Figure 4.
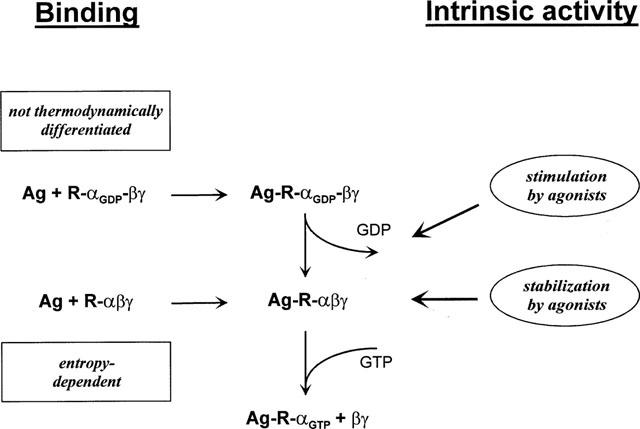
Proposed mechanism of agonist binding to and activation of A1 adenosine receptors. Agonists bind to receptors in the absence (R-αβγ) or presence of GDP (R-αGDPβγ) and may either stimulate the release of GDP or stabilize receptor-G protein-complexes in the GDP-free form. A1 adenosine receptor agonists of different intrinsic activities are not thermodynamically discriminated in binding in the presence of GDP. In the absence of GDP, ligands were thermodynamically discriminated, and a linear correlation between standard entropy and intrinsic activity was observed. This relationship between intrinsic activity and standard entropy only in the absence of GDP possibly indicates that agonist binding to the GDP-free state of the receptor-G protein complex determines the intrinsic activity of the ligand, rather than the stimulation of GDP release.
