Figure 2.
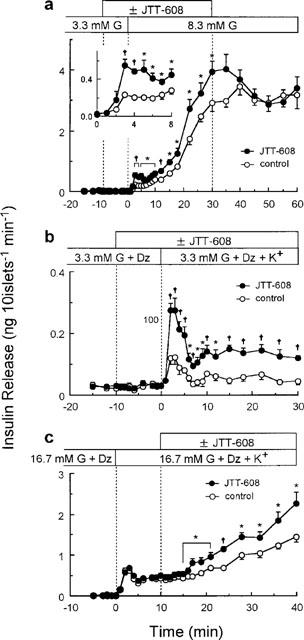
Effects of 100 μM JTT-608 on the time courses of insulin release under different conditions. (a) Effect of JTT-608 on 8.3 mM glucose-induced biphasic insulin release. Two groups of islets were perifused with 5.5 mM glucose for 30 min, and then with 3.3 mM glucose for 30 min to establish a stable rate of secretion. For an additional 30 min, they were stimulated by 8.3 mM glucose with or without 100 μM JTT-608. JTT-608 was applied from 10 min before 8.3 mM glucose stimulation. Withdrawal effect of JTT-608 was also determined for 30 min in the presence of 8.3 mM glucose alone. Inset shows the time course of insulin secretion during the first 8 min after 8.3 mM glucose stimulation in the same experiment. Values are mean±s.e.mean of six observations in the same experiment. (b) Effect of JTT-608 on a depolarizing high K+-stimulated monophasic insulin release. Two groups of islets were perifused with 5.5 mM glucose for 30 min, and then with 3.3 mM glucose and 200 μM diazoxide for 30 min to establish a stable rate of secretion. For an additional 30 min, they were stimulated by 30 mM K+ in the presence of 3.3 mM glucose and 200 μM diazoxide with or without 100 μM JTT-608. JTT-608 was applied from 10 min before 30 mM K+ stimulation. Values are mean±s.e.mean of six observations in the same experiment. (c) Effect of JTT-608 on a sustained insulin release in the presence of 16.7 mM glucose, 200 μM diazoxide, and 30 mM K+. Two groups of islets were perifused with 16.7 mM glucose and 200 μM diazoxide for 30 min, and then stimulated by 30 mM K+ under the same condition. The peak elevation of insulin release was observed after 2–3 min, and was sustained or gradually increased. They were then perifused with or without 100 μM JTT-608 from 10 min after application of 30 mM K+. Values are mean±s.e.mean of seven observations in the same experiment. *P<0.05, †P<0.01 vs corresponding control, respectively. G, glucose; Dz, diazoxide.
