Figure 8.
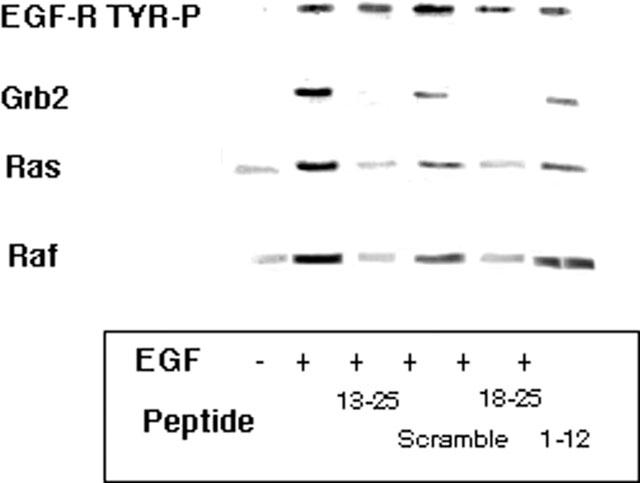
Inhibition of the association of Grb2, p21ras and Raf to activated EGF-R by N-terminal peptide fragments of LC1. A549 cells activated with 10 nM EGF for 30 min were pretreated for 3 h with 100 μg ml−1 of the LC1 peptide sequences indicated. Western blotting of immunoprecipitated EGF-R with specific antibodies to these signalling intermediates showed that the co-association of Grb2 was inhibited by peptide LC113–25 by 95% and by LC118–25 by 100% of EGF alone values. Co-association of p21ras was reduced by LC113–25 by 80% and by LC118–25 by 75% of EGF alone values. Co-association of Raf was reduced by LC113–25 by 80% and by LC118–25 by 70% of EGF alone values. A scrambled sequence of LC113–25 and peptide LC11–12 had no significant effect on the co-association of any of these intermediates.
