3 receptor agonist α,β-methylene ATP (100 μM) reduced the plateau Δ[Ca2+]v during long trains of impulses (Figure 7b), in a minority of varicosities (Figure 7e; nv=8, ns=5 and ne=4), indicating that most varicosities do not possess P2x receptors. The response to α,β-methylene ATP was heterogenous (as described in the Methods). There was no evidence for an action of the P1 receptor agonist adenosine (200 μM), as this did not affect the size of Δ[Ca2+]v following long trains of impulses (Figure 7f; nv=6, ns=2, ne=2). These observations suggest that P2y receptors exist on sympathetic varicosities for the modulation of Δ[Ca2+]v with only some of these possessing P2x receptors.
Figure 7.
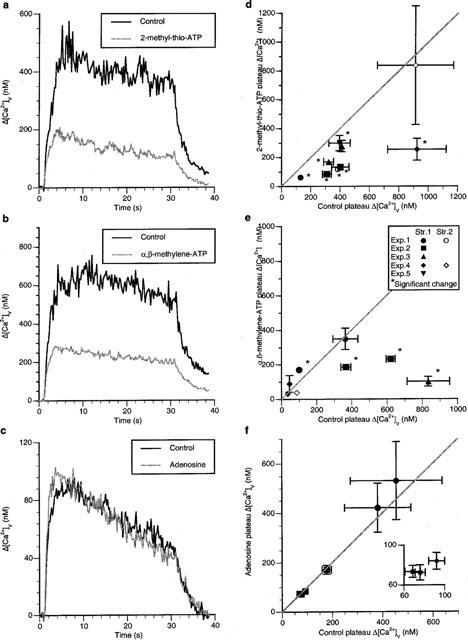
2-methyl-thio-ATP decreases the Δ[Ca2+]v plateau during long trains of impulses whereas neither α,β-methylene ATP nor adenosine has much effect. (a–c) show the effects on Δ[Ca2+]v of stimulating with long trains of impulses (10 Hz for 30 s) in the presence of 2-methyl-thio-ATP (100 μM), α,β-methylene ATP (100 μM) and adenosine (200 μM) respectively. (d–f) give the changes in the size of Δ[Ca2+]v in the presence of 2-methyl-thio-ATP, α,β-methylene ATP and adenosine respectively for a number of varicosities. Each symbol gives the average changes in the amplitude of Δ[Ca2+]v in the presence of the drug plotted against the average value of Δ[Ca2+]v for the same varicosity in the control solution.
The effect of purinergic receptor antagonists on Δ[Ca2+]v
The effects of the P2 receptor antagonist suramin were next determined in order to check if Δ[Ca2+]v is modulated through endogenously released ATP acting on P2 receptors on varicosities. Suramin (100 μM) increased the peak Δ[Ca2+]v significantly following a single impulse in the majority of varicosities (Figure 8a). In three strings of the four examined, each possessed at least one varicosity that was affected by suramin, which increased Δ[Ca2+]v by up to 100% independent of the peak Δ[Ca2+]v in the control solution (Figure 8d; nv=10, ns=4, ne=3). Suramin increased the peak Δ[Ca2+]v significantly during short trains of impulses in nearly all varicosities (Figure 8b). All strings possessed varicosities that showed substantial increases in Δ[Ca2+]v, sometimes over 100% (Figure 8e; nv=11, ns=3, ne=3). The plateau Δ[Ca2+]v was also increased substantially during long trains of impulses in many varicosities (Figure 8c). In six varicosities there were significant increases in Δ[Ca2+]v of about 80% (nv=14, ns=6, ne=3), although all the varicosities on three of the strings examined failed to increase plateau Δ[Ca2+]v. The response to suramin was heterogenous (as described in the Methods) following short trains and long trains of impulses. Suramin did not affect the rates of calcium sequestration after single impulses or short trains of impulses (for single impulses: control component amplitudes of 53±9 and 8±5 nM with time constants of 2.5±0.3 and 0.9±0.5 s respectively; suramin component amplitudes of 70±12 and 33±10 nM with time constants of 4.1±0.7 and 0.8±0.2 s respectively. For short trains of impulses: control component amplitudes of 103±22 and 18±8 nM with time constants of 1.7±0.2 and 0.04±0.14 s respectively; suramin component amplitudes of 210±57 and 32±57 nM with time constants of 1.8±0.4 and 0.26±0.20 s respectively).
Figure 8.
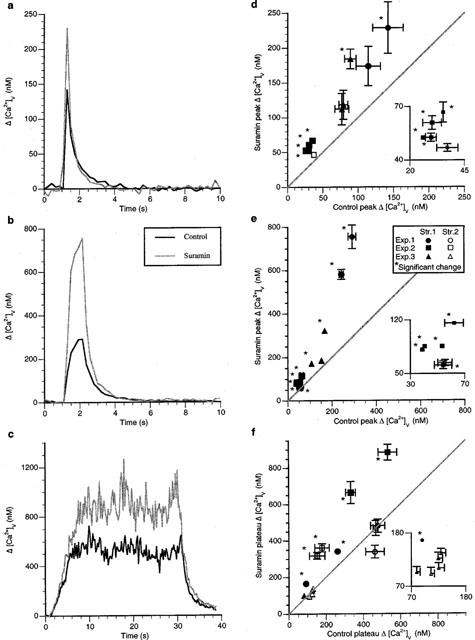
Suramin increases Δ[Ca2+]v following an impulse and during short and long trains of impulses in most varicosities. (a–c) show the typical changes in Δ[Ca2+]v during and following a single impulse, a short train of impulses (five impulses at 5 Hz) and a long train of impulses (30 s of stimulation at 10 Hz) in a varicosity of response class A in response to suramin (100 μM). (d–f) give the changes in the size of Δ[Ca2+]v in the presence of suramin for a number of varicosities following a single impulse, a short train of impulses and a long train of impulses respectively. Each symbol gives the average changes in the amplitude of Δ[Ca2+]v in the presence of idazoxan plotted against the average value of Δ[Ca2+]v for the same varicosity in the control solution.
These observations on the effects of suramin indicate that P2 receptors are under tonic control by endogenous ATP. Given the observations that 2-methyl-thio-ATP affects the plateau Δ[Ca2+]v in most varicosities, described above, the results with suramin suggest that it is the P2y subclass of P2 receptors on varicosities which are under the control of endogenously released ATP. In order to check this, the effects of the P2y antagonist reactive blue (100 μM) on the Δ[Ca2+]v of varicosities was next examined. Reactive blue did not increase the Δ[Ca2+]v due to a single impulse in any of the varicosities studied (Figure 9a,d; nv=9, ns=6, ne=3). Reactive blue also had little effect on the peak Δ[Ca2+]v reached during short trains of impulses (Figure 9b), with only one varicosity showing a significant increase in Δ[Ca2+]v (Figure 9e; nv=14, ns=7, ne=4). The plateau Δ[Ca2+]v reached during long trains was increased by reactive blue at a greater number of varicosities than for short trains (Figure 9c).
Figure 9.
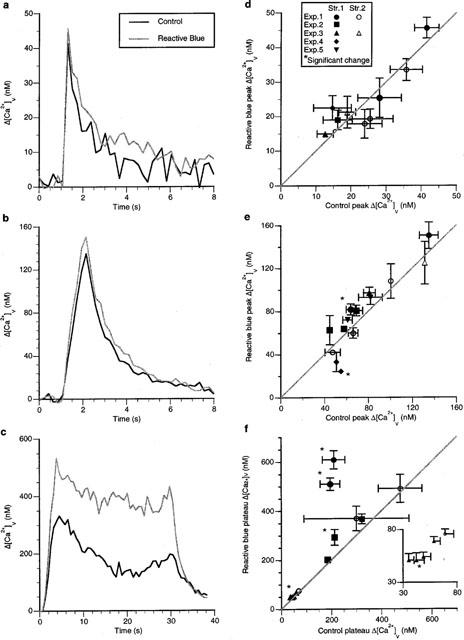
Reactive blue does not increase Δ[Ca2+]v during an impulse or following a short train of impulses but does in a minority of varicosities during long trains of impulses. Typical changes in Δ[Ca2+]v response during and following a single impulse (a), a short train of impulses (b; five impulses at 5 Hz) and a long train of impulses (c) 30 s of stimulation at 10 Hz) in a varicosity of response class A in response to reactive blue (100 μM). (d–f) give the changes in the Δ[Ca2+]v peak or plateau in the presence of reactive blue for a number of varicosities following a single impulse, a short train of impulses and a long train of impulses respectively. Each symbol gives the average changes in the amplitude of Δ[Ca2+]v in the presence of reactive blue plotted against that for the same varicosity in the control solution. Reactive blue had no effect on the rates of sequestration of calcium following either an impulse or short trains of impulses (for single impulses: control amplitudes of 16±2 and 12±5 nM with time constants of 6.8±3.9 and 0.17±0.06 s respectively; reactive blue amplitudes of 17±6 and 12±3 nM with time constants of 5.8±4.0 and 0.17±0.04 s respectively. For short trains of impulses: control amplitudes of 49±7 and 27±5 nM with time constants of 4.1±2.6 and 0.11±0.07 s respectively; reactive blue amplitudes of 45±7 and 33±7 nM with time constants of 1.7±0.3 and 0.20±0.07 s respectively).
Four varicosities showed a significant increase in Δ[Ca2+]v (nv=12, ns=4, ne=3), with two of these occurring on the same string and each of the others on a single string, so that one string possessed varicosities that showed no effects at all (Figure 9f). Overall, the response to Reactive blue was heterogenous (as described in the Methods) following all stimulus protocols. These results show that most varicosities are not affected by reactive blue at any of the protocols of stimulation used whereas they are by suramin, indicating that types of P2 receptors other than P2y might be involved in the modulation of Δ[Ca2+]v.
The effect of ATPase antagonists on Δ[Ca2+]v
In order to see if ecto-ATPases limit the amount of ATP acting on the P2y autoreceptors, the effects of the ATP ectoenzyme antagonist ARL 67157 on Δ[Ca2+]v were determined. ARL 67157 (50 μM) significantly decreased the plateau Δ[Ca2+]v following a long trains of impulses by about 45% in all four varicosities on two strings in one preparation but did not decrease the plateau Δ[Ca2+]v in any of the other varicosities on three other strings in three other preparations (nv=12, ns=5, ne=4; Figure 10).
Figure 10.
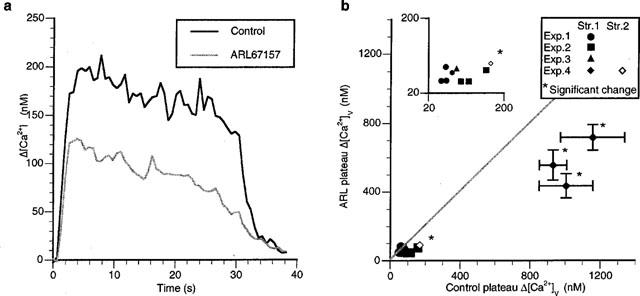
Ecto-ATPase antagonist (ARL 67157) decreases Δ[Ca2+]v during long trains of impulses in a small minority of varicosities. (a) shows the typical changes in Δ[Ca2+]v during and following a long train of impulses (30 s of stimulation at 10 Hz) in a varicosity of response class A in response to ARL 67157 (50 μM). (b) gives the changes in the Δ[Ca2+]v in the presence of ARL 67157 for a number of varicosities following a long train of impulses. Each symbol gives the average changes in the amplitude of Δ[Ca2+]v in the presence of ARL 67157 plotted against the average value of Δ[Ca2+]v for the same varicosity in the control solution.
Discussion
While the most common type of response of a varicosity to prolonged stimulation was the establishment of a relatively stable plateau of elevated Δ[Ca2+]v (i.e. a class A response), this was not the only type of response. In some varicosities the Δ[Ca2+]v began to decline from its peak before the end of the stimulus train (class B and C responses). It may be that in these varicosities the decline in the peak corresponds with the onset of presynaptic autoinhibition of the calcium influx (which may be adrenergic, purinergic or peptidergic). Alternatively, the kinetics of calcium removal or sequestration may change during the stimulus train. For example, the activity of the calcium pump may be upregulated in the presence of an increase in intracellular calcium concentration via a Ca2+-calmodulin-dependent pathway (for review, see Monteith & Roufogalis, 1995), which would lead to either the class B or C responses. It is also possible that the class B and C responses occur as a result of an increasing number of failures to initiate action potentials during the course of prolonged stimulation. We have observed such failures during long trains of stimuli with line scanning confocal microscopy through single varicosities (unpublished observation). The class D responses (which were relatively rarely observed) may occur in varicosities in which calcium sequestration mechanisms begin to fail as the stimulus train progresses.
A linear relationship between the stimulation frequency and the plateau Δ[Ca2+]v is theoretically expected if calcium is removed from the varicosities by a first order process (see the Appendix). Using the measured value of the stimulus frequency versus Δ[Ca2+]v plateau of 25 nM s−1, and assuming that only the first component of Δ[Ca2+]v sequestration (time constant of 0.42±0.05 s; Brain & Bennett, 1997) is significant in determining the plateau concentration, then the change in calcium concentration per impulse is predicted from the derivation in the Appendix to be about 60 nM. This is in agreement with the Δ[Ca2+]v peak of 65±12 nM measured following single impulses that we have previously reported (Brain & Bennett, 1997). The observed non-linearity of the relationship between frequency and plateau amplitude may be due to more complicated kinetics of calcium sequestration than assumed by the simple first order model.
The effect of α-receptor agonists and antagonists on Δ[Ca2+]v
Clonidine on average decreased Δ[Ca2+]v during short trains of impulses by about 40% and during long trains by an even greater percentage in the large majority of varicosities without changing the kinetics of sequestration of calcium, indicating that most varicosities possess α2 receptors. It is known that prazosin has considerably affinity for both α2B and α2C adrenoceptors, with pKi for the human isoforms of 7.0 and 7.1 respectively (Marjamäki et al., 1993). As the present experiments were carried out in 3 μM prazosin, with the aim of blocking contraction via inhibition of α1 receptors, we are unable to comment on the presence or action of either α2B or α2C adrenoceptor mechanisms in the varicosities. The failure of clonidine to decrease Δ[Ca2+]v due to a single impulse, except in a small minority of varicosities, was surprising. Although it has been shown previously that clonidine can decrease Δ[Ca2+]v due to a single impulse (Brain & Bennett, 1997), the proportion and disposition of varicosities which behave in this way was not established. It seems that the agonist effects of clonidine can only be detected when there is a substantial influx of calcium into the varicosities as occurs during trains of impulses. However, even during trains of impulses these effects of clonidine on Δ[Ca2+]v were quite heterogeneous across varicosities, with some strings of these remaining entirely unaffected and other strings possessing some affected varicosities next to unaffected varicosities. The unaffected strings existed in too high a number to be attributed to sensory endings, and furthermore all their varicosities showed calcium transients on arrival of a nerve impulse, so that the possibility exists that these strings might belong to terminals that do not release noradrenaline. It is likely that clonidine modulates Δ[Ca2+]v by decreasing the calcium influx through an N-type like voltage-dependent calcium channel. α2 receptors on sympathetic neurons inhibit calcium influx through an N-type calcium channel (Boehm & Huck, 1996) although in sympathetic nerve terminals the α2 receptor mediated changes in transmitter release occur via an ω-conotoxin GVIA resistant channel, which is nevertheless a voltage-sensitive calcium channel as it is blocked by cadmium ions (Brock & Cunnane, 1995; Smith & Cunnane, 1996; 1998).
Idazoxan on average increased Δ[Ca2+]v for short trains of impulses without changing Δ[Ca2+]v during a single impulse, consistent with the previously reported action of the α2-antagonist yohimbine (Brain & Bennett, 1997). These results are also consistent with electrophysiological analysis of the effects of these agents on junction potentials in the vas deferens, with clonidine depressing all the junction potentials during a train (Illes & Starke, 1983; Blakeley et al., 1984) and yohimbine increasing all the junction potentials in a train except for the first one (Illes & Starke, 1983; Brock et al., 1990). These results are to be expected given the fact that the cotransmitter noradrenaline released by an impulse does not act on α2 autoreceptors to modulate transmitter release by the same impulse (Story et al., 1981; Mayer et al., 1988). The observation that Δ[Ca2+]v was unaffected by idazoxan during long trains of impulses in whole strings of varicosities is consistent with the failure of clonidine to affect whole strings of varicosities under the same stimulation paradigm, and reinforces the conclusion that some terminals do not possess α2 adrenoceptors at all.
The effect of purinergic receptor agonists and antagonists on Δ[Ca2+]v
The P1 receptor agonist adenosine had no significant effect on the great majority of varicosities. On the other hand the P2y agonist 2-methyl-thio-ATP had a profound effect in decreasing Δ[Ca2+]v during long trains of impulses in nearly all varicosities, pointing to a widespread distribution of this receptor on varicosities. The P2x receptor agonist α,β-methylene ATP affected a minority of varicosities, indicating that this receptor is not widely distributed over varicosities.
The significant effect of the P2 receptor antagonist suramin on varicosities indicates the activation of the P2y receptor by endogenous ATP. Suramin increased the amplitude of Δ[Ca2+]v for single impulses as well as for short and long trains of impulses in a high proportion of varicosities without affecting the kinetics of sequestration. Suramin is an antagonist at P2-purinoceptors (Dunn & Blakeley, 1988), blocking membrane currents due to the effects of exogenous ATP acting at P2x receptors on isolated smooth muscle cells (Hoiting et al., 1990), the purinergic (P2x) phase of the contraction of the vas deferens (von Kugelgen et al., 1989b; Mallard et al., 1992) as well as the excitatory junction potential in the vas deferens due to ATP acting on P2x receptors (Sneddon, 1992). However suramin tends to potentiate the contractions produced by exogenous ATP on the vas deferens (Bailey & Hourani, 1994; Reilly & Hirst, 1996). This apparent contradiction is probably resolved by the discovery that suramin has direct inhibitory effects on ectonucleotidases (Hourani & Chown, 1989; Stout & Kirley, 1995; Ziganshin et al., 1995; Kennedy & Leff, 1995; also see below). Exogenous ATP is hydrolysed to adenosine by membrane bound ectonucleotidases; the inhibition of the ectonucleotidases with suramin will protect the ATP from hydrolysis and so enhance the contractile response. However the potentiating effect of suramin on Δ[Ca2+]v is unlikely to be due to Δ[Ca2+]v being protected from a negative feedback effect normally exerted by adenosine but failing to occur in the presence of this inhibitor of the ectonucleotidases, as exogenous adenosine had little effect on Δ[Ca2+]v in any varicosity studied. It is interesting in this regard that Blakeley et al. (1988) could find no evidence for an endogenous effect of adenosine in modulating transmission in the vas deferens.
The Δ[Ca2+]v due to a single impulse was increased significantly by suramin in most varicosities. As it is unlikely that ATP released by an impulse can act back on the varicosity from which it is released to modulate secretion by the same impulse, it seems that there is an ambient level of ATP (or another endogenous P2-receptor agonist) present at the varicosities in the absence of impulses that regulates the Δ[Ca2+]v. There is evidence to suggest that suramin increases the overflow of transmitter from the vas deferens for long trains of impulses, but not for short trains, an observation that has been interpreted as showing the necessity for prolonged nerve activity before the negative feedback mediated by the P2y autoreceptors is operative (von Kugelgen et al., 1993). It might be that a sustained release of ATP from varicosities occurs in the absence of nerve impulses, as indicated by the spontaneous excitatory junction potentials in the vas deferens (Burnstock & Holman, 1962), or that there is a spontaneous release of ATP from smooth muscle cells (Kurz et al., 1994; Henery et al. 1999). This spontaneous ATP release could provide a tonic inhibitory effect on evoked release of transmitter through a tonic down regulation of calcium channels on varicosities. During trains of impulses evoked release probably adds to the spontaneous tonic release that affects Δ[Ca2+]v. This would explain the increase in the extent to which suramin enhanced Δ[Ca2+]v during a short train of impulses (on average about 105%) compared with that for a single impulse (on average about 90%).
Given the extensive effects of 2-methyl-thio-ATP and suramin on the Δ[Ca2+]v transient in most varicosities, the paucity of effects of the P2y antagonist reactive blue 2 (Burnstock & Warland, 1987) was surprising. Reactive blue 2 acts in the mouse vas deferens to greatly increase the overflow of stimulation-evoked noradrenaline (von Kugelgen et al., 1994). However reactive blue had little effect in potentiating Δ[Ca2+]v compared with that of suramin for either a single impulse or short trains of impulses, indicating that it is unlikely that suramin is only blocking P2y receptors on the varicosities, and perhaps pointing to a more effective role of P2x receptors on varicosities than revealed in the present study.
The effect of ARL 67157 on Δ[Ca2+]v
The newly synthesized ATPase ectonucleotidase inhibitor ARL 67157 (formerly FPL 67156) decreased Δ[Ca2+]v during trains of impulses for some varicosities but not for others. This result is to be expected if the released ATP in the vas deferens is partly metabolized by ATPases before it acts on P2y autoreceptors to inhibit evoked transmitter release: protecting ATP from metabolism then increases the availability of ATP to act on these autoreceptors and so down-regulate the voltage-dependent calcium channels that control the evoked influx of calcium ions and hence Δ[Ca2+]v. The fact that ARL 67157 also potentiates the contraction of the vas deferens in response to exogenous ATP (Crack et al., 1995) is simply explained on the effect of this agent in protecting the exogenous ATP from metabolism, and hence increasing its concentration at the postjunctional P2x receptors. The recent discovery that soluble nucleotidases are secreted along with ATP during evoked transmitter release and that these nucleotidases are inhibited by suramin and ARL 67157 adds a new dimension to the pharmacological analysis of purinergic transmission (Todorov et al., 1997). ATP metabolism would be prevented by inhibition of this secreted nucleotidase, thus giving a greater depression of Δ[Ca2+]v during trains of impulses than for single impulses, as is observed.
Heterogeneity of autoreceptors on varicosities
In this investigation, changes in Δ[Ca2+]v have been used to determine the actions of adrenergic and purinergic agents on single varicosities. Of course if a particular agent exerts its effect on the varicosities by other means than altering calcium influx or sequestration then this approach is of no use. Adenosine, which is known in many synapses to modulate calcium currents (see for example Bennett & Ho, 1992) failed to have any effect on Δ[Ca2+]v in any varicosities but one. It seems most likely that the P1 receptors that mediate the action of adenosine are rarely present on varicosities.
The possibility that the calcium influx from one varicosity contributes to the Δ[Ca2+]v recorded in adjacent varicosities has been examined previously using line scans through the varicosities and shown not to occur following a single impulse; there was, however, some evidence for diffusion of calcium between varicosities during relatively sustained spontaneous elevations in Δ[Ca2+]v (lasting over seconds) that sometimes occur in varicosities (Brain & Bennett, 1997). These observations suggest that changes in Δ[Ca2+]v that occur in a varicosity during long trains might be coupled to the changes in Δ[Ca2+]v that occur in adjacent varicosities. Thus, the present observations of diverse changes in Δ[Ca2+]v plateau in different varicosities exposed to adrenergic and purinergic agonists and antagonists point to even greater diversity in the distribution of receptors on varicosities. For example, the actions of clonidine on Δ[Ca2+]v during long trains of impulses indicate that whilst a majority of varicosities were affected there exist whole strings of varicosities that were not and which therefore are unlikely to possess α2 receptors. Furthermore some varicosities on the same string responded to clonidine, while adjacent ones did not, suggesting that any coupling between the varicosities was too weak to remove the effects of receptor distribution diversity. In contrast, the actions of 2-methyl-thio-ATP on Δ[Ca2+]v during long trains indicated that most varicosities possessed P2y receptors.
The effects of the antagonists to α2 and P2 receptors on Δ[Ca2+]v during trains of impulses pointed to the importance of the pooling in the extracellular space of noradrenaline and ATP released from many different sites in the process of autoinhibition. Thus the number of varicosities showing significant effects on Δ[Ca2+]v to idazoxan was increased during long trains of impulses compared with short trains. If there is no significant pooling of calcium between varicosities during long trains, as discussed above, then the increasing effects of idazoxan at longer trains may be attributed to the greater concentration of noradrenaline in the extracellular space.
It is important to consider the presence of non-sympathetic axons and terminals within the vas deferens. In the monkey vas deferens, about 0.1% of varicosities are sensory (Type V varicosities; Leong & Singh, 1989). Acetycholinesterase positive fibres are most densely distributed in the lamina propria, but are also present in the inner part of the smooth musculature. If these results hold for the mouse, then it would be very unlikely that any of the varicosities viewed in these experiments, which are close to the outer surface of the vas deferens, were either sensory or cholinergic.
This work presents a first description of the pharmacology of single varicosities when the agents to be examined might have been expected to exert an effect on intra-varicosity calcium. There are many questions that remain to be addressed. These include whether the number of varicosities examined in the present work are a sufficient sample on which to reach general conclusions, or whether there are pharmacological differences between the subclasses of varicosities defined on the basis of the Δ[Ca2+]v response to long trains of stimuli. A valuable technical addition to the approach here would be to combine confocal imaging of the immunohistochemical localization of receptors on varicosities with the Δ[Ca2+]v response to stimulation determined for the same varicosities. The distribution of purinergic and adrenergic receptors at single varicosities determined immunohistochemically is now under investigation (Hansen et al., 1998; Dutton et al., 1999), so that this combined approach, which would couple structure with function, will soon be possible.
Abbreviations
- ATP
adenosine triphosphate
- BAPTA
bis (O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- Δ[Ca2+]v
change in calcium concentration in a varicosity from its resting level
- class A response
a sustained Δ[Ca2+]v plateau during prolonged stimulation
- class B response
a slowly recovering Δ[Ca2+]v during prolonged stimulation
- class C response
a rapidly recovering Δ[Ca2+]v during prolonged stimulation
- class D response
an increasing Δ[Ca2+]v during prolonged stimulation
- Exp.
experiment
- Str.
string of varicosities
- Oregon-BAPTA
Oregon Green™ 488 BAPTA-1, 10 kD dextran
Appendix
Assume that the calcium concentration returns to its resting level by a first order process, which has a time constant of τ. Hence, at a given time (t) the difference in the calcium concentration from its resting concentration (Δ[Ca2+]v), given the perturbation above the resting concentration at time t=0 (Δ[Ca2+]v), is,
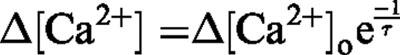 |
Suppose that calcium influx is triggered by a train of stimuli at a frequency of ν. Once dynamic equilibrium has been established within the train, the calcium concentration decline between each pulse must be equal to that added with the next stimulus. If the peak elevation in calcium concentration is Δ[Ca2+]v, then,
Hence, Equation A1
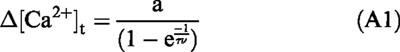 |
To find the average elevation in calcium concentration during the plateau phase, when dynamic equilibrium has been attained, we can average the calcium concentration over one stimulus cycle, giving
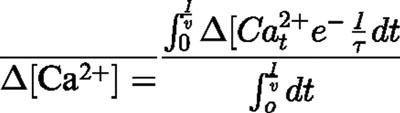 |
and hence,
Substituting Δ[Ca2+] from Equation A1 then gives,
Hence, the plateau Δ[Ca2+] is proportional to change in calcium concentration per impulse, the time constant of calcium removal, and the frequency of stimulation. It can also be noticed that the peak calcium concentration (from Equation A1) is not proportional to the frequency of stimulation, except in the limit as the frequency of stimulation becomes very large.
References
- BAILEY S.J., HOURANI S.M. Differential effects of suramin on P2-purinoceptors mediating contraction of the guinea-pig vas deferens and urinary bladder. Br. J. Pharmacol. 1994;112:219–225. doi: 10.1111/j.1476-5381.1994.tb13055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT M.R., HO S. Adenosine modulation of potassium currents in preganglionic nerve terminals of avian ciliary ganglia. Neurosci. Lett. 1992;137:41–44. doi: 10.1016/0304-3940(92)90293-g. [DOI] [PubMed] [Google Scholar]
- BLAKELEY A.G., CUNNANE T.C., MASKELL T., MATHIE A., PETERSEN S.A. Alpha-adrenoceptors and facilitation at a sympathetic neuroeffector junction. J. Autonomic Pharmacol. 1984;4:53–58. doi: 10.1111/j.1474-8673.1984.tb00433.x. [DOI] [PubMed] [Google Scholar]
- BLAKELEY A.G., DUNN P.M., PETERSEN S.A. A study of the actions of P1-purinoceptor agonists and antagonists in the mouse vas deferens in vitro. Br. J. Pharmacol. 1988;94:37–46. doi: 10.1111/j.1476-5381.1988.tb11497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOEHM S., HUCK S. Inhibition of N-type calcium channels: the only mechanism by which presynaptic alpha 2-autoreceptors control sympathetic transmitter release. Eur. J. Neurosci. 1996;8:1924–1931. doi: 10.1111/j.1460-9568.1996.tb01336.x. [DOI] [PubMed] [Google Scholar]
- BRAIN K.L., BENNETT M.R. Calcium in sympathetic varicosities of mouse vas deferens during facilitation, augmentation and autoinhibition. J. Physiol. 1997;502:521–536. doi: 10.1111/j.1469-7793.1997.521bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK J.A., CUNNANE T.C. Effects of Ca2+ and K+ channel blockers on nerve impulses recorded from guinea-pig postganglionic sympathetic nerve terminals. J. Physiol. 1995;489:389–402. doi: 10.1113/jphysiol.1995.sp021060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK J.A., CUNNANE T.C., STARKE K., WARDELL C.F. Alpha 2-adrenoceptor-mediated autoinhibition of sympathetic transmitter release in guinea-pig vas deferens studied by intracellular and focal extracellular recording of junction potentials and currents. Naunyn-Schmiedebergs' Arch. Pharmacol. 1990;342:45–52. doi: 10.1007/BF00178971. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M.E. The transmission of excitation from autonomic nerves to smooth muscle. J. Physiol. 1962;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., WARLAND J.J. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2y-but not the P2x-purinoceptor. Br. J. Pharmacol. 1987;90:383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEUNG A., BRAIN K.L., BENNETT M.R. Calcium modulation in single sympathetic varicosities of mouse vas deferens during trains of impulses by sequestration and autoinhibition. Proc. Aust. Neurosci. Soc. 1998;9:137. [Google Scholar]
- CRACK B.E., POLLARD C.E., BEUKERS M.W., ROBERTS S.M., HUNT S.F., INGALL A.H., MCKECHNIE K.C., IJZERMAN A.P., LEFF P. Pharmacological and biochemical analysis of FPL 67156, a novel, selective inhibitor of ecto-ATPase. Br. J. Pharmacol. 1995;114:475–481. doi: 10.1111/j.1476-5381.1995.tb13251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRIESSEN B., VON KUGELGEN I., STARKE K. P1-purinoceptor-mediated modulation of neural noradrenaline and ATP release in guinea-pig vas deferens. Naunyn-Schmiedebergs' Arch. Pharmacol. 1994;350:42–48. doi: 10.1007/BF00180009. [DOI] [PubMed] [Google Scholar]
- DUNN P.M., BLAKELEY A.G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br. J. Pharmacol. 1988;93:243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUTTON J.L., HANSEN M., BALCAR V.J., BARDEN J.A., BENNETT M.R. Development of P2x receptor clusters on smooth muscle cells in relation to nerve varicosities in the rat urinary bladder. J. Neurocytol. 1999;28:3–15. doi: 10.1023/a:1007043132537. [DOI] [PubMed] [Google Scholar]
- HANSEN M., BALCAR V., BARDEN J., BENNETT M.R. The distribution of single P2x1 receptor clusters on smooth muscle cells in relation to nerve varicosities in the rat urinary bladder. J. Neurocytol. 1998;27:529–539. doi: 10.1023/a:1006908010642. [DOI] [PubMed] [Google Scholar]
- HENERY R., HANSEN M., BARDEN J., BENNETT M.R. P2x receptors and spontaneous potentials in smooth muscle cells at the intimal surface of rat tail artery. Proc. Aust. Neurosci. Soc. 1999;10:139. [Google Scholar]
- HOITING B., MOLLEMAN A., NELEMANS A., DEN HERTOG A. P2-purinoceptor-activated membrane currents and inositol tetrakisphosphate formation are blocked by suramin. Eur. J. Pharmacol. 1990;181:127–131. doi: 10.1016/0014-2999(90)90253-3. [DOI] [PubMed] [Google Scholar]
- HOURANI S.M., CHOWN J.A. The effects of some possible inhibitors of ectonucleotidases on the breakdown and pharmacological effects of ATP in the guinea-pig urinary bladder. Gen. Pharmacol. 1989;20:413–416. doi: 10.1016/0306-3623(89)90188-2. [DOI] [PubMed] [Google Scholar]
- HOYLE C.H., POSTORINO A., BURNSTOCK G. Pre-and postjunctional effects of diadenosine polyphosphates in the guinea-pig vas deferens. J. Pharm. Pharmacol. 1995;47:926–931. doi: 10.1111/j.2042-7158.1995.tb03272.x. [DOI] [PubMed] [Google Scholar]
- ILLES P., STARKE K. An electrophysiological study of presynaptic alpha-adrenoceptors in the vas deferens of the mouse. Br. J. Pharmacol. 1983;78:365–373. doi: 10.1111/j.1476-5381.1983.tb09402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY C., LEFF P. How should P2x purinoceptors be classified pharmacologically. Trend. Pharm. Sci. 1995;16:168–174. doi: 10.1016/s0165-6147(00)89010-0. [DOI] [PubMed] [Google Scholar]
- KURZ A., BULTMANN R., DRIESSEN B., VON KUGELGEN I., STARKE K. Release of ATP in rat vas deferens: origin and role of calcium. Naunyn Schmiedebergs' Arch. Pharmacol. 1994;350:491–498. doi: 10.1007/BF00173018. [DOI] [PubMed] [Google Scholar]
- LEONG S.K., SINGH G. Innervation of the monkey vas deferens. J. Anat. 1990;171:93–104. [PMC free article] [PubMed] [Google Scholar]
- MALLARD N., MARSHALL R., SITHERS A., SPRIGGS B. Suramin: a selective inhibitor of purinergic neurotransmission in the rat isolated vas deferens. Eur. J. Pharmacol. 1992;220:1–10. doi: 10.1016/0014-2999(92)90004-n. [DOI] [PubMed] [Google Scholar]
- MARJAMÄKI A., LUOMALA K., ALA-UOTILA S., SCHEININ M. Use of recombinant human alpha 2-adrenoceptors to characterize subtype selectively of antagonist binding. Eur. J. Pharmacol. 1993;246:219–226. doi: 10.1016/0922-4106(93)90034-7. [DOI] [PubMed] [Google Scholar]
- MAYER A., LIMBERGER N., STARKE K. Transmitter release patterns of noradrenergic, dopaminergic and cholinergic axons in rabbit brain slices during short pulse trains, and the operation of presynaptic autoreceptors. Naunyn-Schmiedebergs' Arch. Pharmacol. 1988;338:632–643. doi: 10.1007/BF00165627. [DOI] [PubMed] [Google Scholar]
- MONTEITH G.R., ROUFOGALIS B.D. The plasma membrane calcium pump – a physiological perspective on its regulation. Cell. Calcium. 1995;18:459–470. doi: 10.1016/0143-4160(95)90009-8. [DOI] [PubMed] [Google Scholar]
- REILLY M.J., HIRST G.D. Differences in the responses to purinergic nerve stimulation and applied ATP in the guinea-pig vas deferens. J. Autonom. Nerv. Syst. 1996;57:93–100. doi: 10.1016/0165-1838(95)00105-0. [DOI] [PubMed] [Google Scholar]
- SMITH A.B., CUNNANE T.C. Omega-conotoxin GVIA-resistant neurotransmitter release in postganglionic sympathetic nerve terminals. Neuroscience. 1996;70:817–824. doi: 10.1016/s0306-4522(96)83018-1. [DOI] [PubMed] [Google Scholar]
- SMITH A.B., CUNNANE T.C. Omega-conotoxin GVIA-resistant neurotransmitter release from postganglionic sympathetic nerves in the guinea-pig vas deferens and its modulation by presynaptic receptors. Br. J. Pharmacol. 1998;123:167–172. doi: 10.1038/sj.bjp.0701577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNEDDON P. Suramin inhibits excitatory junction potentials in guinea-pig isolated vas deferens. Br. J. Pharmacol. 1992;107:101–103. doi: 10.1111/j.1476-5381.1992.tb14469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNEDDON P., MELDRUM L.A., BURNSTOCK G. Control of transmitter release in guinea-pig vas deferens by prejunctional P1-purinoceptors. Eur. J. Pharmacol. 1984;105:293–299. doi: 10.1016/0014-2999(84)90621-6. [DOI] [PubMed] [Google Scholar]
- STARKE K., GOTHERT M., KILBINGER H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol. Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- STARKE K., VON KUGELGEN I., DRIESSEN B., BULTMANN R. ATP release and its prejunctional modulation. Ciba Found. Symp. 1996;198:239–249. doi: 10.1002/9780470514900.ch14. [DOI] [PubMed] [Google Scholar]
- STORY D.F., MCCULLOCH M.W., RAND M.J., STANDFORD-STARR C.A. Conditions required for the inhibitory feedback loop in noradrenergic transmission. Nature. 1981;293:62–65. doi: 10.1038/293062a0. [DOI] [PubMed] [Google Scholar]
- STOUT J.G., KIRLEY T.L. Inhibition of purified chicken gizzard smooth muscle ecto-ATPase by P2 purinoceptor antagonists. Biochem. Mol. Biol. Int. 1995;36:927–934. [PubMed] [Google Scholar]
- TODOROV L.D., MIHAYLOVA-TODOROVA S., WESTFALL T.D., SNEDDON P., KENNEDY C., BJUR R.A., WESTFALL D.P. Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature. 1997;387:76–79. doi: 10.1038/387076a0. [DOI] [PubMed] [Google Scholar]
- TROUT S.J., BRAIN K.L, , DASS N., CUNNANE T.C. ‘Effects of nicotine on intracellular calcium dynamics in sympathetic nerve terminal varicosities'. Br. J. Pharmacol. 1999;127:53P. [Google Scholar]
- VON KUGELGEN I., BULTMANN R., STARKE K. Effects of suramin and alpha, beta-methylene ATP indicate noradrenaline-ATP co-transmission in the response of the mouse vas deferens to single and low frequency pulses. Naunyn-Schmiedebergs' Arch. Pharmacol. 1989b;340:760–763. doi: 10.1007/BF00169686. [DOI] [PubMed] [Google Scholar]
- VON KUGELGEN I., KURZ K., STARKE K. Axon terminal P2-purinoceptors in feedback control of sympathetic transmitter release. Neuroscience. 1993;56:263–267. doi: 10.1016/0306-4522(93)90330-i. [DOI] [PubMed] [Google Scholar]
- VON KUGELGEN I., KURZ K., STARKE K. P2-purinoceptor-mediated autoinhibition of sympathetic transmitter release in mouse and rat vas deferens. Naunyn-Schmiedebergs' Arch. Pharmacol. 1994;349:125–132. doi: 10.1007/BF00169828. [DOI] [PubMed] [Google Scholar]
- VON KUGELGEN I., SCHOFFEL E., STARKE K. Inhibition by nucleotides acting at presynaptic P2-receptors of sympathetic neuro-effector transmission in the mouse isolated vas deferens. Naunyn-Schmiedebergs' Arch. Pharmacol. 1989a;340:522–532. doi: 10.1007/BF00260607. [DOI] [PubMed] [Google Scholar]
- ZIGANSHIN A.U., ZIGANSHINA L.E., BODIN P., BAILEY D., BURNSTOCK G. Effects of P2-purinoceptor antagonists on ecto-nucleotidase activity of guinea-pig vas deferens cultured smooth muscle cells. Biochem. Mol. Biol. Int. 1995;36:863–869. [PubMed] [Google Scholar]


