Figure 3.
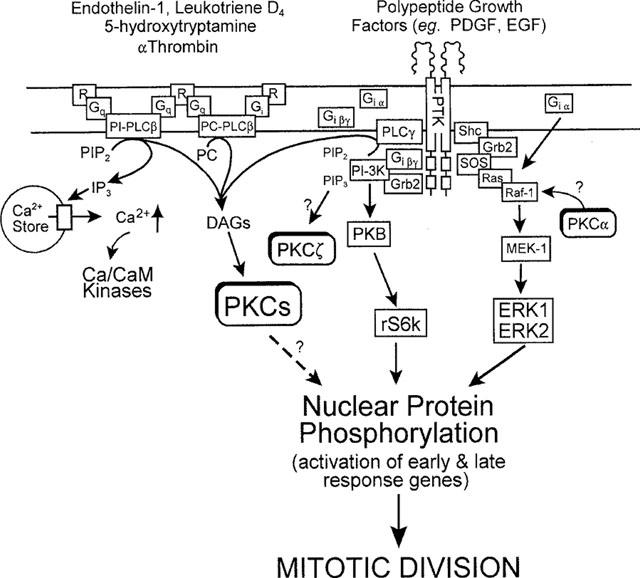
Possible mechanisms that regulate ASM mitogenesis induced by agonists that activate G-protein (G)-coupled receptors (R) and polypeptide growth factor receptor tyrosine kinases (PTK). G protein agonist-induced extracellular signal-regulated kinase (ERK) activation by Gi-coupled, but not PTK-linked receptors, occurs by a pertussis toxin-sensitive, Raf-1-dependent pathway, in which G1α subunits associate with Ras. Alternatively, interaction of polypeptide growth factors with their receptors results in recruitment of the guanine nucleotide releasing binding protein, Grb2 at the membrane. Grb2 localizes the guanine nucleotide exchange factor, Sos, to Ras. Activation of Ras results in recruitment to the membrane of Raf-1 and phosphorylation and activation of MEK-1 (MAP kinase/ERK-kinase activating kinase). MEK-1 directly phosphorylates and activates ERK1/ERK2 which translocate to the cell nucleus. Activation of PKC is implicated in ASM mitogenesis, though the importance of phosphatidylinositol (PI)- or phosphatidylcholine (PC)-dependent-PLC-derived diacylglycerols (DAGs) is disputed. Currently, no clear consensus for the site of integration of PKC activation in activation of ERK in ASM has been established, although in other systems PKCα may phosphorylate and activate Raf-1. The dotted arrow illustrates possible direct effects of PKC on nuclear transcription elements. Recent evidence suggests proliferation induced by certain Gi-coupled receptor agonists (α-thrombin) may be dependent on activation of PI3kinase by Giβγ subunits. Localized generation of PI 3,4,5-trisphosphate (PIP3) by PI-3kinase recruits protein kinase B (PKB) to the plasma membrane where it can be fully activated prior to activation of the S6 ribosomal kinase (p70s6k). PIP3 may also activate PKCζ and this may offer another integration site for PKC in ASM mitogenesis.
