Abstract
Antidepressant drugs are known to inhibit some changes evoked by glucocorticoids, as well as a hyperactivity of hypothalamic-pituitary-adrenal (HPA) axis, often observed in depression.
The aim of present study was to investigate effects of various antidepressant drugs on the glucocorticoid-mediated gene transcription in fibroblast cells, stably transfected with an MMTV promoter (LMCAT cells).
The present study have shown that antidepressants (imipramine, amitriptyline, desipramine, fluoxetine, tianeptine, mianserin and moclobemide), but not cocaine, inhibit the corticosterone-induced gene transcription in a concentration- and a time-dependent manner.
Drugs which are known to augment clinical effects of medication in depressed patients (lithium chloride, amantadine, memantine), do not affect the inhibitory effects of imipramine on the glucocorticoid receptor (GR)-mediated gene transcription.
Inhibitors of phospholipase C (PLC), protein kinase C (PKC), Ca2+/calmodulin-dependent protein kinase (CaMK) and antagonists of the L-type Ca2+ channel also inhibit the corticosterone-induced gene transcription.
Inhibitors of protein kinase A (PKA) and protein kinase G (PKG) are without effect on the GR-induced gene transcription.
Phorbol ester (an activator of PKC) attenuates the inhibitory effect of imipramine on the GR-induced gene transcription.
Imipramine decreases binding of corticosterone-receptor complex to DNA.
It is concluded that antidepressant drugs inhibit the corticosterone-induced gene transcription, and that the inhibitory effect of imipramine depends partly on the PLC/PKC pathway.
Keywords: Antidepressant drugs, GR-mediated gene transcription, fibroblast cells, kinase inhibitors, kinase activators
Introduction
Antidepressant drugs have been used for a long time in the clinic, but their mechanism of action still remains unknown. Most antidepressants increase synaptic concentrations of monoamines; however, there is no correlation between changes in a particular monoamine system and their therapeutic efficacy. Moreover, rapid effects of antidepressants on the synaptic content of monoamines do not correlate with their late therapeutic effects which can be observed only after 2–3 weeks of treatment. These facts suggest that long-term adaptive changes in the neuronal system, possibly at a genomic level, may be important for the explanation of the mechanism of action of antidepressants. Indeed, recent reports draw attention to the effects of antidepressant drugs on intracellular processes connected with the regulation of gene expression. It has been shown that antidepressant drugs affect the transcription factor, cyclic AMP responsive element binding protein (CREB), activated by a cyclic AMP/protein kinase A (PKA) pathway (Nibuya et al., 1996; Schwaninger et al., 1995). Except for PKA, antidepressant drugs are known to influence the activity of other intracellular protein kinases such as protein kinase C (PKC) and the Ca2+/calmodulin-dependent protein kinase (CaM-K) (Bouron & Chatton, 1999; Mann et al., 1995; Nalepa & Vetulani, 1991; Silver et al., 1986).
Phosphorylation systems that are modulated by membrane receptors via G proteins are involved in the regulation of gene transcription not only by transcription factors bound to second messenger responsive elements, but also transcription factors bound to glucocorticoid responsive elements. This fact deserves special consideration, since glucocorticoid receptors (GR) are involved in the regulation of synthesis, metabolism and level of receptors of neurotransmitters and neuromodulators which are disturbed in depression. Beside the disturbed monoaminergic neurotransmission, a hyperactivity of the hypothalamic-pituitary-adrenocortical (HPA) axis is the main biochemical change observed in patients suffering from major depression. Since antidepressant drugs have been shown to inhibit some changes evoked by glucocorticoids or stress (Jackson & Luo, 1998; Nibuya et al., 1995; Schaaf et al., 1998; Watanabe et al., 1992), a question arises whether they affect the glucocorticoid-mediated gene transcription. GR is a hormone-activated transcription factor which binds to a specific DNA sequence (glucocorticoid responsive element, GRE) and acts as a regulator of gene expression. The GR-mediated gene transcription can be modulated by cyclic AMP/PKA- PLC/PKC- and CAM-mediated signal transduction pathways (Maroder et al., 1993; Moyer et al., 1993; Ning & Sanchez, 1995; Nordeen et al., 1994) whose activities are affected by antidepressant drugs (Bouron & Chatton, 1999; Mann et al., 1995; Nalepa & Vetulani, 1991; Nibuya et al., 1996; Schwaninger et al., 1995; Silver et al., 1986). To date, only the effect of desipramine on the GR-mediated transcription has been determined, yet this drug acts in both directions, depending on experimental conditions, i.e., on the concentration and time of its and dexamethasone presence in the medium, and on the presence or absence of steroids in the serum added to an incubation medium (Pariante et al., 1997; Pepin et al., 1992).
The aim of our study was to find out whether antidepressants which differ between themselves in respect of their effect on the monoamine system may have a similar effect on the glucocorticoid receptor-mediated CAT gene expression in LMCAT cells. This cell line contains a chloramphenicol acetyl transferase (CAT) reporter gene, controlled by a mouse mammary tumour virus (MMTV) promoter. Moreover, because fibroblast cell line does not secrete catecholamines, the action of antidepressant drugs on GR-mediated gene expression is rather not connected with their effects on monoamine reuptake. The following antidepressant drugs were investigated in the present study: imipramine and amitriptyline-nonselective serotonin and noradrenaline reuptake blockers, desipramine – a selective noradrenaline reuptake blocker, fluoxetine – a selective serotonin reuptake blocker, tianeptine – a novel tricyclic antidepressant which increases the uptake of serotonin, mianserin – an atypical antidepressant drug which does not affect the uptake of the monoamines, moclobemide – a reversible monoamine oxidase-A inhibitor and, for comparison, cocaine – a releaser of monoamine, yet without an antidepressant action (Laakmann, 1983). The effects of drugs which are known to augment the clinical effects of medication in depressed patients, e.g. lithium chloride, amantadine, memantine alone and with imipramine (the most effective antidepressant drug in the clinic), were also studied.
In order to shed more light on the mechanism of action of antidepressant drugs, in the second phase of our experiment the influence of some kinase or other enzyme inhibitors and activators on imipramine-evoked changes in the corticosterone-induced CAT enzyme activity was determined.
Moreover, since the influence of antidepressant drugs on the GR-mediated gene transcription may also result from the action of these drugs on the binding of the corticosterone-receptor complex to DNA, the effect of imipramine and, for the sake of comparison, cocaine on the DNA-binding activity of the corticosterone-receptor complex was also determined.
Methods
Cell culture conditions and drug treatments
Effects of drugs on the glucocorticoid receptor-mediated gene expression were determined in mouse fibroblast cells (L929), stably transfected with mouse mammary tumour virus-chloramphenicol acetyltransferase (MMTV-CAT) reporter plasmid (LMCAT cells). The LMCAT cell line was generously provided by Dr E.R. Sanchez (Department of Pharmacology, Medical College of Ohio, Toledo, OH, U.S.A.). The cells were grown in DMEM (Gibco-BRL) with a 10% heat-inactivated foetal bovine serum (Gibco-BRL) and a 0.02% Geneticin (Gibco-BRL) at 37°C, at a 5% CO2/95% air atmosphere. In a preliminary experiment, the cells were also grown in medium without steroids, i.e. with charcoal-extracted (a 1% activated charcoal, a 0.1% dextran), heat-inactivated foetal bovine serum.
The LMCAT cells (final confluency 95%) were treated with vehicle, imipramine hydrochloride (Polfa), amitriptyline hydrochloride (Polfa), desipramine hydrochloride (RBI), fluoxetine hydrochloride (Eli Lilly), tianeptine hydrochloride (Servier), mianserin hydrochloride (Organon), moclobemide (Hoffman-La Roche) and cocaine hydrochloride (Merck) for 5 days. The drugs were added at final concentrations of 1, 10 and 100 μM. The medium and drugs were changed every second day. The CAT activity was stimulated by adding 0.1 μM corticosterone for 24 h, or 1 μM corticosterone for 2 h. In the experiment designed to investigate time-dependent drug effects, imipramine, amitriptyline, desipramine, fluoxetine and cocaine were present in the medium at a concentration of 10 μM for 1, 3 or 5 days; the CAT-activity was stimulated by corticosterone 1 μM, for 2 h. In the preliminary experiment, LMCAT cells were treated with imipramine (1–100 μM), and the CAT-activity was stimulated by dexamethasone, 0.1 or 1 μM. Corticosterone and dexamethasone (Sigma) were dissolved in a 20% 2-hydroxypropyl-γ-cyclodextrin; the same vehicle was also added to control cultures.
Effects of amantadine hydrochloride (10 μM, Sigma) and memantine hydrochloride (10 μM, Sigma) – alone and with imipramine hydrochloride (10 μM), and lithium chloride (10, 100, 500 μM, Merck) – alone and at the highest concentration (500 μM) with imipramine (10 μM), present in the medium for 5 days, on the CAT-activity induced by corticosterone at 1 μM for 2 h were also determined.
In the following phase of the experiment, the influence of kinase and some enzyme inhibitors and activators on imipramine-evoked changes in the CAT activity induced by corticosterone at 1 μM for 2 h was determined. Rp-isomer of adenosine-3′,5′-monophosphorothioate sodium salt (Rp-cyclic AMPS), a competitive inhibitor of protein kinase A (types I and II) and Rp -isomer of 8-(4-chlorophenylthio)guanosine-3′,5′-monophosphorothioate sodium salt (Rp-8-pCPT-cyclic GMPS), a potent specific inhibitor of protein kinase G (types Ia, Ib and II) were purchased from BIOLOG. The following compounds were tested: W-13 hydrochloride (Alexis Biochemicals) – specific calcium/calmodulin kinase inhibitor, tamoxifen citrate (Tocris) – an inhibitor of protein kinase C, U-107 (U-73122, RBI) – an inhibitor of phospholipase C and A2, nifedipine (Sigma) – dihydropyridine derivative, and±verapamil – a methoxy-HCl (RBI) – benzenoacetonitrile derivative of L-type Ca2+ channel blockers, forskolin (Calbiochem) – an activator of protein kinase A and phorbol 12-myristate 13-acetate (TPA, RBI) – an activator of protein kinase C.
The modulators were added alone or with imipramine (10 μM) to a culture medium for 3 days. The medium and drugs were changed every day. CAT activity was induced by corticosterone at 1 μM for 2 h. The effect of some modulators added to the culture medium for 4 h on the CAT activity induced by corticosterone (1 μM, 2 h) in control cells and in cells grown with imipramine (10 μM, 3 days) was determined.
We observed that some solvents were able to interfere with the corticosterone-induced CAT-activity. Water and DMSO at a concentration of 0.01% had no effect, but a 0.1% DMSO increased, while a 0.2% 2-hydroxypropyl-γ-cyclodextrin decreased the corticosteroid-induced CAT gene expression. Those data are in line with Nordeen's findings that some solvents were able to interfere with the dexamethasone-induced luciferase activity (Nordeen et al., 1994). Control cultures received an appropriate vehicle at the same time and concentration as cultures treated with a drug and/or a modulator.
A preliminary experiment showed that antidepressant drugs affected the GR-mediated CAT gene expression in LMCAT cells only in the presence of the glucocorticoid hormone – dexamethasone or corticosterone, but had no effect on the low basal transcription level. Imipramine inhibited the CAT activity stimulated by dexamethasone or corticosterone. In successive experiments we determined CAT activity only after stimulation with corticosterone, a natural glucocorticoid hormone in rodents. Imipramine inhibited the CAT activity in LMCAT cells grown in a medium with or without steroids (i.e. containing a heat-inactivated foetal bovine serum or a charcoal-extracted, heat-inactivated foetal bovine serum, respectively) (results not shown). Because the use of a charcoal-extracted serum had been reported to increase the expression of GR (Adcock et al., 1999), in successive experiments an unextracted serum were used.
Proteolytic effects of fluoxetine, amitriptyline and, to a lesser degree, imipramine and mianserin – used at a 100 μM concentration were observed, while the same drugs used at a 10 μM concentration, as well as other drugs at concentrations up to 100 μM were without such an effect. Similar toxic effects of some antidepressants used in high concentration were observed in rat glioma C6 cells (Fowler & Brännström, 1990).
CAT activity
Cell lysates were prepared by a freezing/thawing procedure (Pariante et al., 1997). To determine CAT enzyme activity, aliquots of lysate (after heating for 10 min at 60°C) were incubated in a 0.25 M Tris-HCl buffer (pH=7.8) with 0.25 μCi D-threo-[dichloroacetyl-1-14C]-chloramphenicol and 0.2 mM n-butyryl Coenzyme A for 1 h at 37°C. The butyrylated forms of chloramphenicol (in direct proportion to the CAT gene expression) were extracted twice with xylene, washed with 0.25 M Tris-HCl buffer, and radioactivity was measured in a β-counter (Beckmann LS 335 liquid scintillation counter). The results are presented as d.p.m. of a butyrylated fraction of chloramphenicol per 10 μg of protein per hour of incubation, and are expressed as fold induction in CAT activity (compared to samples without corticosterone), or as a percentage of the control value (compared to samples with corticosterone, but without drugs or modulators). The protein concentration in cell lysates was determined by a method of Lowry et al. (1951).
DNA-cellulose binding assay
DNA-cellulose binding assay was performed as previously described (Webb et al., 1986). Cytosol from the hippocampus or brain cortex of male Wistar rats (1 day after adrenalectomy) was prepared in a buffer without molybdate, and was incubated with 50 nM [3H]-corticosterone (spec. act. 85 Ci mmol−1, Amersham) in the absence (total binding) or presence (non-specific binding) of 25 mM dexamethasone for 20 h at 4°C. Imipramine and cocaine in final concentrations of 0.1, 1, 10 and 100 μM were added to hippocampal cytosol, and in a concentration of 100 μM to cortex cytosol. The corticosterone-receptor complex was activated by heating for 30 min at 25°C in an alkaline buffer (pH=8.5). Aliquots of cytosol were then incubated with DNA-cellulose (24 mg DNA) for 1 h at 0°C. At the end of the incubation, an ice-cold buffer was added, mixed and centrifuged. The washing procedure was repeated three times, and the final DNA-cellulose pellet was dissolved and counted in liquid scintillation. The obtained results are presented as fmol of [3H]-corticosterone binding to DNA-cellulose per mg of protein, and are expressed as a percentage of binding in control (without drugs) samples.
Statistical analysis
The data obtained from cell culture are presented as mean±s.e.mean from 3–5 independent experiments (in duplicate wells), and the significance of differences between the means has been evaluated by the Dunnett's test following one-way or two-way analysis of variance, respectively. The data obtained in DNA binding experiments are presented as mean±s.e.mean from 6–8 samples and the significance of differences between the means has been evaluated by the Dunnett's test following one-way analysis of variance.
Results
The CAT activity induced by corticosterone, 1 μM, in LMCAT cells was significantly inhibited by 1 μM RU 38486 and completely blocked by 10 μM RU 38486, a specific antagonist of type II glucocorticoid receptors, which confirm involvement of the glucocorticoid receptor in this response (Figure 1).
Figure 1.
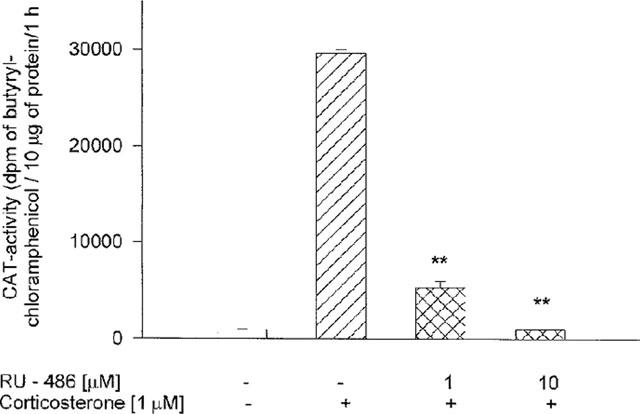
The effect of RU 38486 on the CAT gene transcription induced by corticosterone in LMCAT cells. Corticosterone (1 μM) and RU 38486 (1 and 10 μM) were added 2 h before harvesting the cells for an assay of CAT enzyme activity (presented as d.p.m. of butyrylated form of chloramphenicol per 10 μg of protein per 1 h.). The data are presented as mean±s.e.mean, and the significance of differences between the means was evaluated by the Dunnett's test following a two-way analysis of variance (**P<0.001 vs corticosterone group).
Effect of antidepressant drugs and cocaine on corticosterone-induced gene transcription
Treatment of cells with antidepressant drugs, but not with cocaine, for 5 days inhibited the stimulatory effect of corticosterone on the GR-mediated gene transcription. Addition of corticosterone at a concentration of 1 μM for 2 h increased about 35 fold CAT activity. That potent effect of corticosterone was inhibited by imipramine, amitriptyline, desipramine, fluoxetine, tianeptine and moclobemide, all of them used at concentrations of 10 and 100 μM, while their low concentration, i.e. 1 μM, was without effect. Mianserin inhibited CAT activity at all the concentrations used, while cocaine was without effect (Figure 2).
Figure 2.
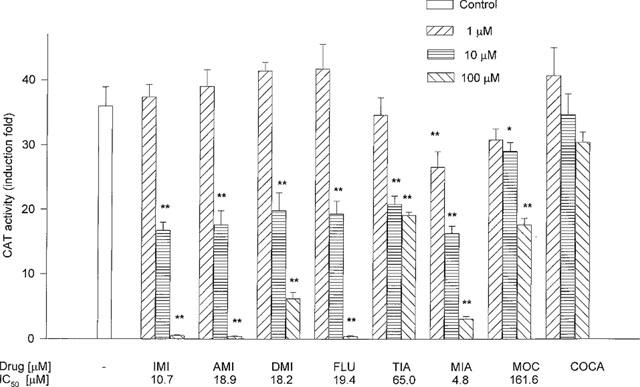
The effect of antidepressant drugs and cocaine on the CAT gene transcription induced by a high concentration of corticosterone (1 μM, 2 h) in LMCAT cells. IMI-imipramine; AMI-amitriptyline; DMI-desipramine; FLU-fluoxetine; TIA-tianeptine; MIA-mianserin; MOC-moclobemide, and COCA-cocaine were applied at the indicated concentrations for 5 days. Corticosterone (1 μM) was added 2 h before harvesting the cells for an assay of CAT enzyme activity (presented as fold induction in CAT activity). The data are presented as mean±s.e.mean, and the significance of differences between the means was evaluated by the Dunnett's test following a one-way analysis of variance (*P<0.05; **P<0.001 vs control group).
Corticosterone at a low concentration of 0.1 μM, present in the medium for 24 h, increased about 6 fold CAT activity. That transcriptional activity of corticosterone was strongly reduced in the presence of amitriptyline or mianserin (10 and 100 μM) and to the lesser extent by imipramine, fluoxetine and moclobemide (100 μM), whereas in the presence of desipramine or tianeptine only an inhibitory tendency was observed. Cocaine had no effect on CAT activity (Figure 3).
Figure 3.
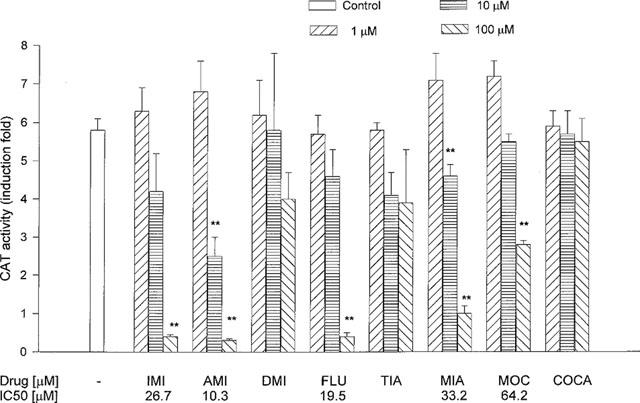
The effect of antidepressant drugs and cocaine on the CAT gene transcription induced by a low concentration of corticosterone (0.1 μM, 24 h) in LMCAT cells. IMI-imipramine; AMI-amitriptyline; DMI-desipramine; FLU-fluoxetine; TIA-tianeptine; MIA-mianserin; MOC-moclobemide, and COCA-cocaine were applied at the indicated concentrations for 5 days. Corticosterone (0.1 μM) was added 24 h before harvesting the cells for an assay of CAT enzyme activity (presented as fold induction in CAT activity). The data are presented as mean±s.e.mean, and the significance of differences between the means was evaluated by the Dunnett's test following a one-way analysis of variance (**P<0.001 vs control group).
A time-course study showed that imipramine present in the medium for 1, 3 or 5 days inhibited in a statistically significant way corticosterone effect on CAT activity; the effect of amitriptyline was significant on days 3 and 5, while those of desipramine and fluoxetine were significant on day 5 only. Cocaine changed CAT activity in no time point (Figure 4).
Figure 4.
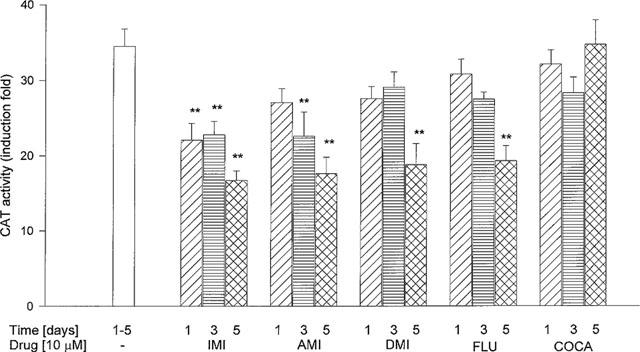
The effect of antidepressant drugs and cocaine on the CAT gene transcription induced by corticosterone (1 μM, 2 h) in LMCAT cells. IMI-imipramine; AMI-amitriptyline; DMI-desipramine; FLU- fluoxetine, and COCA-cocaine were used at a concentration of a 10 μM for 1, 3 or 5 days. Corticosterone (1 μM) was added 2 h before harvesting the cells for an assay of CAT enzyme activity (presented as fold induction in CAT activity). The data are presented as mean±s.e.mean, and the significance of differences between the means was evaluated by the Dunnett's test following a one-way analysis of variance (**P<0.001 vs control group).
No synergistic effect between lithium chloride, amantadine or memantine and imipramine on corticosterone-induced gene transcription was observed
Amantadine and memantine, present in the medium at a concentration of 10 μM for 5 days, had no effect on the CAT activity induced by corticosterone, 1 μM, in cultures with and without imipramine. Lithium chloride only at a concentration of 500 μM reduced CAT activity, but did not enhance imipramine action (Figure 5).
Figure 5.
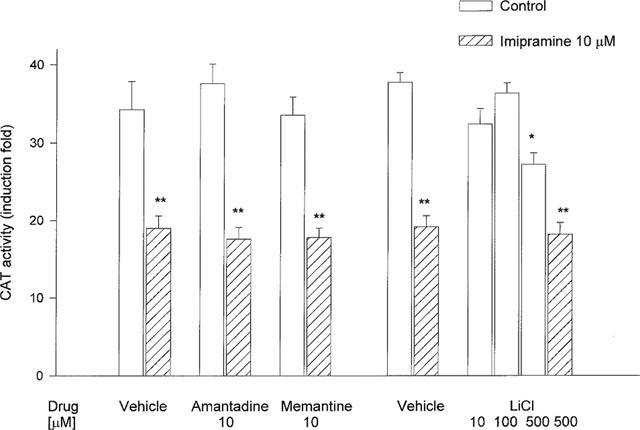
The effect of some adjunctive drugs on the CAT gene transcription induced by corticosterone (1 μM, 2 h) in LMCAT cells. The drugs were used at the indicated concentrations for 5 days. Corticosterone (1 μM) was added 2 h before harvesting the cells for an assay of CAT enzyme activity (presented as fold induction in CAT activity). The data are presented as mean±s.e.mean, and the significance of differences between the means was evaluated by the Dunnett's test following a two-way analysis of variance (*P<0.05; **P<0.001 vs respective control group).
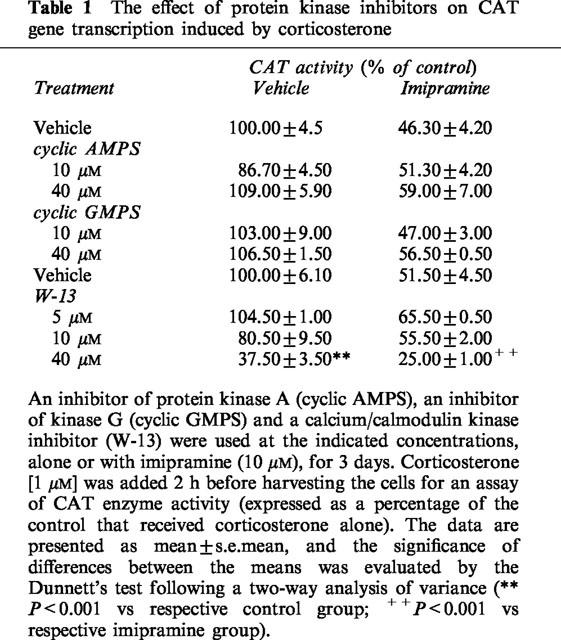
Effect of some kinase or other enzyme inhibitors and activators, present in the medium for 3 days, on the corticosterone-induced CAT activity in control cells and cells incubated with 10 μM of imipramine
The specific inhibitor of protein kinase G (Rp-8-pCPT-cyclic GMPS) and the competitive inhibitor of protein kinase A (Rp-cyclic AMPS) at both the concentrations used (10 and 40 μM) had no effect on the action of corticosterone, or on the activity of corticosterone with imipramine. W-13, a specific calcium/calmodulin kinase inhibitor, only at the highest concentration (40 μM) decreased the effect of corticosterone alone and in combination with imipramine (Table 1).
Table 1.
The effect of protein kinase inhibitors on CAT gene transcription induced by corticosterone
Tamoxifen citrate, an inhibitor of protein kinase C (Horgan et al., 1986) and U-107, an inhibitor of phospholipase C and A2 (Smallridge et al., 1992) (either used at concentrations of 1 and 10 μM) decreased CAT activity in a control incubation. Treatment of cells with imipramine and U-107 (10 μM) decreased CAT activity to a greater extent than did imipramine alone (Figure 6).
Figure 6.
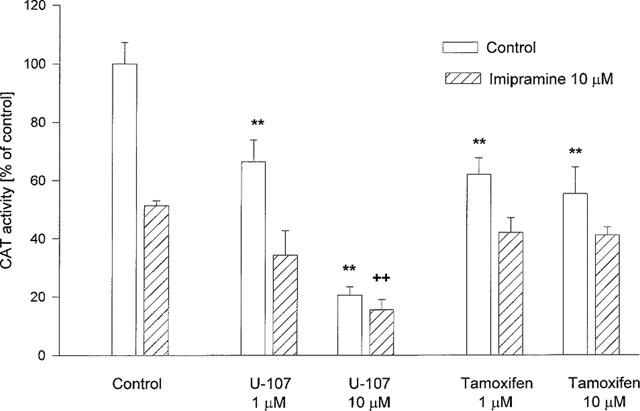
The effect of inhibitors of phospholipase C and protein kinase C on the CAT gene transcription induced by corticosterone (1 μM, 2 h) in LMCAT cells. The phospholipase C inhibitor (U-107) and the protein kinase C inhibitor (tamoxifen) were used at the indicated concentrations, alone or with imipramine (10 μM), for 3 days. Corticosterone (1 μM) was added 2 h before harvesting the cells for an assay of CAT enzyme activity (expressed as a percentage of the control that received corticosterone alone). The data are presented as mean±s.e.mean, and the significance of differences between the means was evaluated by the Dunnett's test following a two-way analysis of variance (**P<0.001 vs control group; ++P<0.001 vs imipramine group).
Nifedipine (1 and 5 μM) and verapamil (5 and 10 μM), L-type Ca2+ channel blockers, diminished the corticosterone-mediated gene transcription, but did not change the effect of imipramine (Figure 7).
Figure 7.
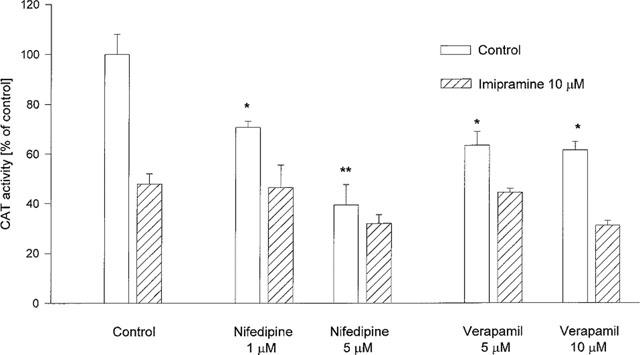
The effect of Ca2+ channel blockers of the L-type on the CAT gene transcription induced by corticosterone (1 μM, 2 h) in LMCAT cells. Nifedipine and verapamil (inhibitors of the L-type Ca2+ channel) were used at the indicated concentrations, alone or with imipramine (10 μM), for 3 days. Corticosterone (1 μM) was added 2 h before harvesting the cells for an assay of CAT enzyme activity (expressed as a percentage of the control that received corticosterone alone). The data are presented as mean±s.e.mean, and the significance of differences between the means was evaluated by the Dunnett's test following a two-way analysis of variance (*P<0.05; **P<0.001 vs control group).
Phorbol ester, an activator of protein kinase C, did not change CAT gene transcription in control cultures; however, when used at a concentration of 1 μM, it attenuated the inhibitory action of imipramine (Figure 8). Phorbol ester at concentrations of 20 and 100 nM had no effect on corticosterone action in either control or imipramine-treated cells (data not shown). Forskolin – an activator of protein kinase A, at concentrations of 1 and 10 μM had no effect on the action of corticosterone alone or combined with imipramine.
Figure 8.
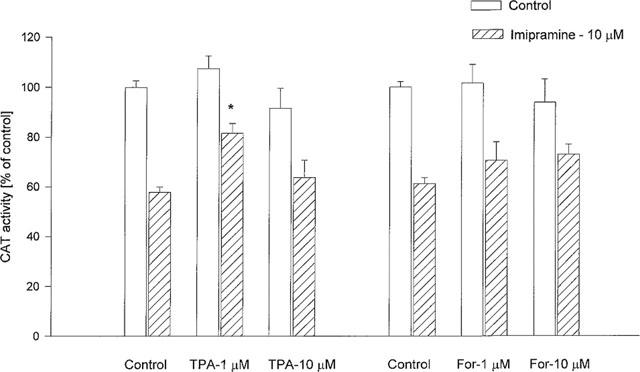
The effect of protein kinase activators on the CAT gene transcription induced by corticosterone (1 μM, 2 h) in LMCAT cells. An activator of protein kinase C (TPA- phorbol 12-myristate 13-acetate) and an activator of protein kinase A (For-forskolin) were used at the indicated concentrations, alone or with imipramine (10 μM), for 3 days. Corticosterone (1 μM) was added 2 h before harvesting the cells for an assay of CAT enzyme activity (expressed as a percentage of the control that received corticosterone alone). The data are presented as mean±s.e.mean, and the significance of differences between the means was evaluated by the Dunnett's test following a two-way analysis of variance (*P<0.05 vs respective imipramine group).
Effect of some modulators, present in the medium for 4 h, on corticosterone-induced CAT activity in control cells and in cells incubated with imipramine
Cyclic AMPS (10 and 40 μM), cyclic GMPS (10 μM), TPA (10 μM), tamoxifen (10 μM) and U-107 (10 μM), present in the incubation medium for 4 h, did not change CAT activity in control cells or in cells incubated with imipramine (10 μM) for 3 days. W-13 (10 and 40 μM) inhibited CAT activity in control cells, and, when used at a higher concentration, it potentiated the inhibitory effect of imipramine. Nifedipine (5 μM) inhibited CAT activity in control cells, but did not change the effect of imipramine. Forskolin (10 μM) decreased the CAT activity induced by corticosterone in cells grown with and without imipramine (Figure 9).
Figure 9.
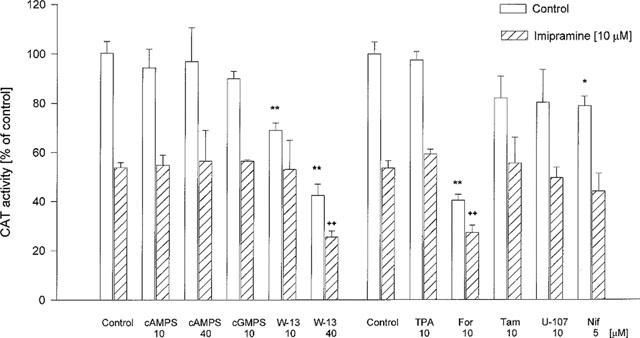
The effects of some modulators on the CAT gene transcription induced by corticosterone (1 μM, 2 h) in LMCAT cells. Imipramine at a concentration of 10 μM was added to the indicated cultures for 3 days. An inhibitor of protein kinase A (cyclic AMPS), an inhibitor of protein kinase G (cyclic GMPS), a calcium/calmodulin kinase inhibitor (W-13), an activator of protein kinase C (TPA), an activator of protein kinase A (For-forskolin), an inhibitor of protein kinase C (Tam-tamoxifen), an inhibitor of phospholipase C (U-107) and an L-type Ca2+ channel blocker (Nif-nifedipine) were used at the indicated concentrations for 4 h. Corticosterone (1 μM) was added 2 h before harvesting the cells for an assay of CAT enzyme activity (expressed as a percentage of the control that received corticosterone alone). The data are presented as mean±s.e.mean, and the significance of differences between the means was evaluated by the Dunnett's test following a two-way analysis of variance (*P<0.05; **P<0.001 vs respective control group; ++P<0.001 vs respective imipramine group).
The effect of imipramine and cocaine on the DNA-binding activity of the corticosterone-receptor complex
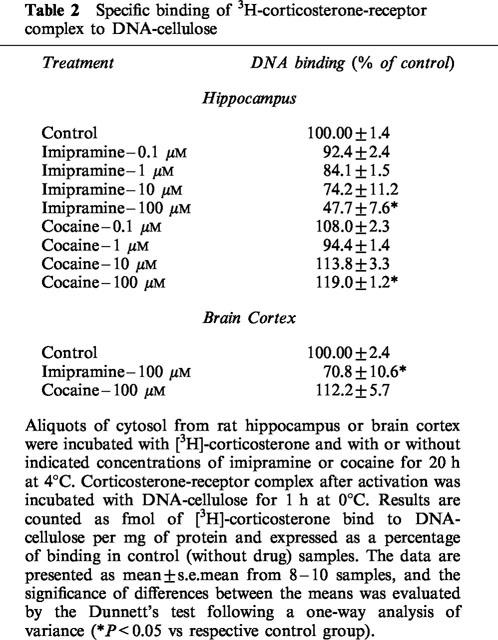
Imipramine in the highest concentration of 100 μM decreased in a statistically significant manner the DNA-binding activity of the corticosterone-receptor complex in both the hippocampus and brain cortex, while its lower concentration, 10 μM, only tended to decrease that activity in the hippocampus. Cocaine, 100 μM, increased the DNA-binding activity of the corticosterone-receptor complex in the hippocampus only (Table 2)
Table 2.
Specific binding of 3H-corticosterone-receptor complex to DNA-cellulose
Discussion
Treatment of cells with antidepressant drugs, but not cocaine, for 5 days inhibited the effect of corticosterone on the GR-mediated gene transcription. The inhibitory effect of those drugs was observed under different experimental conditions, e.g. in the case of CAT activity stimulated by the presence of corticosterone at 0.1 μM for 24 h and corticosterone at 1 μM for 2 h. The effect of antidepressant drugs was dose- and time-dependent. The effect of the higher concentration of corticosterone was potently inhibited by antidepressants in descending order (according to their IC50 values): mianserin, imipramine, desipramine, amitriptyline, fluoxetine and, to a lesser degree, by tianeptine and moclobemide. The effect of the lower concentration of corticosterone was inhibited by those drugs in descending order: amitriptyline, fluoxetine, imipramine, mianserine and, to a lesser degree, by moclobemide. In the case of desipramine and tianeptine only an inhibitory tendency was observed.
The preliminary experiment showed that imipramine also inhibited the CAT activity stimulated by the synthetic glucocorticoid dexamethasone. These findings are in line with results of Pariante et al. (1997), who observed that another antidepressant drug, desipramine, at a concentration of 10 μM inhibited the CAT activity stimulated by dexamethasone (0.1–10 μM) for 1.5 h. However, in the above-cited paper and in that of Pepin et al. (1992), under certain conditions desipramine was also able to increase the dexamethasone-induced CAT activity. In the present study we observed only inhibitory effects of imipramine on CAT enzyme activity in LMCAT cells, grown in a medium with and without steroids. These findings show that there is a difference between the action of desipramine and imipramine on the GR-induced gene transcription.
An inhibitory effect of antidepressant drugs on the corticosterone-induced promoter gene activity was observed after a therapeutical concentration of the drugs under study. The therapeutic concentrations of tricyclic antidepressants are 0.3–1 μM in the plasma, and are about 20–30 times higher in the brain (Glotzbach & Preskorn, 1982; Hrdina & Dubas, 1981; Miyake et al., 1990). All the antidepressants used in the present study (except moclobemide) inhibited, by about 50%, the action of the higher corticosterone concentration, being applied at a concentration of 10 μM, i.e. a therapeutical brain concentration (Glotzbach & Preskorn, 1982; Hrdina & Dubas, 1981). At the above concentration, only moclobemide had a weak effect. However the action of the low corticosterone concentration was inhibited only by therapeutical concentrations of amitriptyline and mianserin, which indicates that all antidepressant drugs can inhibit the effect of a high corticosterone concentration (during stress) on gene expression; although, only amitriptyline and mianserin were also able to attenuate the effect of a low corticosterone concentration. Among the drugs which are known to augment the clinical effects of medication in depressed patients (e.g. lithium chloride, which blocks the conversion of inositol phosphate to inositol; amantadine and memantine, NMDA receptor antagonists), only lithium at the highest concentration decreased the GR-induced gene transcription; however, no drug enhanced the action of imipramine.
The decrease in the glucocorticoid receptor-mediated gene transcription produced by antidepressants may be a mechanism by which these drugs block some effects induced by stress or corticosterone administration.
In the second part of this study we investigated the molecular mechanism by which imipramine inhibits the corticosterone-mediated gene transcription. MMTV promoter, which has a well-characterized glucocorticoid response elements are widely used to study the glucocorticosteroid control of gene expression. A transcriptional cooperations between GR and the signalling pathway activated by cyclic AMP or phospholipase C have been found to participate in regulation of many genes. Activation of protein kinase A and protein kinase C pathways has been shown to potentiate the glucocorticoid-induced gene transcription in some mammary carcinoma cell lines and a few fibroblast cell lines (Maroder et al., 1993; Moyer et al., 1993; Nordeen et al., 1994). In our experiment, forskolin, an activator of PKA, did not enhance the corticosterone-induced CAT activity. Moreover, during a 4-h incubation, an inhibitory action of that modulator was observed. These results are in line with the data obtained with fibroblast cell lines with a full-length MMTV promoter (Nordeen et al., 1993), and they confirm some early conclusions that the transcriptional cooperation between GR and cyclic AMP signalling pathways depends on the promoter/response element combination and is cell-dependent. Similarly, stimulation of PKC with TPA increases glucocorticoid response in some mammary carcinoma cell lines, but has no effect on the CAT activity induced by corticosterone in LMCAT cells (present data) or in fibroblasts transfected with other promoters (Nordeen et al., 1994). PKC activator does not change the corticosterone-induced CAT activity in control cells, but in some experimental conditions it is able to attenuate the inhibitory action of imipramine. Effects of imipramine are not changed by a short-time incubation with TPA (4 h) at a high concentration, nor by a long-term (3 days) incubation with TPA, 20 nM and 100 nM. Only a concentration of 1 μM of TPA, applied for 3 days, was able to attenuate in a statistically significant manner imipramine action. It is not possible to determine whether the action of imipramine results from inhibition of the PKC activity, since in some cells TPA present at this concentration for long time induced down-regulation of some isoforms of PKC (α, βI, ε, δ) (Chen & Chen, 1999). However, in cultured rat astrocytes, a forbol ester used at concentrations ranging from 16 nM to 16 μM increased the corticotropin-releasing factor-binding protein expression and this effect was similar from 1–3 days (Maciejewski et al., 1996). About 10 isoforms of mammalian PKC are presently known and only some of the enzymes are down-regulated after long-time treatment with phorbol ester (Chen et al., 1997; Pfeffer et al., 1991). Therefore, it is possible that imipramine inhibits an isoform of PKC which is not down-regulated in fibroblast cells by TPA, and the presence of TPA for the same time as imipramine is necessary to attenuate the effect of imipramine. Moreover, the inhibition of PKC by tamoxifen, or of phospholipase C (PLC) by U-107 evokes effects similar to these of imipramine and other antidepressant drugs, furthermore, the presence of these inhibitors for a long time (3 days) is necessary to evoke a statistically significant effect.
In line with our assumption, that the effect of antidepressant drugs on corticosterone-induced CAT activity in LMCAT cells may be connected with inhibition of PKC, it has been shown that imipramine inhibits protein kinase C in cerebral cortical slices of the rat (Nalepa & Vetulani, 1991), and amitriptyline inhibits phospholipase C in chick cerebral neurones (Wong et al., 1993). Repeated treatment with desipramine and fluoxetine decreases PKC activity in soluble and particulate fractions from rat cerebral cortex and hippocampus (Mann et al., 1995). On the other hand, some data show that PKC inhibitors block effects of antidepressant drugs, which suggests that these drugs may also stimulate some PKC isoforms (Bouron & Chatton, 1999).
An effect similar to that induced by antidepressant drugs and PLC/PKC inhibitors was observed after treatment of LMCAT cells with a high concentration of W-13, an inhibitor of Ca2+/calmodulin-dependent protein kinase (CaM-kinase). W-13 (40 μM) decreased the corticosterone-induced CAT gene expression in cultures with and without imipramine when it was present in those cultures for both 4 h and 3 days. It was previously shown that CaM-kinase can regulate the activity of steroid receptors (Migliaccio et al., 1984), and our results are in line with some earlier findings that calmodulin inhibitors decrease the GR-mediated gene transcription (Ning & Sanchez, 1995). Since some antidepressant drugs have been shown to inhibit Ca2+/calmodulin-regulated phosphorylation (Silver et al., 1986), the effect of these drugs on corticosterone-induced CAT activity may be connected with inhibition of CaM-K.
Intracellular, free Ca2+ are known to activate PLC, some isoforms of phospholipase A2 (PLA2) and, after binding to calmodulin, CaM-K. Like PLC/PKC and CaM-K inhibitors, in the present study also nifedipine and verapamil, i.e. compounds blocking Ca2+ entry through an L-type Ca2+ channel, inhibited the corticosterone-induced CAT activity. This inhibitory effect is in line with the observation that calcium channel antagonists decrease some effects of stress (Mamczarz et al., 1999). However, the inhibition of Ca2+ entry did not enhance the action of imipramine on CAT activity, this finding differing from other observations, that calcium channel antagonists potentiate some effects of antidepressant drugs (Czyrak et al., 1990).
Another major, PKA-linked phosphorylation system does not seem to be involved in the action of imipramine on the GR-mediated gene transcription. Neither inhibition of PKA (by cyclic AMPS), nor long-time activation (by forskolin) changed the action of corticosterone or imipramine. After a 4-h incubation, an inhibitory effect of forskolin on the corticosterone-induced CAT activity and imipramine action was observed, but no effect of PKA inhibitor suggests that the action of forskolin is rather not connected with adenylate cyclase-PKA pathway. The present results indicate that protein kinase G (PKG) is not crucially involved, either, in the corticosterone-induced gene transcription and imipramine action in fibroblast cells. Inhibition of PKG by Rp-8-pCPT-cyclic GMPS was without effect on corticosterone-induced CAT activity and imipramine action.
It is well known that regulation of gene transcription by modulators is cell- and promoter-specific, a similar mechanism may work in neurones. Some effects evoked by chronic treatment with antidepressant drugs may be explained by inhibition of the GR-mediated gene transcription, especially of genes which have GRE (e.g. β1-adrenergic receptor-, TRH-, GH- and prolactin-genes). It should be mentioned that antidepressants can differently act on GR-mediated gene transcription, but evoked via influencing other, than GRE, DNA sequences i.e. via cyclic AMP-responsive element (CRE) in the corticotropin-releasing hormone coding gene (Guardiola-Diaz et al., 1996). It has been found that chronic treatment with antidepressants decreases the level of CRH in the paraventricular nucleus of the hypothalamus most probably by enhancing the density of GR in rat central nervous system, especially in the hippocampus which is involved in the mediation of an inhibitory effect on CRH secretion in the paraventricular nucleus (Brady et al., 1991; Budziszewska et al., 1994; Okugawa et al., 1999; Peiffer et al., 1991; Przegaliński & Budziszewska, 1993; Seckl & Fink, 1992). These data show that antidepressant drugs inhibit the HPA axis activity in two different, independent ways. They increase the GR level in the CNS and enhance the GR-mediated feedback inhibition, which leads to a decrease in the corticosterone level. Apart from lowering the corticosterone level, they are able to inhibit some corticosterone receptor-mediated gene transcription.
It should be emphasized that the effects of antidepressant drugs and modulators described in the present study are most probably exerted on the GR-dependent GRE activity. Besides binding sites for GR, the MMTV LTR promoter also contains a binding site for the transcription factor NF-1 and other cis-regulatory sequences (of unknown factors), hence the role of other sequences (besides GRE) in the antidepressants action cannot be excluded. However, an analysis of MMTV LTR delation mutants shows that most modulators of protein kinases act on the GR-GRE complex (Maroder et al., 1993).
The antidepressant drugs capable of affecting the GR-induced gene transcription can act on different processes connected with GR action, such as the binding of hormones with receptors, dissociation of the steroid-receptor complex from other cytosol proteins, translocation to the nucleus, phosphorylation of GR, binding to DNA and, finally, action on the transcription complex. The available data on the action of antidepressants on these processes are still incomplete. Antidepressants do not change the binding of steroids to receptors, but their effect on dissociation of the corticosterone-GR complex from cytosol proteins and on GR phosphorylation has not been studied so far. It has already been found that desipramine induces GR translocation and potentiates the dexamethasone-induced GR translocation (Pariante et al., 1997), but effects of other antidepressant drugs have not been determined. The data about the action of antidepressants on the GR binding to DNA are also sparse. Chronic treatment with desipramine enhances the GRE binding in rat hippocampus, while fluoxetine is ineffective (Frechilla et al., 1998). In the present experiment, imipramine inhibited the DNA-binding activity of corticosterone-receptor complex, possibly by affecting the process of activation, translocation or binding to DNA. Nonetheless, the effect of imipramine on the GR binding to DNA seems to be too weak to be solely responsible for changes in the transcription, but it may participate in inhibitory action on the corticosterone-mediated CAT transcription.
In conclusion, the present data show that different classes of antidepressant drugs, but not cocaine, dose-dependently inhibit the corticosterone-induced CAT gene expression in fibroblast cells. An inhibitory effect is also observed after treatment of LMCAT cells with inhibitors of the PLC/PKC pathway, with an inhibitor of Ca2+/calmodulin-dependent protein kinase, and with inhibitors of the L-type Ca2+ channel. The action of antidepressant drugs on the GR-mediated gene transcription seems to be an important mechanism by which these drugs inhibit some effects exerted by glucocorticoids whose level in depression is elevated.
Acknowledgments
The authors are grateful to Dr E.R. Sanchez for generously providing an LMCAT cell line and wish to thank Servier for a gift of tianeptine hydrochloride. They are also indebted to Ms E. Smolak, MA, for the English translation of the manuscript, and to Ms B. Korzeniak for her skillful technical assistance.
Abbreviations
- CaM-K
Ca2+/calmodulin-dependent protein kinase
- CAT
chloramphenicol acetyl transferase
- CREB
cyclic AMP-responsive element binding protein
- CRH
corticotropin-releasing hormone
- GR
glucocorticoid receptor
- GRE
glucocorticoid responsive element
- HPA
hypothalamic-pituitary-adrenal
- LMCAT cells
fibroblast cells stably transfected with pMMTV
- MMTV
mouse mammary tumour virus
- PKA
protein kinase A
- PKC
protein kinase C
- PKG
protein kinase G
- PLC
phospholipase C
- TPA
phorbol 12-myristate 13-acetate
References
- ADCOCK I.M., NASUHARA Y., STEVENS D.A., BARNES P.J. Ligand-induced differentiation of glucocorticoid receptor (GR) trans-repression and transactivation: preferential targetting of NF-κB and lack of I-κB involvement. Br. J. Pharmacol. 1999;127:1003–1011. doi: 10.1038/sj.bjp.0702613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOURON A., CHATTON J.Y. Acute application of the tricyclic antidepressant desipramine presynaptically stimulates the exocytosis of glutamate in the hippocampus. Neuroscience. 1999;90:729–736. doi: 10.1016/s0306-4522(98)00480-1. [DOI] [PubMed] [Google Scholar]
- BRADY L.S., WHITFIELD H.J., FOX R.J., GOLD P.W., HERKENHAM M. Long-term antidepressant administration alters corticotropin-releasing hormone, tyrosine hydroxylase, and mineralocorticoid receptor gene expression in rat brain. J. Clin. Invest. 1991;87:831–837. doi: 10.1172/JCI115086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUDZISZEWSKA B., SIWANOWICZ J., PRZEGALIŃSKI E. The effect of chronic treatment with antidepressant drugs on the corticosteroid receptor levels in the rat hippocampus. Pol. J. Pharmacol. 1994;46:147–152. [PubMed] [Google Scholar]
- CHEN W.-C., CHEN C.-C. Signal transduction of arginine vasopressin-induced arachidonic acid release in H9c2 cardiac myoblasts: role of Ca2+ and the protein kinase C-dependent activation of p42 mitogen-activated protein kinase. Endocrinology. 1999;140:1639–1648. doi: 10.1210/endo.140.4.6654. [DOI] [PubMed] [Google Scholar]
- CHEN C.C., WANG J.K., CHEN W.C. TPA induces translocation but not down-regulation of new PKC isoform ζ in macrophages, MDCK cells and astrocytes. FEBS Lett. 1997;412:30–34. doi: 10.1016/s0014-5793(97)00697-2. [DOI] [PubMed] [Google Scholar]
- CZYRAK A., MOGILNICKA E., SIWANOWICZ J., MAJ J. Some behavioural effects of repeated administration of calcium channel antagonists. Pharmacol. Biochem Behav. 1990;35:557–560. doi: 10.1016/0091-3057(90)90289-t. [DOI] [PubMed] [Google Scholar]
- FOWLER C.J., BRÄNNSTRÖM G. Reduction in β-adrenoceptor density in cultured rat glioma C6 cells after incubation with antidepressants is dependent upon the culturing conditions used. J. Neurochem. 1990;55:245–250. doi: 10.1111/j.1471-4159.1990.tb08845.x. [DOI] [PubMed] [Google Scholar]
- FRECHILLA D., OTANO A., DEL RIO J. Effect of chronic antidepressant treatment on transcription factor binding activity in rat hippocampus and frontal cortex. Prog. Neuro-Psychopharmacol. Biol. Psychiat. 1998;22:787–802. doi: 10.1016/s0278-5846(98)00040-2. [DOI] [PubMed] [Google Scholar]
- GLOTZBACH R.K., PRESKORN S.H. Brain concentration of tricyclic antidepressants: single-dose kinetics and relationship to plasma concentrations in chronically doses rats. Psychopharmacology. 1982;78:25–27. doi: 10.1007/BF00470582. [DOI] [PubMed] [Google Scholar]
- GUARDIOLA-DIAZ H.M., KOLINSKE J.S., GATES L.H., SEASHOLTZ A.F. Negative glucocorticoid regulation of cyclic adenosine 3′,5′-monophosphate-stimulated corticotropin-releasing hormone-reporter expression in AtT-20 cells. Mol. Endocrinol. 1996;10:317–329. doi: 10.1210/mend.10.3.8833660. [DOI] [PubMed] [Google Scholar]
- HORGAN K., COOKE E., HALLETT M.B., MANSEL R.E. Inhibition of protein kinase C mediated signal transduction by tamoxifen. Bioch. Pharmacol. 1986;35:4463–4465. doi: 10.1016/0006-2952(86)90764-1. [DOI] [PubMed] [Google Scholar]
- HRDINA P.D., DUBAS T.C. Brain distribution and kinetics of desipramine in the rat. Can. J. Physiol. Pharmacol. 1981;59:163–167. doi: 10.1139/y81-027. [DOI] [PubMed] [Google Scholar]
- JACKSON I.M., LUO L.G. Antidepressants inhibit the glucocorticoid stimulation of thyrotropin releasing hormone expression in cultured hypothalamic neurons. J. Investig. Med. 1998;46:470–474. [PubMed] [Google Scholar]
- LAAKMANN G.Antidepressants Clinical Psychopharmacology 1983Amsterdam, Oxford, Princeton: Excerpta Medica; 86–96.ed. Hippius, H. & Winokur, G. pp [Google Scholar]
- LOWRY O.H., ROSENBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MACIEJEWSKI D., CROWE P.D., DE SOUZA E.B., BEHAN D.P. Regulation of corticotropin-releasing factor-binding protein expression in cultured rat astrocytes. J. Pharmacol. Exp. Ther. 1996;278:455–461. [PubMed] [Google Scholar]
- MAMCZARZ J., BUDZISZEWSKA B., ANTKIEWICZ-MICHALUK L., VETULANI J. The Ca2+channel blockade changes the behavioral and biochemical effects of immobilization stress. Neuropsychopharmacology. 1999;20:248–254. doi: 10.1016/S0893-133X(98)00071-2. [DOI] [PubMed] [Google Scholar]
- MANN C.D., VU T.B., HRDINA P.D. Protein kinase C in rat brain cortex and hippocampus: effect of repeated administration of fluoxetine and desipramine. Br. J. Pharmacol. 1995;115:595–600. doi: 10.1111/j.1476-5381.1995.tb14973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARODER M., FARINA A.R., VACCA A., PIA FELLI M., MECO D., SCREPANTI I., FRATI L., GULINO A. Cell-specific bifunctional role of jun oncogene family members on glucocorticoid receptor-dependent transcription. Mol. Endocrinol. 1993;7:570–584. doi: 10.1210/mend.7.4.8388998. [DOI] [PubMed] [Google Scholar]
- MIGLIACCIO A., ROTONDI A., AURICCHIO F. Calmodulin-stimulated phosphorylation of 17 β-estradiol receptor on tyrosine. Proc. Natl. Acad. Sci. USA. 1984;81:5921–5925. doi: 10.1073/pnas.81.19.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAKE K., FUKUCHI H., KITAURA T., KIMURA M., SARAI K., NAKAHARA T. Pharmacokinetics of amitriptyline and its demetylated metabolite in serum and specific brain regions of rats after acute and chronic administration of amitriptyline. J. Pharmaceut. Sci. 1990;79:288–291. doi: 10.1002/jps.2600790403. [DOI] [PubMed] [Google Scholar]
- MOYER M.L., BORROR K.C., BONA B.J., DEFRANCO D.B., NORDEEN S.K. Modulation of cell signaling pathways can enhance or impair glucocorticoid-induced gene expression without altering the state of receptor phosphorylation. J. Biol. Chem. 1993;268:22933–22940. [PubMed] [Google Scholar]
- NALEPA I., VETULANI J. Involvement of protein kinase C in the mechanism of in vitro effects of imipramine on generation of second messengers by noradrenaline in cerebral cortical slices of the rat. Neuroscience. 1991;44:585–590. doi: 10.1016/0306-4522(91)90079-4. [DOI] [PubMed] [Google Scholar]
- NIBUYA M., MORINOBU S., DUMAN R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neuroscience. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIBUYA M., NESTLER E.J., DUMAN R.S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neuroscience. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NING Y.-M., SANCHEZ E.R. Evidence for a functional interaction between calmodulin and the glucocorticoid receptor. Bioche. Biophys. Res. Commun. 1995;208:48–54. doi: 10.1006/bbrc.1995.1303. [DOI] [PubMed] [Google Scholar]
- NORDEEN S.K., BONA B.J., MOYER M.L. Latent agonist activity of the steroid antagonist, RU486, is unmasked in cells treated with activators of protein kinase A. Mol. Endocrinol. 1993;7:731–742. doi: 10.1210/mend.7.6.8395651. [DOI] [PubMed] [Google Scholar]
- NORDEEN S.K., MOYER M.L., BONA B.J. The coupling of multiple signal transduction pathways with steroid response mechanisms. Endocrinology. 1994;134:1723–1732. doi: 10.1210/endo.134.4.8137736. [DOI] [PubMed] [Google Scholar]
- OKUGAWA G., OMORI K., SUZUKAWA J., FUJISEKI Y., KINOSHITA T., INAGAKI C. Long-term treatment with antidepressants increases glucocorticoid receptor binding and gene expression in cultured rat hippocampal neurones. J. Neuroendocrinol. 1999;11:887–895. doi: 10.1046/j.1365-2826.1999.00405.x. [DOI] [PubMed] [Google Scholar]
- PARIANTE C.M., PEARCE B.D., PISELL T.L., OWENS M.J., MILLER A.H. Steroid independent translocation of the glucocorticoid receptor by the antidepressant desipramine. Mol. Pharmacol. 1997;52:571–581. doi: 10.1124/mol.52.4.571. [DOI] [PubMed] [Google Scholar]
- PEIFFER A., VEILLEUX S., BARDEN N. Antidepressant and other centrally acting drugs regulate glucocorticoid receptor messenger RNA levels in rat brain. Psychoneuroendocrinology. 1991;16:505–515. doi: 10.1016/0306-4530(91)90034-q. [DOI] [PubMed] [Google Scholar]
- PFEFFER L.M., EISENKRAFT B.L., REICH N.C., IMPROTA T., BAXTER G., DANIEL-ISSAKANI S., STRULOVICI B. Transmembrane signaling by interferon α involves diacylglycerol production and activation of the ε isoform of protein kinase C in Daudi cells. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7988–7992. doi: 10.1073/pnas.88.18.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEPIN M.-C., GOVINDAN M.V., BARDEN N. Increased glucocorticoid receptor gene promoter activity after antidepressant treatment. Mol. Pharmacol. 1992;41:1016–1022. [PubMed] [Google Scholar]
- PRZEGALIŃSKI E., BUDZISZEWSKA B. The effect of long-term treatment with antidepressant drugs on the hippocampal mineralocorticoid and glucocorticoid receptors in rats. Neurosci. Lett. 1993;161:215–218. doi: 10.1016/0304-3940(93)90297-x. [DOI] [PubMed] [Google Scholar]
- SCHAAF M.J.M., DE JONG J., DE KLOET E.R., VREUGDENHIL E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813:112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- SCHWANINGER M., SCHOFL C., BLUME R., ROSSIG L., KNEPEL W. Inhibition by antidepressant drugs of cyclic AMP response element-binding protein/cyclic AMP response element-directed gene transcription. Mol. Pharmacol. 1995;47:1112–1118. [PubMed] [Google Scholar]
- SECKL J.R., FINK G. Antidepressants increase glucocorticoid and mineralocorticoid receptor mRNA expression in rat hippocampus in vivo. Neuroendocrinology. 1992;55:621–626. doi: 10.1159/000126180. [DOI] [PubMed] [Google Scholar]
- SILVER P.J., SIGG E.B., MOYER J.A. Antidepressants and protein kinases: inhibition of Ca2+ regulated myosin phosphorylation by fluoxetine and iprindole. Eur. J. Pharmacol. 1986;121:65–71. doi: 10.1016/0014-2999(86)90393-6. [DOI] [PubMed] [Google Scholar]
- SMALLRIDGE R.C., KIANG J.G., GIST I.D., FEIN H.G., GALLOWAY R.J. U-73122, an aminosteroid phospholipase C antagonist, noncompetitively inhibits thyrotropin-releasing hormone effects in GH3 rat pituitary cells. Endocrinology. 1992;131:1883–1888. doi: 10.1210/endo.131.4.1396332. [DOI] [PubMed] [Google Scholar]
- WATANABE Y., GOULD E., DANIELS D.C., CAMERON H., MCEWEN B.S. Tianeptine attenuates stress-induced morphological changes in the hippocampus. J. Pharmacol. 1992;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- WEBB M.L., FLYNN J.J., SCHMIDT T.J., MARGULES D.L., LITWACK G. Decreased glucocorticoid binding and receptor activation in brain of genetically diabetic (MDB/MDB) mice. J. Steroid Biochem. 1986;25:649–657. doi: 10.1016/0022-4731(86)90007-5. [DOI] [PubMed] [Google Scholar]
- WONG K.L., CHUANG T.Y., BRUCH R.C., FARBMAN A.I. Amitriptyline inhibits neurite outgrowth in chick cerebral neurons: a possible mechanism. J. Neurobiol. 1993;24:474–487. doi: 10.1002/neu.480240406. [DOI] [PubMed] [Google Scholar]


