Abstract
Experiments were done to determine the influence of gender and the oestrous cycle on rat urinary bladder contractility in response to cholinergic stimulation.
Bladder strips from female rats responded to high frequency stimulation with smaller contractile responses than did strips from males, and to low concentrations of carbachol with greater responses. The decreased responsiveness of bladder strips from female rats to electrical field stimulation can be primarily attributed to the rats in the oestrous stage of the oestrous cycle.
Bladder strips from female rats in all stages of the oestrous cycle were more sensitive to carbachol than those from males, but there were no differences in sensitivity to electrical field stimulation.
The contractile responses of strips from both male and female rats to carbachol were antagonized by muscarinic antagonists with the following rank order of affinity (pA2) estimates: 4-DAMP>>pirenzepine>methoctramine, suggesting that the receptor mediating contraction was the M3 subtype. There were no differences in pA2 values between bladder strips from male and female rats.
The data indicate that responsiveness of bladder strips to electrical field stimulation and carbachol is altered in female rats in the oestrous stage of the oestrous cycle. Furthermore, gender influences the sensitivity of rat bladder to muscarinic stimulation.
Keywords: Gender, oestrous cycle, urinary bladder, smooth muscle contraction, electrical field stimulation, muscarinic receptors
Introduction
Oestrogen and progesterone receptors are present in the bladder and urethra (Iosif et al., 1981; Saez & Martin, 1981; Batra & Iosif, 1987; Wolf et al., 1991; Strittmatter et al., 1994; Kuiper et al., 1998). Lower urinary tract function in females is influenced by sex hormones (Miodrag et al., 1988), and some normal women with no previously reported urological dysfunction exhibit changes in micturition frequency (Glenning, 1985), urethral pressure profile (Schreiter et al., 1976), urethral length (Van Geelen et al., 1981), and incidence of incontinence (Stanton, 1977; Wall & Warrell, 1989; DeLancey & Nygaard, 1991) during the menstrual cycle. Shimonovitz and co-workers found lower urinary tract dysfunction more common during the follicular phase of the menstrual cycle when progesterone levels are low, than during the luteal phase when both progesterone and oestradiol levels are high (Shimonovitz et al., 1997), and suggested that the timing of cystometric evaluation could be important in patient evaluation of incontinence. Although oestrogens are reputed to increase and progesterone to decrease smooth muscle tone as a result of effects on autonomic receptors, there is little objective clinical data to support the hypothesis that these mechanisms are important for the maintenance of continence (Salmon et al., 1941; Cardozo, 1990; Fantl et al., 1994; 1996).
The oestrous cycle of the female rat lasts 4–5 days and is divided into four stages, characterized by changes in vaginal cytology and hormone levels. The stages of pro-oestrous and oestrous, which last about 12 h each, correspond to the luteal phase of the human menstrual cycle. During pro-oestrous, serum oestradiol and progesterone levels peak, there is a surge in luteinizing hormone (LH), and ovulation takes place. This is followed by oestrous, during which hormone levels decline and the female is receptive to males. The follicular phase corresponds to metoestrous (≈21 h) and dioestrous (≈57 h), during which oestradiol levels gradually increase until the LH surge occurs again during pro-oestrous (Long & Evans, 1922; Butcher et al., 1974; Smith et al., 1975).
In rats, both oestrogens and progesterone have been shown to decrease bladder contractility when administered in vivo for several months (Elliott et al., 1992; Longhurst et al., 1992b; Ekström et al., 1993; Levin & Longhurst, 1996), but the relationship of the hormone doses used to physiological levels is difficult to evaluate. Elliot and co-workers showed significant decreases in the cholinergic component of the response of female rat bladder strips to nerve stimulation and to carbachol after only 8 days of oestradiol treatment (Elliott et al., 1992). Production of functional changes after short-term administration of oestradiol suggests that bladder function may be altered by the acute changes in sex hormone levels which occur during the menstrual or oestrous cycle. However, the mechanisms involved have not been resolved.
The bladder contains a heterogeneous population of muscarinic receptors, but recent studies have shown that the minority M3 receptors mediate the response of bladder strips to muscarinic stimulation (Longhurst et al., 1995; Hegde et al., 1997). Muscarinic antagonists are often the first line of treatment for urge incontinence, which occurs predominantly in females (Wein, 1995). Although gender differences in responsiveness of the bladder to muscarinic stimulation have been reported (Chun et al., 1990; Longhurst et al., 1992a), the receptor subtypes that are responsible have not been identified, nor has the influence of the oestrous cycle on contractility been evaluated. Thus, the aim of this study was to examine the influence of gender and the oestrous cycle on muscarinic responsiveness of bladder strips.
Methods
Animals
Age-matched adult male and female Sprague Dawley rats obtained from Charles River Laboratories were used throughout the study. All animals received food and water ad libitum. The stage of oestrous for the female rats was determined from vaginal smears taken on at least 2 consecutive days (Long & Evans, 1922). The protocols employed for this investigation were approved by the Stratton Veterans Affairs Medical Center Institutional Animal Care and Use Committee.
Tissue preparation
Rats were anaesthetized with Nembutal (50 mg kg−1, i.p.). The urinary bladder was removed and placed in ice-cold modified Krebs-Henseleit buffer of the following composition (mM): NaCl 113, KCl 4.8, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, dextrose 5.6, containing 10 μM indomethacin. Longitudinal strips of approximately 2×10 mm were cut from the bladder body, suspended on 000 sutures between a pair of platinum ring electrodes, 8 mm apart, and placed in 10 ml organ baths containing Krebs-Henseleit solution equilibrated with 95% O2, 5% CO2 at 37°C. The sutures were connected to Grass force displacement transducers (FT03) and the resting tension was adjusted to 2 g. Responses were recorded on a Grass Model 7E polygraph. All tissues were then given a 30 min equilibration period during which they were washed and the resting tension was adjusted every 10 min.
Contractile studies
Frequency-response curves (0.5–32 Hz) were generated by stimulating the strips for 15 s with pulses of 0.05 ms width at 100 V every 3 min with a Grass S88 stimulator. These responses have previously been shown to be sensitive to tetrodotoxin and therefore neurogenic (Tammela et al., 1994). The maximal response, which results primarily from cholinergic stimulation, was measured. Concentration-response curves to carbachol were generated non-cumulatively. Strips were washed at least twice between each incremental concentration. Strips were then incubated with a single concentration of antagonist (pirenzepine, methoctramine, or 4-DAMP methiodide) for 30 min before repeating the frequency and concentration-response curves (Longhurst et al., 1995).
Drugs
Carbamylcholine chloride (carbachol) and indomethacin, were obtained from Sigma Chemical Company (St. Louis, MO, U.S.A.). 4-Diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP), methoctramine tetrahydrochloride, and pirenzepine dihydrochloride were obtained from Research Biochemicals International (Natick, MA, U.S.A.).
Statistical analyses
All data are expressed as means±s.e.mean or 95% confidence limit (CL) with n=number of rats. Contractile responses were normalized for changes in strip size by dividing the change in tension by the cross-sectional area. Cross-sectional area was calculated from the equation;
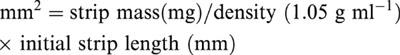 |
The frequency of stimulation producing 50% of maximal response (EF50) was determined by linear regression. F′/F is the frequency-ratio determined at 50% of maximal response to electrical field stimulation before (F) and after (F′) treatment. Geometric mean EC50 values were obtained by probit analysis (Fleming et al., 1972; Kenakin, 1984) and are reported as pD2 values (−log EC50=pD2). pA2 values for the antagonists were determined by Schild regression (Arunlakshana & Schild, 1959). Confidence limits for pA2 values were calculated as described by Tallarida & Murray (1987). A′/A is the agonist concentration-ratio determined at 50% of maximal response to carbachol before (A) and after (A′) antagonist treatment. Comparisons between groups were made using the Student's t-test or Bonferroni analysis, where appropriate. In all cases, a P value <0.05 was considered significant.
Results
General characteristics
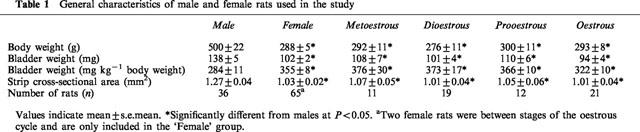
As previously reported (Longhurst et al., 1992a; Eika et al., 1994), male rats weighed significantly more than age-matched female rats and had significantly heavier bladders and bladder strips with larger cross-sectional areas (Table 1). Relative to body weight the bladders from females were significantly heavier than those from males (Table 1). These differences between males and females were seen during all stages of the oestrous cycle (Table 1).
Table 1.
General characteristics of male and female rats used in the study
Responses to electrical field stimulation
When the data from all female rats were combined, bladder strips from the females responded to electrical field stimulation with significantly smaller responses than did strips from males at 8, 16 and 32 Hz, but there were no differences in sensitivity to electrical field stimulation, as determined by comparison of EF50 values (Table 2). When the data from the females were grouped relative to the stage of the oestrous cycle, there were differences in the maximal responses to electrical field stimulation with male⩾metoestrous⩾dioestrous⩾prooestrous>oestrous (Figure 1). Strips from females in oestrous responded to stimulation at 4, 8, 16 and 32 Hz with significantly smaller responses than strips from males or females in metoestrous (Figure 1). At 8, 16 and 32 Hz, strips from females in oestrous were also significantly less responsive than those from females in dioestrous. There were no significant differences in EF50 values based on stage of the oestrous cycle (Table 2).
Table 2.
Responsiveness and sensitivity of bladder strips from male and female rats to electrical field stimulation and carbachol
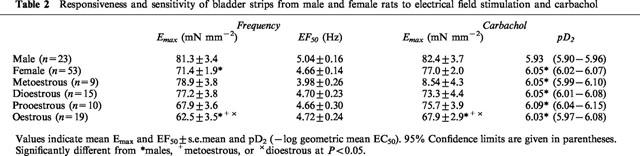
Figure 1.
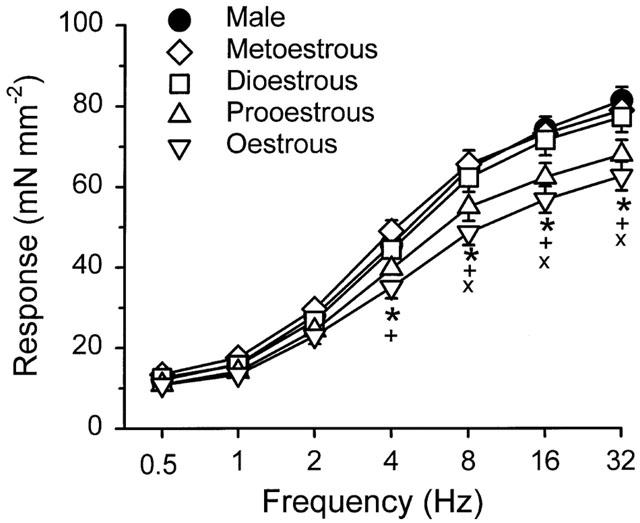
Frequency-response relationship of bladder body strips from male rats and female rats during different stages of the oestrous cycle. Each point represents the mean±s.e.mean (n=9–23). Significantly different from *males, +metoestrous, or ×dioestrous at P<0.05.
Carbachol
Bladder strips from female rats responded to 0.1 and 0.3 μM carbachol with significantly larger contractions than strips from males and were significantly more sensitive to carbachol than those from males (Table 2). When the females were grouped relative to the stage of the oestrous cycle, strips from the rats in metoestrous, dioestrous, and oestrous responded to 0.1 and 0.3 μM carbachol with significantly larger contractions than strips from males. The rank order of maximal response was metoestrous⩾dioestrous⩾male⩾prooestrous>oestrous. Strips from rats in oestrous responded to 30 μM carbachol with significantly smaller contractions than those from males or females in metoestrous and dioestrous (Figure 2). However, strips from all groups of females were significantly more sensitive to carbachol than were strips from males (Table 2).
Figure 2.
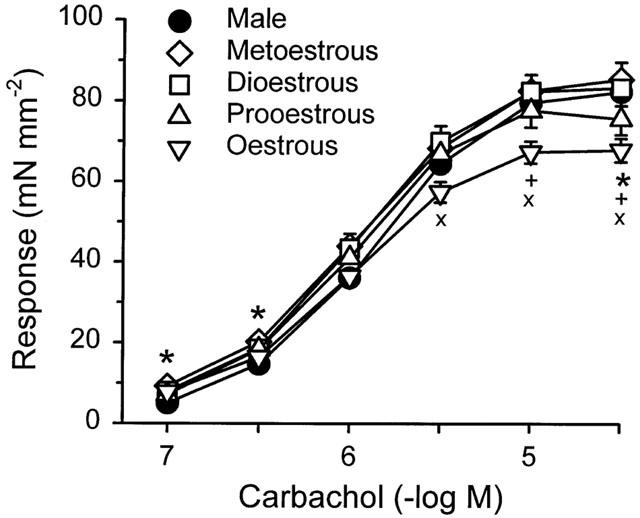
Concentration-response relationship for carbachol of bladder body strips from male rats and female rats during different stages of the oestrous cycle. Each point represents the mean±s.e.mean (n=9–23). Significantly different from *males, +metoestrous, or ×dioestrous at P<0.05.
Effects of time on consecutive responses
Responses of bladder strips to carbachol were reproducible with time. The concentration-ratio, A′/A, was 1.15±0.07 (n=21) in strips from males and 1.32±0.09 (n=34) in strips from females. There were no differences in the Emax values for two consecutive concentration-response curves to carbachol (males: 90.2±8.5 and 91.3±8.5 mN mm2; females: 68.6±3.8 and 66.2±3.4 mN mm2). As we previously reported there was a small but significant decrease in maximal response (Emax males: 86.5±6.5 and 75.8±5.6 mN mm2; females: 69.1±4.2 and 59.9±4.3 mN mm2) and sensitivity to electrical field stimulation during consecutive frequency-response curves (Longhurst et al., 1995). The frequency-ratio, F′/F was 1.71±0.11 (n=27) for strips from male rats and 2.41±0.34 (n=43) for strips from females.
Effects of pretreatment with pirenzepine
Pirenzepine caused parallel concentration-dependent rightward shifts of concentration-response curves to carbachol in bladder strips from both male (Figure 3a) and female rats (Figure 3b). There were no differences in pA2 values for pirenzepine between strips from males (pA2: 7.02; 95% CL: 6.77–7.27; n=8) or females (pA2: 6.76; 95% CL: 6.60–6.92; n=8), and the slopes of the Schild plots were not significantly different from unity. Despite the concentration-dependent parallel shifts in responses to carbachol produced by pirenzepine, all concentrations of pirenzepine produced similar degrees of inhibition of the response to electrical field stimulation (Figure 3c,d). This inhibition was significantly greater than the decrease in response observed in strips where electrical field stimulation was repeated in the absence of pirenzepine (≈15% suppression of maximal response). In strips from male rats, pirenzepine suppressed the maximal response by approximately 70% and in females by 60%. The influence of the oestrous cycle on responsiveness to pirenzepine was not evaluated. Strips were taken from one rat in metoestrous, three rats in dioestrous, two rats in prooestrous, and two rats in oestrous.
Figure 3.
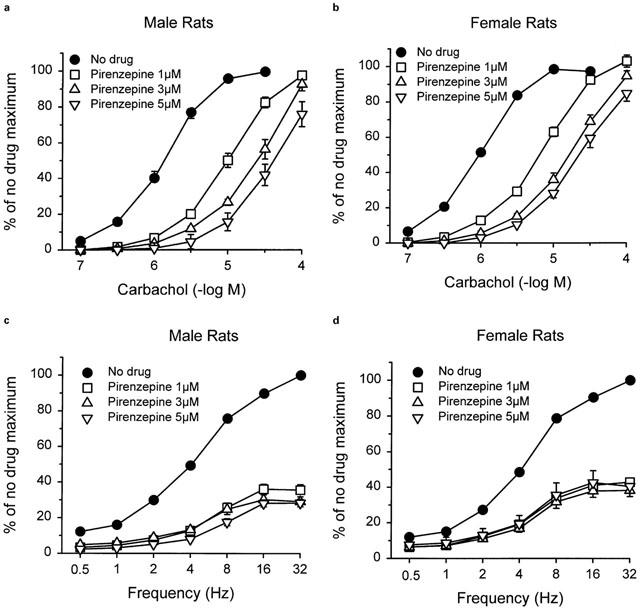
Effects of pretreatment with pirenzepine on the contractile response of bladder strips from male (a) and female (b) rats to carbachol and male (c) and female (d) rats to electrical field stimulation. Each point represents the mean±s.e.mean (n=8).
Effects of pretreatment with methoctramine
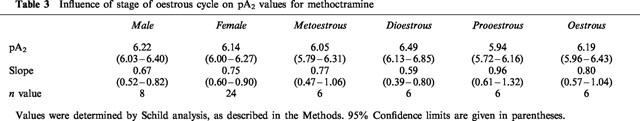
Methoctramine caused parallel concentration-dependent rightward shifts of carbachol concentration-response curves in bladder strips from both male (Figure 4a) and female (Figure 4b) rats. pA2 values for methoctramine were similar in bladder strips from males (6.22) and females (6.14) (Table 3). However, in both cases, the slope of the Schild plot was significantly less than unity (Table 3). The pA2 values were not altered by the stage of the oestrous cycle (Table 3). Despite the significant methoctramine-induced inhibition of the concentration-response curve to carbachol, low concentrations of methoctramine (1 and 3 μM) had no greater effects on the frequency response curve than was observed after repeat stimulation in the absence of methoctramine (≈25%; Figures 4c,d). In the presence of 10 μM methoctramine, the maximal response of strips from male rats to electrical field stimulation was suppressed by approximately 35%; and that of strips from females was suppressed by 42%. There was no difference in the degree of suppression of the frequency response curve when the female rats were grouped according to the stage of the oestrous cycle.
Figure 4.
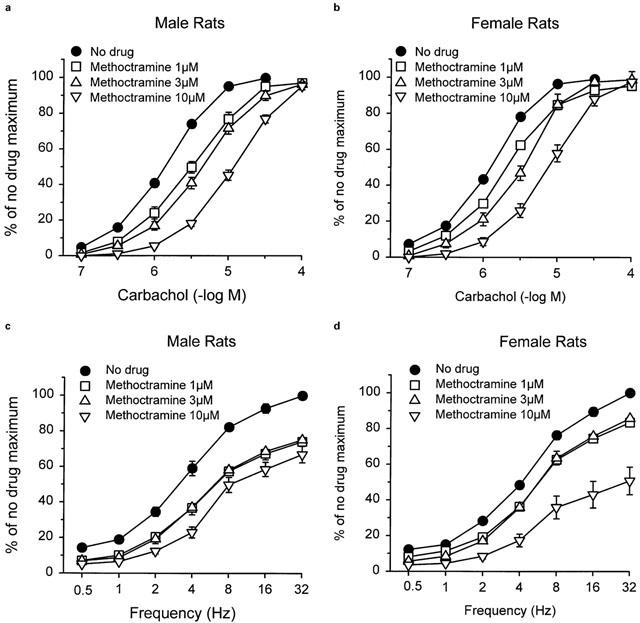
Effects of pretreatment with methoctramine on the contractile response of bladder strips from male (a) and female (b) rats to carbachol and male (c) and female (d) rats to electrical field stimulation. Each point represents the mean±s.e.mean (n=8 (male) and 24 (female)).
Table 3.
Influence of stage of oestrous cycle on pA2 values for methoctramine
Effects of pretreatment with 4-DAMP
4-DAMP caused concentration-dependent rightward shifts of carbachol concentration-response curves in bladder strips from both male (Figure 5a) and female (Figure 5b) rats. pA2 values for 4-DAMP were similar in bladder strips from males (9.08) and females (9.19) (Table 4). The slope of the Schild plots was not different from unity. The stage of the oestrous cycle had no influence on inhibition by 4-DAMP (Table 4). All concentrations of 4-DAMP produced significant inhibition of the response to electrical field stimulation compared to the time controls (≈20%). Inhibition of the response to electrical field stimulation by 4-DAMP was similar in strips from males (Figure 5c) and females (Figure 5d) and was not changed when females were grouped depending on the stage of the oestrous cycle. The rank order of antagonist affinities against the carbachol response was 4-DAMP>>pirenzepine>methoctramine. The profile was characteristic of responses mediated by M3 receptors. Neither gender nor the oestrous cycle affected the affinity of the antagonists for the muscarinic receptor.
Figure 5.
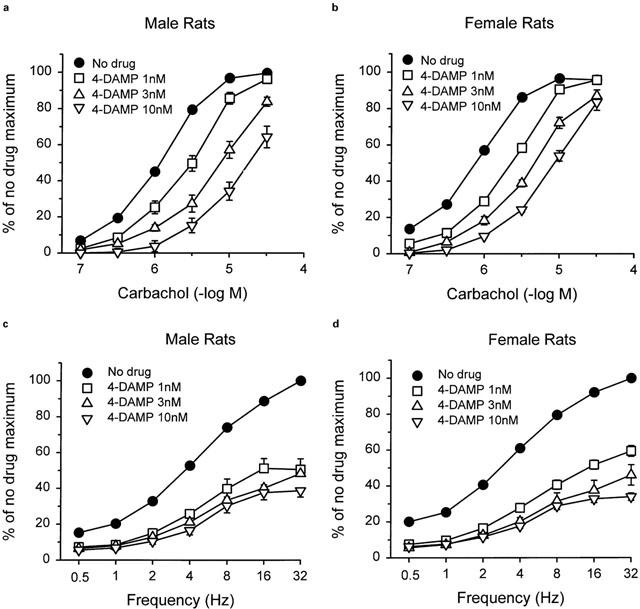
Effects of pretreatment with 4-DAMP on the contractile response of bladder strips from male (a) and female (b) rats to carbachol and male (c) and female (d) rats to electrical field stimulation. Each point represents the mean±s.e.mean (n=5 (male) and 20 (female)).
Table 4.
Influence of stage of oestrous cycle on pA2 values for 4-DAMP

Discussion
Gonadal hormones have been shown to alter bladder function, but little is known about the influence of the oestrous cycle on the bladder. Chun and associates concluded that increases in circulating estrogens during maturation caused an increased responsiveness of bladders from female rats to cholinergic stimulation (Chun et al., 1990). They speculated that an increase in muscarinic receptor numbers was responsible for the change. Previous studies from our laboratory found no gender differences in responses of bladder strips to either electrical field stimulation or to carbachol, but a relatively small number of animals was used in those studies and we did not monitor the stages of the oestrous cycle (Longhurst et al., 1992a; Eika et al., 1994). The reason we now find gender differences is probably because of the large numbers of animals used. Whether the statistically significant difference between responses of males and females is of biological relevance is conjectural. However, our findings of significant differences in bladder strip responsiveness based on the stage of the oestrous cycle, suggest that rapid changes in endogenous gonadal hormone levels can cause rapid functional changes.
The mechanism(s) responsible for the changes in bladder function associated with the oestrous cycle remain to be determined. Although the major actions of sex hormones result from binding to the cytosolic steroid receptors followed by gene transcription and protein synthesis, non-genomic modulation of calcium influx may also occur. These non-genomic actions are probably mediated through cell-surface receptors linked to voltage-dependent L-type calcium channel currents (Kitazawa et al., 1997). Although blockade of calcium channels could explain the decreases in responsiveness found in the strips from rats in oestrous, it seems unlikely that these effects result from non-genomic actions, since the concentration of oestradiol required to inhibit the calcium current and contraction is probably many orders of magnitude greater than that found in plasma (Kitazawa et al., 1997).
In this study, the most prominent changes in bladder function were noted during the stage of oestrous, which occurs 12–24 h after the sex hormone levels peak during pro-oestrous. The responses of strips from rats in pro-oestrous were also decreased, but the differences were not significant. The stimulation of protein synthesis by oestrogens occurs fairly rapidly. Medlock and co-workers showed that administration of physiological doses of 17β-oestradiol to rats increased uterine nuclear oestrogen receptor levels within 1–3 h and uterus weight within 3–6 h (Medlock et al., 1991). Therefore, the changes observed in the bladders coincide with a time when sex hormones can stimulate protein synthesis. However, whether the mechanisms responsible for the observed changes result from genomic actions of sex hormones remains to be determined.
Alterations in muscarinic receptor density could explain decreases in sensitivity and maximal response to carbachol. Shapiro found that implantation of oestradiol pellets for 21 days significantly reduced muscarinic receptor density in rabbit bladder body (Shapiro, 1986). Similarly, Batra and Andersson found that oestradiol treatment significantly decreased rabbit bladder muscarinic receptor density within 1 week, and was associated with an increase in the EF50, an increase in the atropine-sensitive component of the neurogenic response, and a decreased response to KCl, but no change in responsiveness to carbachol (Batra & Andersson, 1989). Batra and Andersson speculated that the contractile changes resulted from decreases in muscarinic receptor density accompanied by oestrogen-induced effects on calcium influx. Similar conclusions were reached by Elliott and co-workers (Elliott et al., 1992). Whether the variations in hormone levels during the oestrus cycle also alter receptor density is unknown. However, the absence of differences in pA2 values for carbachol among female rats in the present study suggest that receptor density changes are not responsible for the observed decreases in responsiveness.
In previous studies, we and others have demonstrated that the functional muscarinic receptor in the rat bladder is the M3 subtype (Longhurst et al., 1995; Hegde et al., 1997). In the current study we used the rank order of affinities of the muscarinic antagonists, pirenzepine, methoctramine, and 4-DAMP methiodide, to characterize the functional muscarinic receptor. The receptor mediating contraction in bladders from both male and female rats had a low affinity for methoctramine, intermediate affinity for pirenzepine, and high affinity for 4-DAMP, and thus most closely resembled the M3 subtype (Caulfield, 1993; Longhurst et al., 1995; Hegde et al., 1997). As reported previously, only a portion of the contractile response of bladder strips to electrical field stimulation is sensitive to inhibition by muscarinic antagonists (Longhurst et al., 1984; 1995; Sibley, 1984; Brading & Williams, 1990). We previously showed that the M2 antagonist, methoctramine, was less effective at inhibiting the neurogenic response than were M1 or M3 antagonists (Longhurst et al., 1995). Similar findings are reported in this study. This effect may be due to the blockade of prejunctional M2 receptors which inhibit the release of acetylcholine (Somogyi & de Groat, 1992).
Cyclic changes in progesterone levels also occur during the oestrus cycle. Little is known of the effects on progesterone on smooth muscle function. Elliott and Castleden found that in vitro administration of progesterone reduced the response of bladder strips to electrical field stimulation and KCl, but progesterone was much less potent than diethylstilboestrol (Elliott & Castleden, 1994). Long-term in vivo treatment with progesterone increased the maximal response of rabbit bladder strips to electrical field stimulation and increased sensitivity to bethanechol compared to ovariectomized controls (Ekström et al., 1993). In rats, in vivo treatment with progesterone decreased the contractile response of bladder strips to acetylcholine accompanied by a decrease in muscarinic receptor density (Tong et al., 1995). Therefore, qualitatively similar effects of progesterone and oestradiol are seen on bladder function, but progesterone is much less potent than the oestrogens. The changes observed during the oestrous cycle are unlikely, therefore, to result from the cyclic alterations in progesterone levels.
Although modest, our findings of increased sensitivity of bladder strips from female rats to the muscarinic agonist, carbachol, may have clinical relevance. A similar increase in sensitivity of the human bladder to the cholinergic transmitter, acetylcholine, could cause hyperactivity and/or involuntary bladder contractions. An increased sensitivity would explain why muscarinic antagonists are the most effective medical treatment for urge incontinence, which occurs predominantly in females. It would be interesting to know whether the efficacy of anticholinergic therapy varies during the menstrual cycle or with menopausal status.
In conclusion, the contractile responses of rat bladder strips to electrical field stimulation and muscarinic stimulation were altered in female rats in the oestrous stage of the oestrous cycle. Furthermore, gender influenced sensitivity of the rat bladder to muscarinic stimulation. Alterations in muscarinic responsiveness in human bladders could explain the greater incidence of incontinence and bladder dysfunction in females.
Acknowledgments
This work was supported in part by USPHS grant DK51384.
Abbreviations
- Carbachol
carbamylcholine chloride
- CL
confidence limits
- 4-DAMP
4-diphenylacetoxy-N-methylpiperidine methiodide
- LH
luteinizing hormone
References
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Brit. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATRA S., ANDERSSON K.-E. Oestrogen-induced changes in muscarinic receptor density and contractile responses in the female rabbit urinary bladder. Acta Physiol. Scand. 1989;137:135–141. doi: 10.1111/j.1748-1716.1989.tb08729.x. [DOI] [PubMed] [Google Scholar]
- BATRA S.C., IOSIF C.S. Progesterone receptors in the female lower urinary tract. J. Urol. 1987;138:1301–1304. doi: 10.1016/s0022-5347(17)43588-9. [DOI] [PubMed] [Google Scholar]
- BRADING A.F., WILLIAMS J.H. Contractile responses of smooth muscle strips from rat and guinea-pig urinary bladder to transmural stimulation: effects of atropine and α,β-methylene ATP. Brit. J. Pharmacol. 1990;99:493–498. doi: 10.1111/j.1476-5381.1990.tb12956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTCHER R.L., COLLINS W.E., FUGO N.W. Plasma concentrations of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- CARDOZO L. Role of estrogens in the treatment of female urinary incontinence. J. Am. Geriatr. Soc. 1990;38:326–328. doi: 10.1111/j.1532-5415.1990.tb03513.x. [DOI] [PubMed] [Google Scholar]
- CAULFIELD M.P. Muscarinic receptors–characterization, coupling and function. Pharmacol. Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- CHUN A.L., WEIN A.J., HARKAWAY R., LEVIN R.M. Comparison of urinary bladder function in sexually mature and immature male and female rats. J. Urol. 1990;143:1267–1271. doi: 10.1016/s0022-5347(17)40252-7. [DOI] [PubMed] [Google Scholar]
- DELANCEY J.O.L., NYGAARD I. Exercise-induced urinary incontinence. JAMA. 1991;265:514. doi: 10.1001/jama.265.4.514. [DOI] [PubMed] [Google Scholar]
- EIKA B., LEVIN R.M., LONGHURST P.A. Modulation of urinary bladder function by sex hormones in streptozocin-diabetic rats. J. Urol. 1994;152:537–543. doi: 10.1016/s0022-5347(17)32789-1. [DOI] [PubMed] [Google Scholar]
- EKSTRÖM J., IOSIF C.S., MALMBERG L. Effects of long-term treatment with estrogen and progesterone on in vitro muscle responses of the female rabbit urinary bladder and urethra to autonomic drugs and nerve stimulation. J. Urol. 1993;150:1284–1288. doi: 10.1016/s0022-5347(17)35761-0. [DOI] [PubMed] [Google Scholar]
- ELLIOTT R.A., CASTLEDEN C.M. Effect of progestogens and oestrogens on the contractile response of rat detrusor muscle to electrical field stimulation. Clin. Sci. 1994;87:337–342. doi: 10.1042/cs0870337. [DOI] [PubMed] [Google Scholar]
- ELLIOTT R.A., CASTLEDEN C.M., MIODRAG A. The effect of in vivo oestrogen pretreatment on the contractile response of rat isolated detrusor muscle. Brit. J. Pharmacol. 1992;107:766–770. doi: 10.1111/j.1476-5381.1992.tb14521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANTL J.A., BUMP R.C., ROBINSON D., MCCLISH D.K., WYMAN J.F., ELSER D.M., FURBERG C.D., LENTZ S.F., MORGAN T.M., SHUMAKER S.A., THEOFRASTOUS J.P. Efficacy of estrogen supplementation in the treatment of urinary incontinence. Obstet. Gynecol. 1996;88:745–749. doi: 10.1016/0029-7844(96)00281-5. [DOI] [PubMed] [Google Scholar]
- FANTL J.A., CARDOZO L., MCCLISH D.K. Estrogen therapy in the management of urinary incontinence in postmenopausal women – a meta-analysis – first report of the hormones and urogenital therapy committee. Obstet. Gynecol. 1994;83:12–18. [PubMed] [Google Scholar]
- FLEMING W.W., WESTFALL D.P., DE LA LANDE I.S., JELLETT L.B. Log-normal distribution of equieffective doses of norepinephrine and acetylcholine in several tissues. J. Pharmacol. exp. Ther. 1972;181:339–345. [PubMed] [Google Scholar]
- GLENNING P.P. Urinary voiding patterns of apparently normal women. Aust. NZ J. Obstet. Gynaecol. 1985;25:62–65. doi: 10.1111/j.1479-828x.1985.tb00606.x. [DOI] [PubMed] [Google Scholar]
- HEGDE S.S., CHOPPIN A., BONHAUS D., BRIAUD S., LOEB M., MOY T.M., LOURY D., EGLEN R.M. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Brit. J. Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOSIF C.S., BATRA S., EK A., ÅSTEDT B. Estrogen receptors in the human female lower urinary tract. Am. J. Obstet. Gynecol. 1981;141:817–820. doi: 10.1016/0002-9378(81)90710-9. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.P. The classification of drugs and drug receptors in isolated tissues. Pharmacol. Rev. 1984;36:165–222. [PubMed] [Google Scholar]
- KITAZAWA T., HAMADA E., KITAZAWA K., GAZNABI A.K.M. Non-genomic mechanism of 17 beta-oestradiol-induced inhibition of contraction in mammalian vascular smooth muscle. J. Physiol. Lond. 1997;499:497–511. doi: 10.1113/jphysiol.1997.sp021944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUIPER G.G.J.M., SHUGHRUE P.J., MERCHENTHALER I., GUSTAFSSON J.A. The estrogen receptor β subtype: A novel mediator of estrogen action in neuroendocrine systems. Front. Neuroendocrin. 1998;19:253–286. doi: 10.1006/frne.1998.0170. [DOI] [PubMed] [Google Scholar]
- LEVIN R.M., LONGHURST P.A.Pharmacological basis of bladder and urethral function in the female Female Urology 2nd edn. 1996Philadelphia, PA: W.B. Saunders Co; 43–56.ed. S. Raz, pp [Google Scholar]
- LONG J.A., EVANS H.M. The Oestrus Cycle in the Rat and its Associated Phenomena. Berkeley: University of California Press; 1922. [Google Scholar]
- LONGHURST P.A., BELIS J.A., O'DONNELL J.P., GALIE J.R., WESTFALL D.P. A study of the atropine-resistant component of the neurogenic response of the rabbit urinary bladder. Eur. J. Pharmacol. 1984;99:295–302. doi: 10.1016/0014-2999(84)90136-5. [DOI] [PubMed] [Google Scholar]
- LONGHURST P.A., EIKA B., LEGGETT R.E., LEVIN R.M. Comparison of urinary bladder function in 6 and 24 month male and female rats. J. Urol. 1992a;148:1615–1620. doi: 10.1016/s0022-5347(17)36981-1. [DOI] [PubMed] [Google Scholar]
- LONGHURST P.A., KAUER J., LEGGETT R.E., LEVIN R.M. The influence of ovariectomy and estradiol replacement on urinary bladder function in rats. J. Urol. 1992b;148:915–919. doi: 10.1016/s0022-5347(17)36777-0. [DOI] [PubMed] [Google Scholar]
- LONGHURST P.A., LEGGETT R.E., BRISCOE J.A.K. Characterization of the functional muscarinic receptors in the rat bladder. Brit. J. Pharmacol. 1995;116:2279–2285. doi: 10.1111/j.1476-5381.1995.tb15065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEDLOCK K.L., FORRESTER T.M., SHEEHAN D.M. Short-term effects of physiological and pharmacological doses of estradiol on estrogen receptor and uterine growth. J. Recept. Res. 1991;11:743–756. doi: 10.3109/10799899109064677. [DOI] [PubMed] [Google Scholar]
- MIODRAG A., CASTLEDEN C.M., VALLANCE T.R. Sex hormones and the female urinary tract. Drugs. 1988;36:491–504. doi: 10.2165/00003495-198836040-00006. [DOI] [PubMed] [Google Scholar]
- SAEZ S., MARTIN P.M. Evidence of estrogen receptors in the trigone area of human urinary bladder. J. Steroid Biochem. 1981;15:317–320. doi: 10.1016/0022-4731(81)90291-0. [DOI] [PubMed] [Google Scholar]
- SALMON U.J., WALTER R.I., GEIST S.H. The use of estrogens in the treatment of dysuria and incontinence in postmenopausal women. Am. J. Obstet. Gynecol. 1941;42:845–851. [Google Scholar]
- SCHREITER F., FUCHS P., STOCKAMP K. Estrogenic sensitivity of α-receptors in the urethra musculature. Urol. Int. 1976;31:13–19. doi: 10.1159/000280026. [DOI] [PubMed] [Google Scholar]
- SHAPIRO E. Effect of estrogens on the weight and muscarinic cholinergic density of the rabbit bladder and urethra. J. Urol. 1986;135:1084–1087. doi: 10.1016/s0022-5347(17)45980-5. [DOI] [PubMed] [Google Scholar]
- SHIMONOVITZ S., MONGA A.K., STANTON S.L. Does the menstrual cycle influence cystometry. Int. Urogynecol. J. 1997;8:213–216. doi: 10.1007/BF02765816. [DOI] [PubMed] [Google Scholar]
- SIBLEY G.N.A. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J. Physiol. 1984;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH M.S., FREEMAN M.E., NEILL J.D. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: Prolactin, gonadotropin and steroid levels associated with the rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- SOMOGYI G.T., DE GROAT W.C. Evidence for inhibitory nicotinic and facilatory muscarinic receptors in cholinergic nerve terminals of the rat urinary bladder. J. Auton. Nerv. Syst. 1992;37:89–98. doi: 10.1016/0165-1838(92)90237-b. [DOI] [PubMed] [Google Scholar]
- STANTON S.L. Female Urinary Incontinence. London: Lloyd-Luke (Medical Books) Ltd; 1977. [Google Scholar]
- STRITTMATTER H.-J., WISCHNIK A., POLLOW K., WEIGEL M., VOGES G., MELCHERT F. Steroid hormone receptors in the female urogenital tract. Int. Urogynecol. J. 1994;5:146–153. [Google Scholar]
- TALLARIDA R.J., MURRAY R.B. Manual of Pharmacologic Calculations 1987New York: Springer- Verlag; 2nd edn [Google Scholar]
- TAMMELA T.L.J., BRISCOE J.A.K., LEVIN R.M., LONGHURST P.A. Factorsunderlying the increased sensitivity to field stimulation of urinary bladder strips from streptozotocin- diabetic rats. Brit. J. Pharmacol. 1994;113:195–203. doi: 10.1111/j.1476-5381.1994.tb16193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONG Y.C., HUNG Y.C., LIN J.S.N., HSU C.T., CHENG J.T. Effects of pregnancy and progesterone on autonomic function in the rat urinary bladder. Pharmacology. 1995;50:192–200. doi: 10.1159/000139282. [DOI] [PubMed] [Google Scholar]
- VAN GEELEN J.M., DOESBURG W.H., THOMAS C.M.G., MARTIN C.B., JR Urodynamic studies in the normal menstrual cycle: the relationship between hormonal changes during the menstrual cycle and the urethral pressure profile. Am. J. Obstet. Gynecol. 1981;141:384–392. doi: 10.1016/0002-9378(81)90599-8. [DOI] [PubMed] [Google Scholar]
- WALL L.L., WARRELL D.W. Detrusor instability associated with menstruation. Case report. Brit. J. Obstet. Gynaecol. 1989;96:737–738. doi: 10.1111/j.1471-0528.1989.tb03294.x. [DOI] [PubMed] [Google Scholar]
- WEIN A.J. Pharmacology of Incontinence. Urol. Clin. North Am. 1995;22:557–577. [PubMed] [Google Scholar]
- WOLF H., WANDT H., JONAT W. Immunohistochemical evidence of estrogen and progesterone receptors in the female lower urinary tract and comparison with the vagina. Gynecol. Obstet. Invest. 1991;32:227–231. doi: 10.1159/000293038. [DOI] [PubMed] [Google Scholar]


