Abstract
The aim of this study was to investigate the involvement of peroxynitrite, reactive metabolite originating from nitric oxide and superoxide, in preconditioning of the ischaemic myocardium in rat isolated hearts.
Isolated hearts perfused with Krebs-Henseleit solution were preconditioned either by 3 min of coronary artery occlusion (CAO) or by peroxynitrite administration at three different concentrations (0.1, 1, 10 μM) for 3 min, followed by 10 min reperfusion and 30 min of CAO. Peroxynitrite, at 1 μM concentration, decreased the incidence of VT from 100% (n=14) to 62% (n=13) and abolished the occurrence of VF (50% in the control group).
N-2-mercaptopropionylglycine (MPG, 1 μM–10 mM) produced a concentration-dependent inhibition of peroxynitrite signals in luminol chemiluminescence and 67±1% inhibition was observed at 100 μM (n=7). MPG (at 300 μM, n=7) added to the perfusate 10 min prior to ischaemic preconditioning or peroxynitrite infusion and maintained until CAO, significantly reversed the beneficial effects of the ischaemic and peroxynitrite-treated groups. MPG administration in the peroxynitrite-treated group increased the incidence of VT from 62% (n=13) to 100% (n=10) and total VF from 0% (n=0) to 67% (n=10). Similarly, MPG elevated the incidence of VT from 50% (n=10) to 100% (n=8) in the ischaemic preconditioned group. On its own, MPG did not affect the severity of cardiac arrhythmias.
These results suggest that endogenously produced peroxynitrite plays a significant role in the antiarrhythmic effect of ischaemic preconditioning in the rat isolated hearts.
Keywords: Peroxynitrite, preconditioning, N-2-mercaptopropionylglycine, chemiluminescence, rat isolated heart
Introduction
Brief episodes of myocardial ischaemia followed by periods of reperfusion increase the resistance to further ischaemic damage. This is known as myocardial ischaemic preconditioning which was first described by Murry et al. (1986). Preconditioning is a powerful mechanism which protects the heart from ischaemic damage (Murry et al., 1986; Liu & Downey, 1992), reduces the incidence of arrhythmias (Hagar et al., 1991; Vegh et al., 1992a) and preserves contractile function (Kimura et al., 1992). Although the exact mechanism responsible for preconditioning has not been fully elucidated, there is a growing body of evidence which suggests that endogenous myocardial protective substances like adenosine, bradykinin, prostaglandins and nitric oxide (NO) may play a pivotal role (Parratt & Vegh, 1996).
NO formation is increased and, at the same time, endothelial production of superoxide radical is accelerated in the early phase of reperfusion (Wang & Zweier, 1996). Beckman et al. (1990) have shown that NO and superoxide radical can rapidly combine to form peroxynitrite which is a powerful oxidant and can exert many cytotoxic effects (Beckman & Koppenol, 1996). It is generally accepted that during the early reperfusion period peroxynitrite formation occurs, since reperfusion of ischaemic myocardium is a good source of NO and superoxide. This concept is supported by a number of observations demonstrating increasing formation of NO and/or peroxynitrite in piglet hearts during hypoxia and reoxygenation (Morita et al., 1994), and during ischaemia/reperfusion in rat and dog hearts (Zweier et al., 1995; Wang & Zweier, 1996; Yasmin et al., 1997). In contrast to the cytotoxic effects of peroxynitrite, it has recently been demonstrated that it also exerts beneficial effects, at low micromolar concentrations, including inhibition of platelet aggregation (Moro et al., 1994), and relaxation of coronary arteries (Liu et al., 1994). It has also been shown that peroxynitrite exerts significant cardioprotective effects on myocardial ischaemia-reperfusion injury by reducing myocardial infarct size, preserving coronary endothelial function, and inhibition of neutrophil-endothelium interactions (Lefer et al., 1997; Nossuli et al., 1997; 1998). Our previous observation in rat isolated hearts showed that exogenous administration of peroxynitrite is able to reduce the severity of reperfusion arrhythmias (Altuğ et al., 1999). Thus, it might be hypothesized that exposure to peroxynitrite formed after brief periods of ischaemia might be one mechanism underlying preconditioning.
The involvement of peroxynitrite during ischaemic preconditioning has not been investigated. The aims of the present study, therefore, were to determine the effect of exogenously administered peroxynitrite on ischaemia-induced arrhythmias, and to explore the role of ischaemic preconditioning in the rat isolated hearts by the use of the thiol compound N-2-mercaptopropionylglycine (MPG), a peroxynitrite scavenger.
Methods
Peroxynitrite synthesis
Peroxynitrite was synthesized in our laboratory from acidified nitrite and hydrogen peroxide (H2O2) according to the method of Beckman et al. (1994). Briefly, an aqueous solution of 0.6 M sodium nitrite was rapidly mixed with an equal volume of 0.6 M H2O2 containing 0.7 M HCl and immediately quenched with the same volume of 1.2 M NaOH. All reactions were performed on ice. Excess H2O2 was removed by addition of manganese dioxide (MnO2) powder to the peroxynitrite solution. The mixture was then shaken for 5 min and MnO2 was removed by passage over a cellulose acetate disposable filter. The final concentration of peroxynitrite was determined spectrophotometrically (ε302=1670 M−1 cm−1) as described previously (Yildiz et al., 1998). Fresh dilutions were made with Krebs-Henseleit solution (lacking glucose and sodium pyruvate) just before use and the pH of this solution was adjusted to 8.4 by addition of an appropriate volume of 0.1 N NaOH (Nossuli et al., 1997). The stock solutions were aliquoted and stored at −20°C for a week.
Chemiluminescence
Luminol-enhanced chemiluminescence (CL) was measured as described previously (Yildiz et al., 1998). Phosphate buffered saline (10 mM KH2PO4 and 150 mM NaCl, pH 7.4) was mixed with luminol (250 μM) in a cuvette. After adding catalase (50 u ml−1) to the cuvette to remove H2O2 left after MnO2 treatment, peroxynitrite at 20 nM was injected and the CL produced was measured at 37°C using a chemiluminometer (Bio-Orbit 1250 Luminometer, Turku, Finland). The CL generated was measured continuously and recorded on a computer using the Luminometer 1250 program (version 1.12, Bio-Orbit) for 3 min. The effects of various concentrations of MPG were examined by addition to the mixture before peroxynitrite.
Preparation of isolated hearts
Male Wistar rats (200–400 g) were acclimatized with 12 h light/dark cycles at a room temperature of 25°C, and supplied with standard laboratory diet and tap water ad libitum. Animals were anaesthetized intraperitoneally with injection of thiopental (60 mg kg−1). After induction of anaesthesia, the abdomen was opened and heparin (500 U) was given through the renal vein. After 3 min of heparin injection, the abdominal aorta was cut to reduce the blood volume in the heart. The thorax was opened and the heart was quickly excised and put into a petri dish containing an ice cold Krebs-Henseleit solution of the following composition (mM): NaCl 118; KCl 3.2, CaCl2 2.52; MgSO4 1.66; NaHCO3 26.88; KH2PO4 1.18; glucose 5.55 and sodium pyruvate 2.0 (Piacentini et al., 1993). The pH of the solution was corrected to 7.4. Then it was perfused retrogradely via aorta by means of a modified Langendorff apparatus at a constant flow by a peristaltic pump (Harward Apparatus, Model 1203, Kent, England) of between 8 and 10 ml min−1 with a Krebs-Henseleit solution maintained at 37°C and gassed with 95% O2 and 5% CO2 (Piacentini et al., 1993). The flow rate was determined according to animal weight using the formula: flow (ml min−1)=x0.56×7.43 (x is the heart weight), heart weight=0.0027 y+0.6 (y is the body weight). A loose ligature was immediately placed round the left anterior descending (LAD) coronary artery; both ends of the ligature were then passed through a short piece of polythene tubing (1 mm i.d. and 1.5 mm long) to form a snare. Following a stabilization period of 15 min, the snare around the LAD coronary artery was tightened and held in place with a small clip. An increase in coronary perfusion pressure indicated successful ligation, likewise a decrease in perfusion pressure indicated successful reperfusion. The electrocardiogram (ECG) was recorded by two electrodes placed on right atrium and apex throughout the experiment by using a computerized data acquisition system (TDA 95 Maycom, Turkey) (Altuğ et al., 1999). Coronary perfusion pressure was measured via a pressure transducer and recorded continuously by the same data acquisition system.
Experimental protocol for isolated hearts
After completing surgical procedures, all hearts were allowed to stabilize for 15 min prior to the experimental protocol. These protocols are diagrammatically represented in Figure 1. In the first group of experiments (protocol 1, control, n=14), hearts were subjected to 30 min LAD coronary artery occlusion. In the second group of experiments (protocol 2, preconditioned, n=10) hearts were preconditioned with single 3 min of occlusion of LAD followed by 10 min reperfusion and subsequent 30 min occlusion. In the third group of experiments (protocol 3, peroxynitrite-induced preconditioning) hearts were subjected to 3 min infusion of peroxynitrite at three different concentrations (0.1, 1, 10 μM) followed by 10 min washout and then 30 min occlusion. Peroxynitrite was infused into the perfusion solution through the rubber tubing placed just proximal to the heart at a rate of 1 ml min−1 to achieve concentrations of 0.1, 1 or 10 μM reaching the heart. Stock solutions of peroxynitrite were kept on ice, and the infusion lines were wrapped in aluminium foil to reduce exposure to light. In protocols 4 and 5 the peroxynitrite scavenger MPG (300 μM) was infused either alone (n=9) or in combination with the ischaemic preconditioning protocol (n=8) respectively. For the last series of experiments (protocol 6), the concentration of 1 μM of peroxynitrite was chosen since it was shown that this concentration could precondition hearts. In this group of experiments (MPG + peroxynitrite-induced preconditioning, n=10), hearts were preconditioned with peroxynitrite as in protocol 3 but received MPG for 23 min starting 10 min prior to peroxynitrite infusion and continued until the onset of the 30 min occlusion period.
Figure 1.
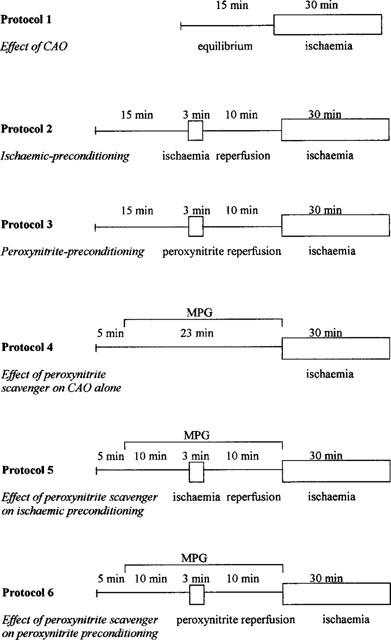
Experimental protocols for the study: Isolated rat hearts perfused with Krebs-Henseleit solution were subjected to 30 min ischaemia (protocol 1). Rat isolated hearts were preconditioned against 30 min ischaemia by a 3 min period of coronary occlusion and 10 min reperfusion (protocol 2). Three different concentrations of peroxynitrite, 0.1, 1 and 10 μM for 3 min were infused in order to precondition the heart (protocol 3). MPG, a peroxynitrite scavenger, (300 μM) added to the perfusate for 23 min prior to the ischaemic period (protocol 4), 10 min prior to the ischaemic preconditioning (protocol 5) or peroxynitrite infusion (protocol 6) and maintained until the starting of 30 min of occlusion (total 23 min).
Measured parameters
For all the groups, heart rate was measured from the recordings of electrocardiogram and the incidences of arrhythmias were registered, in accordance with the Lambeth conventions (Walker et al., 1988), as ventricular tachycardia (VT), ventricular fibrillation (VF), and ventricular ectopic beat (VEB). VEB is defined as a discrete and identifiable premature QRS complex. VT was diagnosed as four or more consecutive VEBs. VF was diagnosed when the ECG recording showed chaotic activity with an amplitude less than that of the normal ECG. Irreversible VF was defined as VF which did not reverse within the 10 min period of reperfusion. The onset and duration of arrhythmias were also measured. At the end of experiments, the LAD was occluded again at the same site as previously, and 3 ml of a 2% solution of Evans blue was infused into the aortic cannula to estimate the area at risk. This was expressed as a percentage of left ventricular free wall.
Materials
MPG, sodium nitrite, Evans blue, luminol and catalase (from bovine liver) were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Hydrogen peroxide was purchased from Merck (Darmstadt, Germany).
Statistical analysis
Values were presented as mean±s.e.mean or the percentage incidence. A Fischer's extract Chi-square test was used to detect significant differences in the incidence of VT, VF and irreversible VF between control and drug-treated groups. In the chemiluminescence studies, results were calculated as peak CL or a percentage of the peak CL. Duplicate assays were performed in CL experiments. Statistical comparison of more than two groups was performed by a one-way analysis of variance followed by Student-Newman-Keuls test. In all tests, P values less than 0.05 was considered to be statistically significant.
Results
Effects of peroxynitrite on ischaemic preconditioning
Preconditioning the hearts with 3 min of ischaemia suppressed arrhythmias during the 30 min occlusion period. VEB number was significantly lower than controls. The incidences of VT and VF were also significantly lower than in the control hearts (Table 1). In order to study the effects of peroxynitrite infusion on ischaemic preconditioning, three different concentrations of peroxynitrite were studied (0.1, 1, 10 μM). All concentrations of peroxynitrite studied decreased VEB number, but the most pronounced cardioprotective effect was seen with 1 μM. At this concentration, the incidences of VT and VF were significantly reduced and none of the hearts had an irreversible VF (Table 1). Therefore this concentration was chosen for the further peroxynitrite-induced preconditioning experiments. Peroxynitrite administration did not cause any significant change in coronary perfusion pressure throughout the experimental protocol (Table 2). Similarly pressure rate index was not modified by peroxynitrite administration (data not shown).
Table 1.
Effects of 3 min period of preconditioning by ischaemia or peroxynitrite infusion on the severity of arrhythmias induced by 30 min of coronary artery occlusion
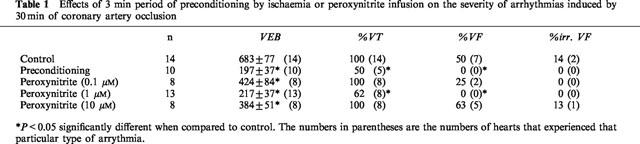
Table 2.
Mean coronary perfusion pressure values (mmHg) during coronary occlusion and reperfusion in rat isolated hearts
Effects of MPG on peroxynitrite-induced chemiluminescence
In luminol CL, MPG inhibited the peroxynitrite-induced response in a concentration-dependent manner (Figure 2). No inhibition was evident at 1 μM MPG (3±1%, n=10) whereas the highest concentration tested (10 mM) caused 79±3% (n=8) inhibition.
Figure 2.
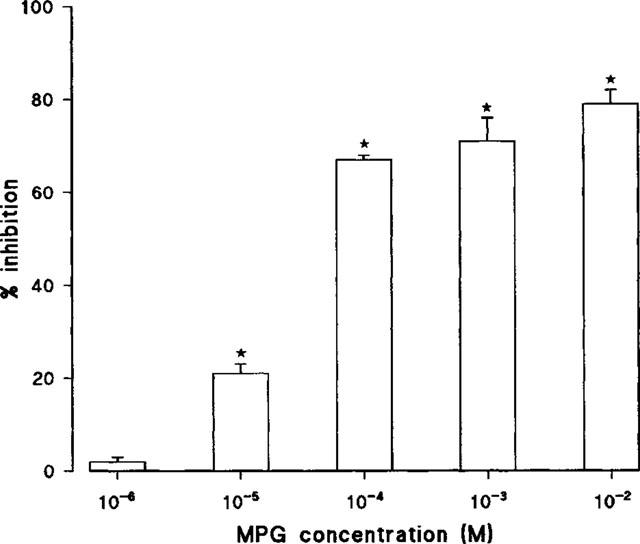
Concentration-dependent effects of MPG (n=6–9) on peroxynitrite-induced luminol chemiluminescence. Data are shown as mean±s.e.mean. *P<0.05 significantly less than its control value.
Effects of MPG on ischaemic and peroxynitrite-induced preconditioning
Administration of the peroxynitrite scavenger MPG (300 μM) reversed beneficial effects of ischaemic preconditioning on ischaemia-induced arrhythmias (Table 3). Both VT and total VF times were increased from 21±10 s (n=4) to 101±33 s (n=8) and from none to 166±83 s (n=5) respectively. MPG (300 μM) also reversed the beneficial effects of peroxynitrite-induced preconditioning on ischaemia-induced arrhythmias. It increased the incidence of VT from 62% (n=8) to 100% (n=10) and total VF from 0% (n=0) to 67% (n=6) (Table 3). Also total VF time was increased from 0 to 270±109 s (n=6).
Table 3.
Effects of peroxynitrite scavenger MPG (300 μM) infusion on preconditioning induced by 3 min ischaemia and by 3 min peroxynitrite infusion at 1 μM concentration
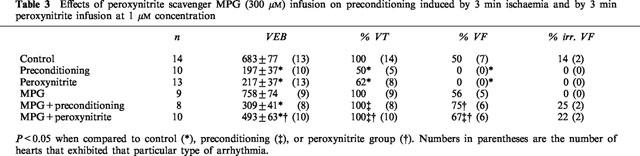
MPG administration did not cause any significant change on coronary perfusion pressure (Table 2) and pressure rate index measured at certain time points throughout the experiment (data not shown).
Area at risk measurements
No significant differences were noted in the left ventricular area at risk between control hearts (32±4%) and those infused with 1 μM peroxynitrite for 3 min (34±6%). The area at risk was similar in hearts from preconditioned (33±5%), MPG alone (31±2%), MPG+preconditioning (34±7%) or MPG+peroxynitrite preconditioning groups (33±9%).
Discussion
In this study we have shown that exposure to 1 μM concentration of peroxynitrite on its own was capable of mimicking the beneficial effects of ischaemic preconditioning. Peroxynitrite at this concentration markedly reduced the severity of ischaemia-induced arrhythmias. This effect was lost in the presence of the peroxynitrite scavenger, MPG. MPG treatment also attenuated the beneficial effects of ischaemic preconditioning. To our knowledge, this is the first evidence that peroxynitrite generated during brief initial ischaemia and reperfusion can initiate a protective response against arrhythmias and involve the signalling cascade of preconditioning.
It has been proposed that maximally achievable concentration of peroxynitrite formed in in vivo conditions is in the low micromolar range (i.e., 2–5 μM), and concentrations above these levels would probably not be formed in vivo (Nossuli et al., 1998). Therefore, the concentrations of peroxynitrite (0.1 and 1 μM) used in the present experiment are highly likely to be reached in in vivo conditions. We have observed that 1 μM peroxynitrite afforded significant cardioprotective effects and reduced both the number of VEBs, the incidence of VT and VF during the 30 min occlusion period. The lower concentration of peroxynitrite (0.1 μM) was less effective than 1 μM only in reducing the ectopic activity.
The mechanism responsible for the beneficial effects of preconditioning is not well established and it seems that more than a single mechanism is involved. Many endogenous substances are released and/or operative during ischaemic preconditioning including adenosine, acetylcholine, catecholamines, angiotensin II, bradykinin, endothelin, nitric oxide and opioids which have all been identified either as a trigger or a mediator (Parratt & Vegh, 1996; Yellon et al., 1998). There is considerable evidence that bradykinin is released early in ischaemia and has been considered as a trigger for the release of NO and peroxynitrite (Kooy & Royall, 1994; Parratt & Vegh, 1996). A constitutive NOS is present in cardiac myocytes (Belhassen et al., 1996; Depre et al., 1997) and increased synthesis of NO by this enzyme may occur during preconditioning, since ischaemia increases intracellular calcium levels and elevates the availability of NADPH whereas reperfusion provides the oxygen required for NO generation. There is increasing evidence that NOS may produce peroxynitrite rather than NO under conditions in which the local tissue levels of L-arginine decline (Xia et al., 1996). If this were the case during brief myocardial ischaemia/reperfusion, the trigger or mediator for preconditioning could be an increased generation of peroxynitrite by NOS.
It is known that endothelial production of superoxide radical is accelerated in the early phase of reperfusion (Wang & Zweier, 1996). There is also evidence for increased superoxide levels immediately after brief, intermittent anoxic preconditioning in rat isolated myocytes (Zhou et al., 1996). It has been demonstrated that production of reactive oxygen species during preconditioning may trigger a protective response that renders the endothelial cells more resistant to subsequent reperfusion injury (Kaeffer et al., 1997). Furthermore, it has been shown that free radicals generated from hypoxanthine plus xanthine oxidase reaction induced protection against infarct size in rabbit hearts (Baines et al., 1997). Additionally, NO formation is also markedly increased during ischaemia and/or reperfusion in rat isolated heart (Zweier et al., 1995; Liu et al., 1997) probably through activation of the constitutive heart NO synthase (Depre et al., 1997). It has been demonstrated that the generation of NO contributes to the marked antiarrhythmic effects of preconditioning in canine myocardium (Vegh et al., 1992b). Taken together, these studies suggest that both superoxide and NO are produced and peroxynitrite are formed in myocardial ischaemia and reperfusion.
It has been suggested that free radicals generated during the first ischaemic insult serve as a trigger for the development of late preconditioning against myocardial infarction (Sun et al., 1996; Qiu et al., 1997). Recently, Takano et al. (1998) showed that NO donors may induce late preconditioning and this phenomenon involves the generation of oxidant species, possibly peroxynitrite and/or hydroxyl radical. It has been proposed that peroxynitrite and/or hydroxyl radical formed after an ischaemic stimulus may activate a protein kinase C-mediated signal transduction cascade that culminates in the development of a protective effect 24 h later (Takano et al., 1998). Thus, peroxynitrite released during brief periods of ischaemia-reperfusion appear to play a role in triggering both the early and the late phases of ischaemic preconditioning. The present study provides new experimental evidence that peroxynitrite is involved in the antiarrhythmic effects of early phase of ischaemic preconditioning in rats.
MPG is proposed to be a cell-permeant antioxidant that reacts rapidly with both peroxynitrite and hydroxyl radical by virtue of its thiol group (Takano et al., 1998). Our data with luminol chemiluminescence showed that MPG is a direct peroxynitrite scavenger. Since peroxynitrite can decompose to form hydroxyl radical (Merenyi et al., 1998), it can be considered that the administration of MPG is useful to scavenge intracellular and extracellular free radicals. Our results demonstrated that MPG completely abolished the preconditioning induced either by ischaemia/reperfusion or peroxynitrite infusion. Therefore, it seems highly likely that peroxynitrite or its byproducts elicit preconditioning in the present study. This conclusion can be further supported by the observations that ischaemic preconditioning can be blocked both by inhibiting NOS (Vegh et al., 1992b) and by scavenging superoxide (Tanaka et al., 1994; Osada et al., 1994). Our results are in agreement with the observations in both in situ and in vitro rabbit hearts that MPG abolished protection afforded by a single, but not multiple, cycle of preconditioning (Baines et al., 1997). Richard et al. (1993) also used MPG in their study to test the role of free radicals in preconditioning, but they failed to block the infarct size limiting effect of ischaemic preconditioning in rats. These differences could be due to the three cycles of ischaemia-reperfusion that were used to induce protection. The multiple cycles may have caused multiple mediators to be released such that elimination of the free radical signal would not have brought the stimulation to a subthreshold level (Baines et al., 1997).
Peroxynitrite can freely cross phospholipid membranes (Marla et al., 1997) and is capable of diffusing across erythrocyte membranes via anion channels and passive diffusion (Denicola et al., 1998). Therefore, it is considered that peroxynitrite is a significant biological effector molecule not only because of its reactivity but also because of its high diffusibility.
In recent years it has become apparent that relatively low concentrations of peroxynitrite are able to mediate beneficial effects in some physiological processes. Peroxynitrite relaxes various arteries including coronary arteries through stimulation of cyclic GMP (Liu et al., 1994; Wu et al., 1994; Tarpey et al., 1995). Peroxynitrite produces S-nitrosothiols which stimulate guanylyl cyclase and release NO (Moro et al., 1994; 1995; Wu et al., 1994). Peroxynitrite has also been found to be cardioprotective in low micromolar concentrations both in in vivo and in vitro experiments (Lefer et al., 1997; Nossuli et al., 1997; 1998; Altuğ; et al., 1999). Although the exact mechanism is not known, there are several possible mechanisms whereby peroxynitrite could induce a cardioprotective action. Firstly, peroxynitrite can S-nitrosylate glutathione or other thiol-containing substances in tissues causing the formation of S-nitrosothiols (Moro et al., 1994; Wu et al., 1994). S-nitrosothiols can directly activate guanylyl cyclase and also release NO over sustained periods of time (Wu et al., 1994). Secondly, peroxynitrite forms intermediates that act as NO donors in the presence of plasma, proteins, glucose or glutathione (Moro et al., 1994; 1995; Balazy et al., 1998). Thirdly, peroxynitrite causes vasodilation of vascular smooth muscle via direct activation of guanylyl cyclase (Tarpey et al., 1995) or poly(ADP-ribose) synthase (Chabot et al., 1997). Lastly, peroxynitrite might also activate other cardioprotective mechanisms. In this regard, Wei et al. (1996) have shown that peroxynitrite is able to activate KATP channels in vascular smooth muscle. Therefore, activation of KATP channels in peroxynitrite-induced preconditioning could be involved although there is evidence that these channels are not involved in preconditioning in rats (Parratt & Kane, 1994). Since peroxynitrite administration did not cause any significant change on coronary perfusion pressure and pressure rate index measured at certain time points throughout the experiment, the cardioprotective effect seen with peroxynitrite administration was not due to an effect on these variables.
In conclusion, the results of this study showed that exogenously administered peroxynitrite at 1 μM concentration was able to mimic the beneficial effects of ischaemic preconditioning. Furthermore, we observed that MPG, a peroxynitrite scavenger, given prior to initial occlusion also blocked the protective effects of ischaemic preconditioning in rat isolated heart. Thus, it is likely that formation of peroxynitrite is an important step in the development of preconditioning in the rat and that peroxynitrite might be one of the possible mediators of cardioprotection. The present study provides new insights into the mechanism of ischaemic preconditioning.
Acknowledgments
This study was supported by a project from Gazi University (SBE-11/99-05). The chemiluminometer used in this study was provided by a project (SBAG-1243) of the Scientific and Technical Research Council of Turkey (TÜBITAK).
Abbreviations
- CAO
coronary artery occlusion
- CL
chemiluminescence
- ECG
electrocardiogram
- H2O2
hydrogen peroxide
- LAD
left anterior descending
- MnO2
manganese dioxide
- MPG
N-2-mercaptopropionylglycine
- NO
nitric oxide
- VEB
ventricular ectopic beat
- VF
ventricular fibrillation
- VT
ventricular tachycardia
References
- ALTUğ S., DEMIRYUREK A.T., ÇAKICI İ., KANZIK İ. The beneficial effects of peroxynitrite on ischaemia-reperfusion arrhythmias in rat isolated hearts. Eur. J. Pharmacol. 1999;384:157–162. doi: 10.1016/s0014-2999(99)00682-2. [DOI] [PubMed] [Google Scholar]
- BAINES C.P., GOTO M., DOWNEY J.M. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J. Mol. Cell. Cardiol. 1997;29:207–216. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- BALAZY M., KAMINSKI P.M., MAO K., TAN J., WOLIN M.S. S-nitroglutathione, a product of the reaction between peroxynitrite and glutathione that generates nitric oxide. J. Biol. Chem. 1998;273:32009–32015. doi: 10.1074/jbc.273.48.32009. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN J., MARSHALL B.A., FREEMAN B.A. Apparent OH radical production from peroxynitrite: implications for endothelial injury by nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMAN J.S., CHEN J., ISCHIROPOULOS H., CROW J.P. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., KOPPENOL W.H. Nitric oxide, superoxide and peroxynitrite: The good, the bad and the ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- BELHASSEN L., KELLY R.A., SMITH T.W., BALLIGAND J.L. Nitric oxide synthase (NOS3) and contractile responsiveness to adrenergic and cholinergic agonists in the heart. Regulation of NOS3 transcription in vitro and in vivo by cyclic adenosine monophosphate in rat cardiac myocytes. J. Clin. Invest. 1996;97:1908–1915. doi: 10.1172/JCI118622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHABOT F., MITCHELL J.A., QUINLAN G.J., EVANS T.W. Characterization of the vasodilator properties of peroxynitrite on rat pulmonary artery: role of poly (adenosine 5′-diphosphoribose) synthase. Br. J. Pharmacol. 1997;121:485–490. doi: 10.1038/sj.bjp.0701162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENICOLA A., SOUZA J.M., RADI R. Diffusion of peroxynitrite across erythrocyte membranes. Proc. Natl. Sci. U.S.A. 1998;95:3566–3571. doi: 10.1073/pnas.95.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEPRE C., FIERAIN L., HUE L. Activation of nitric oxide synthase by ischaemia in the perfused heart. Cardiovasc. Res. 1997;33:82–87. doi: 10.1016/s0008-6363(96)00176-9. [DOI] [PubMed] [Google Scholar]
- HAGER J.M., HALE S., KLONER R.A. Effect of preconditioning ischemia and reperfusion arrhythmias after coronary artery occlusion and reperfusion in the rat. Circ. Res. 1991;68:61–68. doi: 10.1161/01.res.68.1.61. [DOI] [PubMed] [Google Scholar]
- KAEFFER N., RICHARD V., THUILLEZ C. Delayed coronary endothelial protection 24 hours after preconditioning. Role of free radicals. Circulation. 1997;96:2311–2316. doi: 10.1161/01.cir.96.7.2311. [DOI] [PubMed] [Google Scholar]
- KIMURA Y., IYENGAR J., SUBRAMANIAN R., CORDIS G.A., DAS D.K. Preconditioning of the heart by repeated stunning: attenuation of post-ischemic dysfunction. Basic Res. Cardiol. 1992;87:128–138. doi: 10.1007/BF00801960. [DOI] [PubMed] [Google Scholar]
- KOOY N.W., ROYALL J.A. Agonist-induced peroxynitrite production from endothelial cells. Arch. Biochem. Biophys. 1994;310:352–359. doi: 10.1006/abbi.1994.1178. [DOI] [PubMed] [Google Scholar]
- LEFER D.J., SCALIA R., CAMPBELL B., NOSSULI T., HAYWARD R., SALAMON M., GRAYSON J., LEFER A.M. Peroxynitrite inhibits leukocyte-endothelial cell interactions and protects against ischemia-reperfusion injury in rats. J. Clin. Invest. 1997;99:684–691. doi: 10.1172/JCI119212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU P., HOCK C.E., NAGELE R., WONG P.Y.-K. Formation of nitric oxide, superoxide and peroxynitrite, in myocardial ischemia-reperfusion injury in rats. Am. J. Physiol. 1997;272:H2327–H2336. doi: 10.1152/ajpheart.1997.272.5.H2327. [DOI] [PubMed] [Google Scholar]
- LIU S., BECKMAN J.S., KU D. Peroxynitrite, a product of superoxide and nitric oxide, produces coronary vasorelaxation in dogs. J. Pharmacol. Exp. Ther. 1994;268:1114–1121. [PubMed] [Google Scholar]
- LIU Y., DOWNEY J.M. Ischemic preconditioning protects against infarction in rat heart. Am. J. Physiol. 1992;263:H1107–H1112. doi: 10.1152/ajpheart.1992.263.4.H1107. [DOI] [PubMed] [Google Scholar]
- MARLA S.S., LEE J., GROVES J.T. Peroxynitrite rapidly permeates phospholipid membranes. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14243–14248. doi: 10.1073/pnas.94.26.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERENYI G., LIND J., GOLDSTEIN S., CZAPSKI G. Peroxynitrous acid homolyzes into ·OH and ·NO2 radicals. Chem. Res. Toxicol. 1998;11:712–713. doi: 10.1021/tx980043h. [DOI] [PubMed] [Google Scholar]
- MORITA K., IHNKEN K., BUCKBERG G.D., SHERMAN M.P., YOUNG H.H., IGNARRO L.J. Role of controlled cardiac reoxygenation in reducing nitric oxide production and cardiac oxidant damage in cyanotic infantile hearts. J. Clin. Invest. 1994;93:2658–2666. doi: 10.1172/JCI117279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORO M.A., DARLEY-USMAR V.M., GOODWIN D.A., READ N.G., ZAMORO-PINO R., FEELISCH M., MONCADA S. Paradoxical fate and biological action of peroxynitrite on human platelets. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6702–6706. doi: 10.1073/pnas.91.14.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORO M.A., DARLEY-USMAR V.M., LIZASOAIN I., SU Y., KNOWLES R.G., RADOMSKI M.W., MONCADA S. The formation of nitric oxide donors from peroxynitrite. Br. J. Pharmacol. 1995;116:1999–2004. doi: 10.1111/j.1476-5381.1995.tb16404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRY C.E., JENNINGS R.B., REIMER K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemia myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- NOSSULI T.O., HAYWARD R., JENSEN D., SCALIA R., LEFER A.M. Mechanisms of cardioprotection by peroxynitrite in myocardial ischemia and reperfusion injury. Am. J. Physiol. 1998;275:H509–H519. doi: 10.1152/ajpheart.1998.275.2.H509. [DOI] [PubMed] [Google Scholar]
- NOSSULI T.O., HAYWARD R., SCALIA R., LEFER A.M. Peroxynitrite reduces myocardial infarct size and preserves coronary endothelium after ischemia and reperfusion in cats. Circulation. 1997;96:2317–2324. doi: 10.1161/01.cir.96.7.2317. [DOI] [PubMed] [Google Scholar]
- OSADA M., TAKEDA S., SATO T., KOMORI S., TAMURA K. The protective effect of preconditioning on reperfusion-induced arrhythmia is lost by treatment with superoxide dismutase. Japan Circ. J. 1994;58:259–263. doi: 10.1253/jcj.58.259. [DOI] [PubMed] [Google Scholar]
- PARRATT J.R., KANE K.A. KATP channels in ischaemic preconditioning. Cardiovasc. Res. 1994;28:783–787. doi: 10.1093/cvr/28.6.783. [DOI] [PubMed] [Google Scholar]
- PARRATT J.R., VEGH A. Endothelial cells, nitric oxide and ischaemic preconditioning. Basic Res. Cardiol. 1996;91:27–30. doi: 10.1007/BF00788857. [DOI] [PubMed] [Google Scholar]
- PIACENTINI L., WAINWRIGHT C.L., PARRATT J.R. The antiarrhythmic effect of ischaemic preconditioning in isolated rat heart involves a pertussis toxin sensitive mechanism. Cardiovasc. Res. 1993;27:674–680. doi: 10.1093/cvr/27.4.674. [DOI] [PubMed] [Google Scholar]
- QIU Y., RIZVI A., TANG X.-L., MANCHIKALAPUDI S., TAKANO H., JADOON A.K., WU W.J., BOLLI R. Nitric oxide triggers late preconditioning against myocardial infarction in conscious rabbits. Am. J. Physiol. 1997;273:H2931–H2936. doi: 10.1152/ajpheart.1997.273.6.H2931. [DOI] [PubMed] [Google Scholar]
- RICHARD V., TRON C., THUILLEZ C. Ischaemic preconditioning is not mediated by oxygen derived free radicals in rats. Cardiovasc. Res. 1993;27:2016–2021. doi: 10.1093/cvr/27.11.2016. [DOI] [PubMed] [Google Scholar]
- SUN J.-Z., TANG X.-L., PARK S.-W., QIU Y., TURRENS J.F., BOLLI R. Evidence for an essential role of reactive oxygen species in the genesis of late preconditioning against myocardial stunning in conscious pigs. J. Clin. Invest. 1996;97:562–576. doi: 10.1172/JCI118449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKANO H., TANG X.-L., QIU Y., GUO Y., FRENCH B.A., BOLLI R. Nitric oxide donors induce late preconditioning against myocardial stunning and infarction in conscious rabbits via an antioxidant-sensitive mechanism. Circ. Res. 1998;83:73–84. doi: 10.1161/01.res.83.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA M., FUJIWARA H., YAMASAKI K., SASAYAMA S. Superoxide dismutase and N-2-mercaptopropionyl glycine attenuate infarct size limitation effect of ischaemic preconditioning in the rabbit. Cardiovasc. Res. 1994;28:980–986. doi: 10.1093/cvr/28.7.980. [DOI] [PubMed] [Google Scholar]
- TARPEY M.M., BECKMAN J.S., ISCHIROPOULOS H., GORE J.Z., BROCK T.A. Peroxynitrite stimulates vascular smooth muscle cell cycle GMP synthesis. FEBS Lett. 1995;364:314–318. doi: 10.1016/0014-5793(95)00413-4. [DOI] [PubMed] [Google Scholar]
- VEGH A., ROMON S., SZEKERES L., PARRATT J. Antiarrhythmic effects of preconditioning in anaesthetised dogs and rats. Cardiovasc. Res. 1992a;107:487–495. doi: 10.1093/cvr/26.5.487. [DOI] [PubMed] [Google Scholar]
- VEGH A., SZEKERES L., PARRATT J. Preconditioning of the ischaemic myocardium: involvement of the L-arginine nitric oxide pathway. Br. J. Pharmacol. 1992b;107:648–652. doi: 10.1111/j.1476-5381.1992.tb14501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER M.J.A., CURTIS M.J., HEARSE D.J., CAMPBELL R.W.F., JANSE M.J., YELLON D.M., COBBE S.M., COKER S.J., HARNESS J.B., HARRON D.W.G., HIGGINS A.J., JULIAN D.G., LAB M.J., MANNING A.S., NORTHOVER B.J., PARRATT J.R., RIEMERSMA R.A., RIVA E., RUSSELL D.C., SHERIDAN D.J., WINSLOW E., WOODWARD B. The Lambeth Conventions: Guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovasc. Res. 1988;2:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- WANG P., ZWEIER J.L. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. J. Biol. Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- WEI E.P., KONTOS H.A., BECKMAN J.S. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide and peroxynitrite. Am. J. Physiol. 1996;271:H1262–H1266. doi: 10.1152/ajpheart.1996.271.3.H1262. [DOI] [PubMed] [Google Scholar]
- WU M., PRITCHARD K.A., KAMINSKI P.M., FAYNGERSH R.P., HINTZE T.H., WOLIN M.S. Involvement of nitric oxide and nitrosothiols in relaxation of pulmonary arteries to peroxynitrite. Am. J. Physiol. 1994;266:H2108–H2113. doi: 10.1152/ajpheart.1994.266.5.H2108. [DOI] [PubMed] [Google Scholar]
- XIA Y., DAWSON V.L., DAWSON T.M., SNYDER S.H., ZWEIER J.L. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YASMIN W., STRYNADKA K.D., SCHULZ R. Generation of peroxynitrite contributes to ischaemia-reperfusion injury in isolated rat hearts. Cardiovasc. Res. 1997;33:422–432. doi: 10.1016/s0008-6363(96)00254-4. [DOI] [PubMed] [Google Scholar]
- YELLON D.M., BAXTER G.F., GARCIA-DORADO D., HEUSCH G., SUMERAY M.S. Ischaemic preconditioning: present position and future directions. Cardiovasc. Res. 1998;37:21–33. doi: 10.1016/s0008-6363(97)00214-9. [DOI] [PubMed] [Google Scholar]
- YILDIZ G., DEMIRYÜREK A.T., SAHİN-ERDEMLİ İ., KANZIK İ. Comparison of antioxidant activities of aminoguanidine, methylguanidine and guanidine by luminol-enhanced chemiluminescence. Br. J. Pharmacol. 1998;124:905–910. doi: 10.1038/sj.bjp.0701924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU X., ZHAI X., ASHRAF M. Direct evidence that initial oxidative stress triggered by preconditioning contributes to second window of protection by endogenous antioxidant enzyme in myocytes. Circulation. 1996;93:1177–1184. doi: 10.1161/01.cir.93.6.1177. [DOI] [PubMed] [Google Scholar]
- ZWEIER J.L., WANG P., KUPPUSAMY P. Direct measurement of nitric oxide generation in the ischemic heart using electron paramagnetic resonance spectroscopy. J. Biol. Chem. 1995;270:304–307. doi: 10.1074/jbc.270.1.304. [DOI] [PubMed] [Google Scholar]


