Abstract
To determine the influence of the myocardial infarction (MI) on bradykinin B1-receptor (B1R) regulation, we studied its expression in the left ventricle (LV) after MI. Rats were submitted to a permanent occlusion of the left coronary artery. Six hours, 24 h and 6 days after MI or after sham operation, left ventricular pressure (LVP) and dP/dtmax were measured. LV-total RNA was extracted and B1R expression was analysed by a RNase-protection assay (each group n=6). LVP and dP/dtmax were impaired at all time points after MI. Basal B1R expression was not detectable in controls. Six hours after MI, the B1R expression was upregulated and reached a maximum 24 h after MI (4 fold vs 6 h). Six days post-MI, B1R expression returned to levels found 6 h after MI. These data are the first demonstration for an induced myocardial B1R expression in an in vivo model of MI.
Keywords: Bradykinin, B1 receptor, myocardial infarction
Introduction
It is well known that the biological effects of kinins are mediated through the stimulation of two receptors, classified as B1 and B2 receptors. Bradykinin (BK) and kallidin are the natural agonists of the B2R but they are weak B1R ligands. However, the cleavage of these two kinins by arginin-carboxidases produces the high affinity B1R agonists des-Arg9-BK and des-Arg10-kallidin, respectively (for review: Marceau et al., 1998).
Kinins are endogenous vasoactive substances that are released from the myocardium during myocardial ischaemia and contribute to the impact of ischaemic damage (for review: Tschöpe et al., 1997). Several beneficial effects of BK under these conditions have been studied by various authors. Many but not all of these effects are mediated by the stimulation of B2R, which is constitutively expressed under basal conditions and suffers rapid internalization and hence apparent desensitization (Austin et al., 1997). In contrast, B1R, which is usually undetectable under basal conditions, is rapidly upregulated in tissue injury such as inflammation or oxidative stress (Marceau et al., 1998). However, the role of B1R after MI is still under investigation. The present study was conducted to investigate the regulation of the myocardial B1R expression after acute MI using an in vivo model.
Methods
Animals and experimental protocol
Experiments were performed in male Sprague Dawley rats weighing 300–330 g (Charles River, Germany) that had free access to water and standard chow under a 12 h light/dark cycle.
Surgical procedures
MI was induced by permanent ligation of the left descending coronary artery as described by Tschöpe et al. (2000). To correlate the magnitude of the induced MI between the different groups, 6 h, 24 h, and 6 days after MI, the animals were anaesthetized and LVP and dP/dtmax were determined via a Millar-Tip catheter (Tschöpe et al., 2000).
Molecular-biological investigations
Total RNA was isolated after homogenization of the LV of six animals per group using Trizol reagent (Gibco, Eggenstein, Germany) following the manufacturer's directions. To detect cardiac B1R expression, a RNase-protection assay (RPA) was performed using the Ambion RPA II kit (ITC Biotechnology, U.S.A.). Anti-sense RNA probes were generated by T7-polymerase transcription using linearized plasmids containing a fragment of the B1R cDNA. In addition, GAPDH cDNA was used as an internal control. The probes were radiolabelled with [32P]-UTP and approximately 5×104 c.p.m. of each probe were hybridized together with 25 μg of total RNA per sample. After RNase A/T1 digestion, 280 bp of the B1R cDNA and 80 bp for GAPDH were protected. The hybridized fragments were separated by electrophoresis and analysed by a FUJIX BAS2000 phosphor-imager system. Quantitative analysis was performed by measuring the B1R band intensity normalized by the GAPDH intensity.
Statistical analysis of data
All data are expressed as means±s.e.mean. Data were analysed using a two-sided analysis of variance in conjunction with the Student's t-test. P values <0.05 were accepted as significant.
Results
Haemodynamic measurements
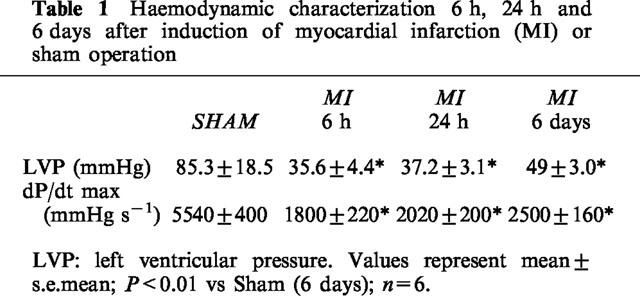
As shown in Table 1, LVP and myocardial dP/dtmax were impaired at all time points after MI. Six hours and 24 h after MI, both the LVP and dP/dtmax were reduced by 60 and 65%, respectively, compared with the controls (Table 1). After 6 days, we observed an improvement in LVP and dP/dtmax up to 25% compared with rats 6 h and 24 h after MI. However, in contrast to values of the LVP, the increase in dP/dtmax after 6 days was not significant vs 6 h (P<0.07). The mortality after coronary occlusion was 30%.
Table 1.
Haemodynamic characterization 6 h, 24 h and 6 days after induction of myocardial infarction (MI) or sham operation
Myocardial B1R expression
In the LV of sham-operated rats, B1R expression could not be detected using at least 25 μg of total RNA, however the house-keeping gene (GAPDH) was detectable in each of the samples (Figure 1A).
Figure 1.
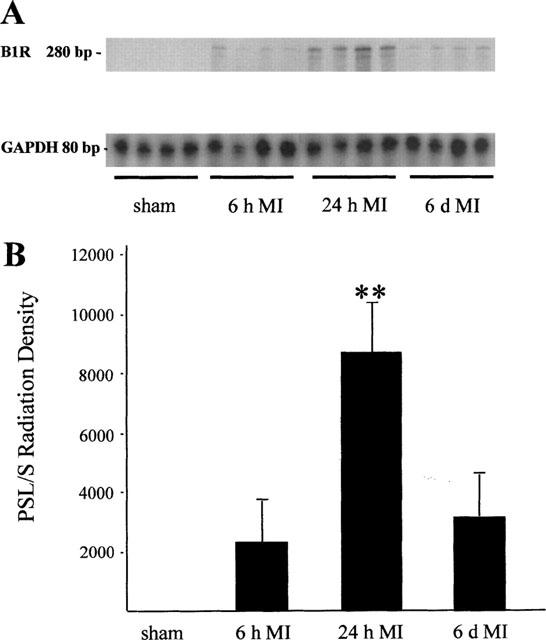
B1R expression in the left ventricle (LV) 6 h, 24 h, and 6 days after MI or after sham operation. (A) Representative RNase-protection assay (n=4, each group) showing B1R (280 bp) vs GAPDH (80 bp) expression. (B) Quantitation of B1R expression after autoradiographic signal analysis. Data are shown as multiples after normalization to GAPDH-mRNA levels (n=6), **P<0.005.
Six hours post MI, B1R expression was first detected. Twenty-four hours after MI, the upregulation of B1R expression reached a 4 fold increase compared with 6 h post MI (P<0.005). Six days after MI the B1R expression was not significantly increased compared with the level measured 6 h after MI (Figure 1B).
An increase in B1R expression in time-matched controls could be excluded in additional experiments (data not shown).
Discussion
Various authors have shown induction of B1R in pathological conditions such as tissue trauma, inflammation or anoxia (for review: Marceau et al., 1998). However, virtually nothing was known about B1R regulation after acute MI. In agreement with previous findings (Marceau et al., 1998), the present study did not detect cardiac B1R mRNA in sham-operated rats. Our findings reveal that acute MI induces B1R expression, which reaches its maximum in the early periods after MI. After 6 days B1R is still induced but less markedly so.
It is known that acute MI can also be assessed by an activation of the kallikrein-kinin system (KKS) (Lamontagne et al., 1995; Tschöpe et al., 1997; 2000) Several of these effects could be abolished by B2R antagonists, indicating B2R involvement. On the other hand, a large body of evidence shows that the B1R can be rapidly and stablly induced in many species after certain types of injuries (for review: Marceau et al., 1998). However, the involvement of the B1R axis within KKS in cardiovascular injuries is still poorly investigated. B1R expression by cardiomyocytes under ischaemic conditions has been identified only indirectly by using B1R agonists and antagonists in in vitro and ex vivo models (Marceau et al., 1998). However, induced B1R expression by post-isolation stress could not be excluded in these studies.
Since the function of B1R after MI has not been proven, we can only speculate about whether haemodynamic or tissue repair mechanisms are involved in B1R regulation after acute MI. It is well known that B1R is strongly expressed in injured tissue by inflammatory mediators such as bacterial lipopolysaccharides or interleukin-1β, which is also released with a peak in the early exsudative (6 h post MI) and inflammatory phases (24 h post MI) of MI (Yue et al., 1998). Furthermore, des-Arg9 BK was found to strongly contract myofibroblasts in granulation tissue (Appleton et al., 1994) and to be involved in coronary endothelial cell proliferation (Morbidelli et al., 1998). Both could be relevant in retraction of scar tissue and angiogenesis after MI.
Our data are the first to demonstrate B1R regulation in an in vivo MI model. The early stages of cardiac wound-healing after MI are accompanied by B1R upregulation at the mRNA level. This indicates a role of B1R in tissue repair and remodelling after MI.
Acknowledgments
This study was supported by grants from the Deutsche Forschungsgemeinschaft (TS-64/2-1) and Gödecke, Germany.
Abbreviations
- BK
bradykinin
- B1R
B1 receptor
- B2R
B2 receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- KKS
kallikrein-kinin system
- LV
left ventricle
- LVP
left ventricular pressure
- MI
myocardial infarction
- RPA
RNase protection assay
References
- APPLETON I., ROSENBLATT L., WILLOUGHBY D.A. Synergistic effect of recombinant human epidermal growth factor and des-Arg9-bradykinin, on rat croton oil-induced granulation tissue contraction. Br. J. Pharmacol. 1994;111:290P. [Google Scholar]
- AUSTIN K.E., FAUSSNER A., ROBINSON H.E., CHAKRAVARTY S., KYLE D.J., BATHON J.M., PRAUD D. Stable expression of the kinin B1 receptor in Chinese hamster ovary cells. J. Biol. Chem. 1997;271:11420–11425. doi: 10.1074/jbc.272.17.11420. [DOI] [PubMed] [Google Scholar]
- LAMONTAGNE D., NADEAU R., ADAM A. Effect of enalaprilat on bradykinin and des-Arg9-bradykinin release following reperfusion of the ischemic rat heart. Br. J. Pharmacol. 1995;115:476–478. doi: 10.1111/j.1476-5381.1995.tb16357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCEAU F., HESS J.F., BACHVAROV D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- MORBIDELLI L., PARENTI A., GIOVANNELLI L., GRANGER H.J., LEDDA F., ZICHE M. B1 receptor involvement in the effect of bradykinin on venular endothelial cell proliferation and potentiation of FGF-2 effects. Br. J. Pharmacol. 1998;124:1286–1292. doi: 10.1038/sj.bjp.0701943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSCHÖPE C., GOHLKE P., ZHU Y.-Z., LINZ W., SCHÖLKENS B.A., UNGER TH. Antihypertensive and cardioprotective effects after angiotensin-converting enzyme inhibition: role of kinins. J. Card. Fail. 1997;3:134–148. doi: 10.1016/s1071-9164(97)90047-6. [DOI] [PubMed] [Google Scholar]
- TSCHÖPE C. , HERINGER-WALTHER S., KOCH M., SPILLMAN F., WENDORF M., HAUKE D. , BADER M., SCHULTHEISS H.-P., WALTHER T. Myocardical bradykinin B2-receptor expression at different time points after induction of myocardial infarction. J. Hypertens. 2000;18:223–228. doi: 10.1097/00004872-200018020-00014. [DOI] [PubMed] [Google Scholar]
- YUE P., MASSIE B.M., SIMPSON P.C., LONG C.S. Cytokine expression increases in nonmyocytes from rats with postinfarction heart failure. Am. J. Physiol. 1998;44:H250–H258. doi: 10.1152/ajpheart.1998.275.1.H250. [DOI] [PubMed] [Google Scholar]


