Abstract
Brain capillary endothelial cells express a variety of nucleotide receptors, but differences have been reported between culture models. This study reports examination of nucleotide receptors on primary cultured rat brain capillary endothelial cells (RBCEC) grown on a biological extracellular matrix (ECM) to produce a more differentiated phenotype.
Fura-2 fluorescence ratio imaging was used to monitor intracellular free calcium concentration [Ca2+]i. ATP, UTP, and 2-methylthioATP (2-MeSATP) increased [Ca2+]i to similar levels, while 2-MeSADP, ADP and adenosine gave smaller responses.
Removal of extracellular calcium caused no significant change in the [Ca2+]i response to 2-MeSATP, evidence that the response was mediated by a metabotropic (P2Y) receptor.
All cells tested responded to ATP, UTP, 2-MeSATP and ADP, while 63% responded to adenosine and 50% to 2-MeSADP. No cells responded to α,β-methyleneATP. Cells grown on rat tail collagen instead of ECM gave smaller and less uniform [Ca2+]i responses, suggesting that the differentiating effect of the ECM contributed to a more uniform receptor profile.
The [Ca2+]i response to the P2Y1-selective agonist 2-MeSADP was abolished in the presence of the subtype-selective antagonist adenosine 3′-phosphate 5′-phosphosulphate (PAPS).
The P2Y2 antagonist suramin completely blocked the response to ATP and inhibited the response to UTP by 66%.
The A1 subtype-selective adenosine receptor agonist N6-Cyclopentyladenosine (CPA) gave a small but characteristic [Ca2+]i response, while A2A and A2B subtype-selective agonists failed to generate [Ca2+]i changes.
The results are consistent with the presence on RBCEC of a P2Y2-like receptor coupled to phospholipase C, and a P2Y1-like receptor mobilizing intracellular Ca2+. The role of multiple nucleotide receptors in the function of the brain endothelium is discussed.
Keywords: Blood-brain barrier, endothelium, ATP, purinergic, nucleotide, extracellular matrix
Introduction
The blood-brain barrier is formed by the endothelial cells of cerebral microvessels. The barrier function derives from the extremely tight zonulae occludentes (tight junctions) joining adjacent cells, effectively sealing the paracellular pathway, combined with a number of specific transporter and enzymatic mechanisms that regulate the passage of molecules across the cells (Abbott & Romero, 1996; Tamai & Tsuji, 1996; Grant et al., 1998). Receptors on the brain endothelial cells are involved in the regulation of several aspects of the physiological function of the blood-brain barrier, including release of agents across the luminal and abluminal membranes to control blood clotting and smooth muscle tone respectively (Boarder & Hourani, 1998), regulating the permeability of the tight junctions (Abbott & Revest, 1991; Abbott, 2000), and controlling specific transcellular solute transport (O'Donnell et al., 1995). Differences have been reported between the pharmacology of brain and non-brain endothelium (reviewed in Boarder & Hourani, 1998), consistent with the special functions of the blood-brain barrier.
Studies of the receptors and signal transduction pathways of the brain endothelium in situ are complicated by the presence of other cell types (e.g. neurons, smooth muscle, astrocytes, mast cells), making it difficult to establish the site and mechanisms of action of applied chemical agents (Abbott, 2000). Several in vitro models of brain endothelium have been used including non-passaged primary culture, passaged cells and immortalized cell lines, but variability in the findings suggests that differences in preparative and culture methods exert a significant influence on the receptor phenotype observed. It is thus difficult on the basis of studies on a single preparation to draw conclusions about the situation in vivo.
Purine and pyrimidine receptors are of particular interest in relation to the function of the blood-brain barrier, having been shown not only to regulate the release of prostacyclin (PGI2) and nitric oxide from the brain endothelium (Boarder & Hourani, 1998), but also to control blood-brain barrier permeability (Olesen, 1989). Natural ligands for nucleotide receptors, including ATP, ADP and UTP, can be released from a number of cell types in the region of the vessel wall, such as platelets on the blood side, and smooth muscle cells and neurons on the brain side. Moreover, the endothelium itself can release ATP (Gordon, 1986) able to act on nucleotide receptors on astrocytes and neurons (reviewed in Nobles et al., 1995), so the endothelium is able to act as both target and source of nucleotide signals.
Early studies on unpassaged (P0) primary cultured rat brain endothelial cells showed an elevation in cytoplasmic calcium concentration ([Ca2+]i) on application of purinergic agents, with potency in the order ATP>ADP>AMP, and desensitization to repeated application of ATP, characteristic of P2 receptors (Revest et al., 1991). Subsequent studies on these cells showed that UTP and ATP were equally effective, suggesting presence of a P2U subtype (Nobles et al., 1995). More detailed characterization of the P2U receptor on the immortalized rat brain endothelial cell line RBE4 generated by transfection of second passage (P2) primary cultured rat brain endothelium showed no evidence for a separate receptor sensitive to ADP, but responses to the agonists α,β-methyleneATP (α,β-MeATP) and 2-methylthioATP (2-MeSATP) in a proportion of cells suggested presence of other nucleotide receptors (Nobles et al., 1995).
A series of studies on the B7 clone of passaged rat brain endothelial cells introduced by Vigne et al. (1989) demonstrated two types of metabotropic nucleotide receptors, one (P2U) sensitive to ATP and UTP coupled to phospholipase C (PLC), and an ADP- and 2-MeSATP-sensitive P2Y1-like receptor increasing mobilization of a thapsigargin-sensitive calcium pool, but independent of inositol phosphates (Frelin et al., 1993; Vigne et al., 1994a). These studies were pursued in the B10 clone lacking the P2U/P2Y2 receptor, where ATP was shown to act as a weak antagonist on the P2Y1-like receptor (Feolde et al., 1995; Vigne et al., 1998b).
Boarder and co-workers reported studies on primary cultured (P0 to P10) human brain endothelial cells (Purkiss et al., 1994) in which ATP and UTP gave a small stimulation of PLC but 2-MeSATP, ATPγS and β,γ-methylene-ATP had no effect. Their subsequent studies on primary cultured rat brain endothelial cells (Albert et al., 1997) showed evidence for a P2Y2-like receptor coupled to PLC, Ca2+ and mitogen-activated protein kinase (MAPK) pathways, a P2Y1-like receptor linked to Ca2+ mobilization but independent of inositol phosphates, and a further response to ATP by an unidentified receptor producing a rise in cyclic AMP in the presence of forskolin. They observed some differences in the percentage of cells responding to ATP, ADP, UTP and 2-MeSATP but it was not clear whether this reflected the in vivo condition.
There have been significant recent advances in understanding nucleotide receptors, with the cloning of several members of the P2X (ionotropic) family of ligand-gated channels, and of the P2Y (metabotropic) family of G-protein coupled receptors; however, it has proved difficult to identify the cloned receptor(s) with particular pharmacological phenotypes naturally expressed in tissues (King et al., 1998; Vigne et al., 2000). Thus for the brain endothelium, further pharmacological studies of nucleotide receptors are needed to investigate the phenotype regulating blood-brain barrier physiology.
We have recently found that primary cultured rat brain endothelial cells grown on a biological extracellular matrix (ECM) produced by corneal endothelial cells grow more rapidly and uniformly to confluence, and show a more uniform spindle-shaped cell morphology characteristic of differentiated brain endothelial cells (Dömötör et al., 1998). The cells are sufficiently homogeneous in physiological properties for resolution of the role of the plasmalemmal Na+–Ca2+ exchange transporter in regulating basal [Ca2+]i, and in returning [Ca2+]i to the basal level after elevation in response to a brief extracellular pulse of ATP (Dömötör et al., 1999). This preparation has proved ideal for a more detailed pharmacological characterization and separation of nucleotide receptors on brain endothelial cells.
The results allow clear pharmacological identification and separation of P2Y2-like and P2Y1-like receptors, and in addition reveal a small calcium response to adenosine. The cells grown on the biological ECM showed a greater uniformity of pharmacological profile and larger calcium responses than cells grown on rat tail collagen. This study suggests a role for extracellular matrix constituents in regulating the differentiated pharmacological phenotype of brain endothelial cells, and indicates the presence of multiple types of nucleotide receptor, with distinct functions, on the in vivo blood-brain barrier.
Methods
Cell culture
Procedures for obtaining cell cultures were in accordance with Guidelines for Use of Laboratory Animals of the Semmelweis University, Budapest. Three- to four-month-old Wistar rats (∼300 g) were decapitated. Primary cultures of rat brain microvascular endothelium prepared by the method of Abbott et al. (1992) were grown on glass coverslips coated with bovine corneal endothelial extracellular matrix; for assessment of the effect of ECM, cells were grown for comparison on rat tail collagen (see below). Cells were characterized immunocytochemically as endothelial by detection of factor VIII-related antigen. The culture medium was based on a standard Dulbecco's medium composed of Dulbecco's modified Eagle's medium, 20% plasma-derived adult bovine serum (Sigma), 75 μg ml−1 endothelial cell growth supplement, 100 i.u. ml−1 penicillin / 100 μg ml−1 streptomycin, 2 mM glutamine, 80 μg ml−1 heparin grade I and supplement containing vitamin C, glutathione, insulin, transferrin and selenium. Chemicals were purchased from Sigma Aldrich, Hungary. Cells were used after culturing for 5–9 days, when they were close to confluence.
Preparation of biological extracellular matrix
This procedure was a modification of the method described by Gospodarowicz (1984), as detailed previously (Dömötör et al., 1998). Briefly, fresh bovine eyes kept on ice were obtained from an abattoir; primary cultures of corneal endothelium were prepared, passaged 2–3 times and finally cultured on untreated glass coverslips. After reaching confluence, cells were lysed to expose the extracellular matrix by treating the preparations with 20 mM NH4OH in distilled water. Matrix-coated coverslips were kept under sterile conditions in isotonic salt solution for up to 3 months at 4°C.
Preparation of rat tail collagen coated coverslips
Following sterilization with 70% ethanol and flaming, the glass coverslips were coated with 0.4 mg/ml rat tail collagen as previously described (Abbott et al., 1992). They were fixed in wet ammonia vapour for 10 min, then washed three times in Hanks' Balanced Salt Solution (HBSS) before the addition of cells.
Measurement of [Ca2+]i
To monitor changes in [Ca2+]i, cells on coverslips were incubated for 1 h at 37°C with 6 μM fura-2 AM in Dulbecco's modified Eagle's medium. The coverslips were transferred to a perfusion chamber fitted to the stage of an inverted Nikon Diaphot 200 microscope and superfused with normal HEPES buffer at 37°C for 10 min prior to experiments. All drugs and chemicals at 37°C were introduced close to the cells of interest via a glass multitube system (tip diameter 0.5 mm; perfusion rate constant at 10 μl s−1). [Ca2+]i was measured by digital image fluorescence microscopy (objective, Fluor 40/1.3; Nikon). Excitation wavelengths were 340 and 380 nm, generated by a polychromator illumination system with a resolution of 12 nm (Visitron Sv. GmbH, Pucheim, Germany). Fluorescence emission was monitored at 510 nm. Digital imaging and data analysis were carried out using a 512×512 frame transfer CCD camera (Princeton Instruments) and Metafluor software (Universal Imaging Corp., West Chester, PA, U.S.A). A fluorescence ratio image (340/380 nm) was acquired every 2 s. In each experimental condition fluorescence was monitored in cells from two to three coverslips (20–30 cells in frame). Fluorescence ratios were converted to free [Ca2+]i using the equation described by Grynkiewicz et al. (1985). The maximum (Rmax) and minimum (Rmin) ratio value and the ratio of fluorescence for Ca2+-bound/Ca2+-free dye measured at 380 nm (Sf2/Sb2) were determined using an in vitro calibration method and were corrected for viscosity (Poenie, 1994). Experimental results are expressed as [Ca2+]i or fluorescence ratio as indicated.
Solutions and drugs
Normal HEPES buffer contained (mM): NaCl, 135; KCl, 5.4; CaCl2, 1.8; MgCl2, 1.2; glucose, 10; and HEPES, 20; pH adjusted to 7.4. For calcium-free experiments CaCl2 was omitted and replaced by 1.5 mM EGTA. All chemicals were obtained from Sigma Aldrich, Hungary.
Data analysis and statistics
Data analysis was performed with Sigmaplot 4.0 Software. Results are given as means±s.e.mean. Statistical significance was evaluated by Student's t-test and differences with P<0.05 were taken as significant.
Results
Extracellular ATP-induced [Ca2+]i elevation
The present study used brief (8 s) pulses of ATP which we have previously shown caused a [Ca2+]i transient resulting from mobilization of Ca2+ from intracellular stores, with no contribution from influx of extracellular Ca2+ (Dömötör et al., 1999). The first pulse of 100 μM ATP increased [Ca2+]i from the basal level of 166.5±3.6 nM (n=30) to a peak of 791.2±27.7 nM (n=30). Two further applications of ATP at 70 s intervals gave progressively smaller Ca2+ transients, respectively 68% and 53% of the initial response (n=30), showing the desensitization characteristic of nucleotide receptors (Barnard et al., 1996) as previously observed in primary cultured and immortalized rat brain endothelial cells (Revest et al., 1991; Nobles et al., 1995).
The effect of nucleotide agonists on [Ca2+]i
Agents used for this investigation included nucleotide analogues chosen to distinguish between P2Y receptors (agonist potency in natural tissues 2-MeSATP>ATPγS>ATP⩾ADP>>α,β-MeATP, UTP) and P2U receptors (UTP=ATP⩾ATPγS>>ADP, 2-MeSATP, α,β-MeATP), and other agents that have proved valuable in identifying cloned P2Y-like proteins: P2Y1 (2-MeSATP>ADP) and the two main candidates for the P2U phenotypic receptor, P2Y2 (ATP=UTP, suramin-sensitive) and P2Y4 (UTP⩾ATP, suramin insensitive) (King et al., 1998). Some agents need to be used with caution: thus α,β-MeATP has been used as a stereotypic P2X receptor agonist, but it acts on only some P2X subfamilies (Fredholm et al., 1997), and is also a potent agonist of a metabotropic receptor in rat astrocytes (Abbracchio et al., 1999). 2-MeSATP is an agonist at rat P2Y1 receptors (Filippov et al., 2000), but it also acts at some P2X receptor subtypes (Fredholm et al., 1997).
To characterize the nucleotide receptor subtypes present on the surface of primary RBCE cells we challenged the cells with 8 s pulses of ATP and a number of other agonists and measured [Ca2+]i transients. All drugs were applied at 100 μM concentration and the results are shown as changes in the fluorescence ratio (ΔFR) in Figure 1a. The [Ca2+]i transient in response to UTP was slightly larger than that produced by ATP (ΔFR 0.82±0.017 and 0.74±0.018 respectively, n=140, P<0.05). The response to 2-MeSATP was not significantly different from that to ATP (ΔFR 0.69±0.04, n=90) but the response to ADP (ΔFR 0.39±0.04, n=60) was significantly smaller (P<0.05). The potent P2Y1 receptor agonist 2-MeSADP gave a smaller response than ATP, but not significantly different from the response to ADP (ΔFR=0.36±0.02, n=50). Since ADP does not act on P2Y2 receptors (Brown et al., 1995), the ADP-induced [Ca2+]i elevation in our system is further evidence for activation of a P2Y1-like receptor. α,β-MeATP did not cause [Ca2+]i elevation in the RBCE cells (data not shown), while 100 μM adenosine gave a small but characteristic [Ca2+]i response (ΔFR 0.19±0.05, n=50) which was significantly smaller than the ATP-induced [Ca2+]i elevation (P<0.05).
Figure 1.
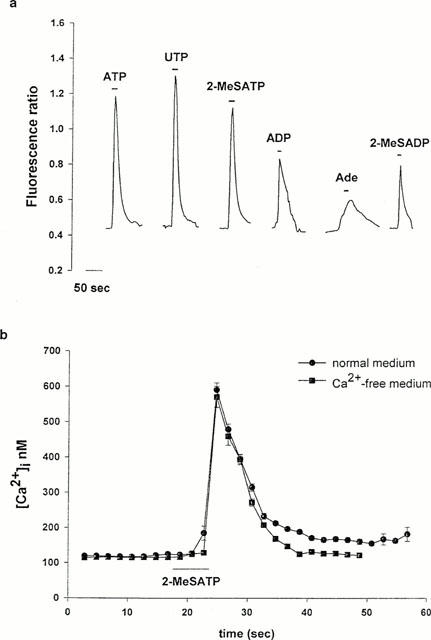
[Ca2+]i response of RBCEC cells to nucleotide receptor agonists. (a) Representative traces of [Ca2+]i changes detected in RBCE cells on stimulation with different agonists, expressed as fluorescence ratio. Pulses of ATP (n=140), UTP (n=147), 2-MeSATP (n=90), 2-MeSADP (n=50), ADP (n=60) or adenosine (Ade) (n=50) were applied at 100 μM and for 8 s as indicated. For each agonist, a fresh coverslip was used. (b) The change in [Ca2+]i caused by 2-MeSATP (100 μM, 8 s) in Ca2+-free medium (442±27.5 nM, n=36) was not significantly different from the response in normal medium (454±27.2 nM, n=37).
All cells tested responded to ATP, UTP, 2-MeSATP and ADP, while adenosine and 2-MeSADP caused an increase in [Ca2+]i in 63% and 50% of the cells, respectively. To confirm that the 2-MeSATP-induced [Ca2+]i response was due to metabotropic and not ionotropic receptor activation, we repeated the experiment in calcium-free medium (Figure 1b). The absence of external calcium did not affect the 2-MeSATP-evoked [Ca2+]i transient; in calcium-containing medium the increase in [Ca2+]i was 454±27.2 nM (n=37), while in calcium-free medium it was 442±27.56 nM (n=36). These results indicate a relatively uniform receptor profile within the population of RBCE cells grown on the biological matrix, and suggest that under these conditions these primary endothelial cells express at least three different nucleotide receptor subtypes: P2U-like, P2Y1-like, and an adenosine receptor.
Figure 2 shows the peak [Ca2+]i signals as a function of the concentration of agonists; ATP, UTP and 2-MeSATP were added in the concentration range 1-1000 μM. The maximal [Ca2+]i responses were reached at 100 μM agonist concentration. The results with ATP and UTP are similar to those reported for RBE4 cells (Nobles et al., 1995); concentration-response curves for 2-MeSATP have not been previously reported for primary brain endothelial cell preparations.
Figure 2.
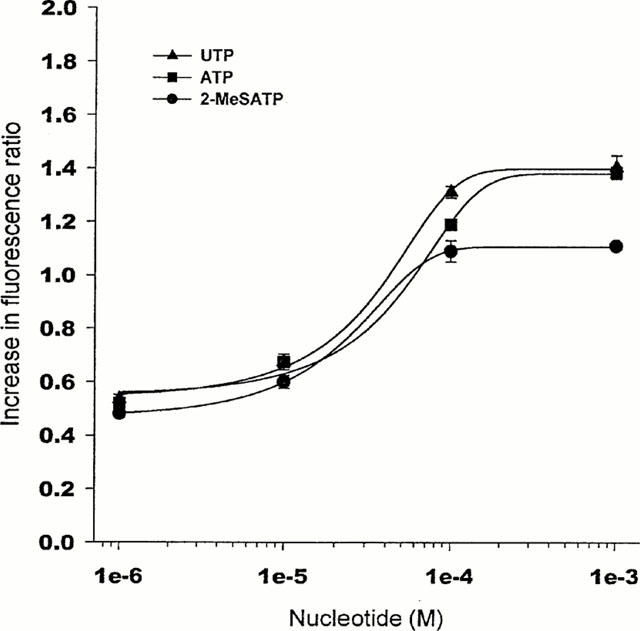
[Ca2+]i response (fluorescence ratio) as a function of agonist concentration. Endothelial cells were simulated with ATP, UTP or 2-MeSATP for 8 s, and the fluorescence ratio measured at the peak of the [Ca2+]i signal. Curves were fitted with the Hill equation, with no parameters fixed. Results are mean±s.e.mean obtained in three different experiments, n>50 number of cells. s.e.mean is not shown where it is smaller than the symbol.
Co-existence of P2Y2 and P2Y1-like purinoreceptors on primary brain endothelial cells
In order to investigate whether ATP and UTP are acting on the same receptors, the Fura 2-loaded cells were challenged with ATP (100 μM) and when the ATP-induced [Ca2+]i signal had returned approximately to the baseline, cells were stimulated with UTP (100 μM) in the continuing presence of ATP. UTP caused a negligible [Ca2+]i elevation under these conditions (Figure 3a). By contrast, the response to 2-MeSATP (100 μM) was not affected by the continuing presence of ATP (Figure 3b). When ATP and UTP were applied together (100 μM each, 10 s, n=40) the [Ca2+]i increase was not significantly different from that caused by ATP (100 μM, 10 s, n=140) or UTP (100 μM, 10 s, n=50) alone, however when ATP was applied together with 2-MeSATP (100 μM each, 10 s, n=50) [Ca2+]i increased to a significantly higher level than that measured after a challenge with ATP alone (P<0.05; Figure 4). These results are further evidence that both P2U-like and P2Y1-like receptors are present on these primary cultured brain capillary endothelial cells.
Figure 3.
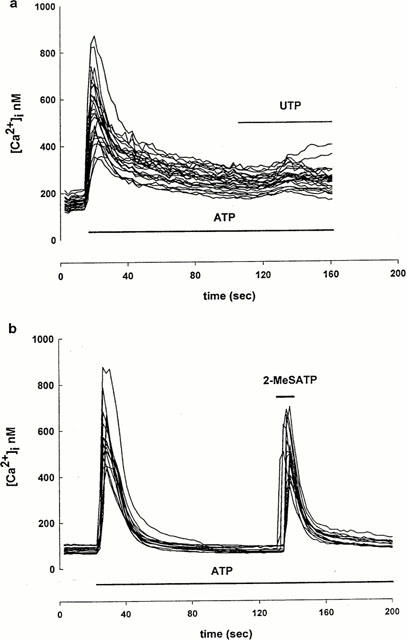
Cross-desensitization between nucleotide agonists. (a) ATP (100 μM) was tested first and when the [Ca2+]i levels returned approximately to the baseline, UTP (a) or 2-MeSATP (b) was added in the continuing presence of 100 μM ATP (n=25 for a; n=20 for b).
Figure 4.
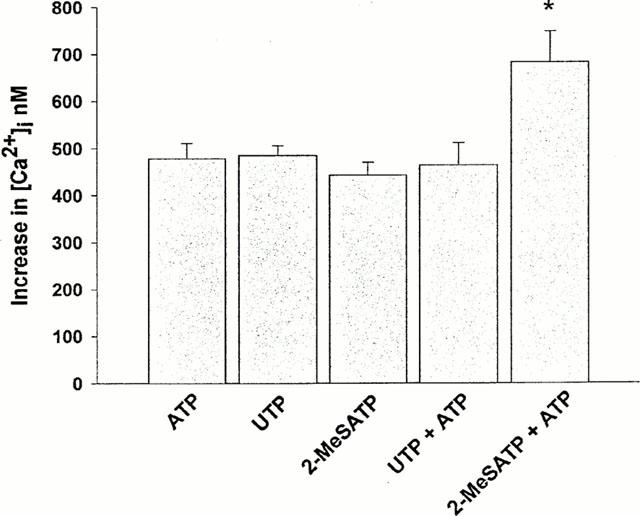
Co-existence of P2U-like and P2Y1-like receptors in primary brain capillary endothelial cells. Differences between the agonist-evoked peak and resting [Ca2+]i levels are shown. ATP (100 μM, 10 s, n=140), UTP (100 μM, 10 s, n=50) and 2-MeSATP (100 μM, 10 s, n=90) alone, or a mixture of ATP/UTP (100 μM for each nucleotide, 10 s, n=40) or a mixture of ATP/2-MeSATP (100 μM for each nucleotide, 10 s, n=50) were applied as indicated. Data are means±s.e.mean *P<0.05, significantly different as compared with response to ATP or 2-MeSATP alone.
We used a further pharmacological manœuvre to check the expression of a P2Y1-like receptor subtype on the rat RBCE cells. Using the subtype-selective antagonist adenosine 3′-phosphate 5′-phosphosulphate (PAPS, Bültmann et al., 1998), we challenged the cells with 2-MeSADP (Figure 5a,b). In the presence of PAPS (5 μM), 2-MeSADP (100 μM, 8 sec, n=37) failed to increase [Ca2+]i (Figure 5a,b), confirming the presence of a P2Y1-like receptor.
Figure 5.
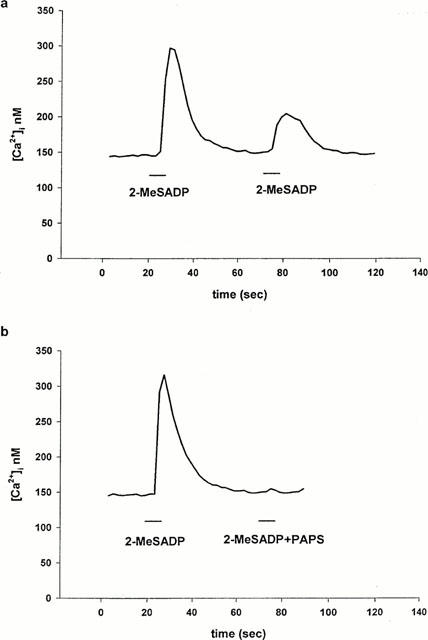
Effect of P2Y1 receptor agonist and antagonist on [Ca2+]i in RBCE cells. (a) Response of RBCE cells (n=37) to 100 μM 2-MeSADP repeated at intervals of 50 s. (b) 2-MeSADP (100 μM) was first tested alone, then 50 s later cells were challenged with 100 μM 2-MeSADP together with 5 μM PAPS (n=35). 2-MeSADP was applied for 10 s as indicated; each trace is representative of two separate experiments.
Pharmacological differentiation between rat P2Y2 and P2Y4 receptors
The cloned rat P2Y2 and P2Y4 receptors are both equally sensitive to ATP and UTP (King et al., 1998). Suramin, a selective antagonist of P2Y2-purinoceptors (King et al., 1998; Bogdanov et al., 1998) was tested on the [Ca2+]i elevation evoked by ATP (Figure 6a). After a first challenge with 100 μM ATP for 10 s, the cells were perfused with medium containing 100 μM suramin for 100 s, then ATP (100 μM, 10 s) was applied again in the presence of the antagonist. As shown in Figure 6a, ATP failed to increase [Ca2+]i under these conditions. When perfusion was switched back to a normal medium lacking suramin, the [Ca2+]i response to ATP (100 μM, 10 s) partially recovered. For control cells we used fragments of the same coverslips broken prior to the experiments, and cells were challenged with ATP under identical conditions but in the absence of suramin. The inset in Figure 6a shows that the third ATP-induced Ca2+-peak was not significantly different from the third control response, but suramin completely blocked the ATP-induced [Ca2+]i elevation. 100 μM suramin inhibited the [Ca2+]i elevation evoked by UTP (100 μM) by 66% (n=38, P<0.05, Figure 6b and inset). The inhibitory effect of suramin on the ATP-induced [Ca2+]i elevation was more pronounced in the present study than in previous studies on the RBE4 brain endothelial cell line (Nobles et al., 1995).
Figure 6.
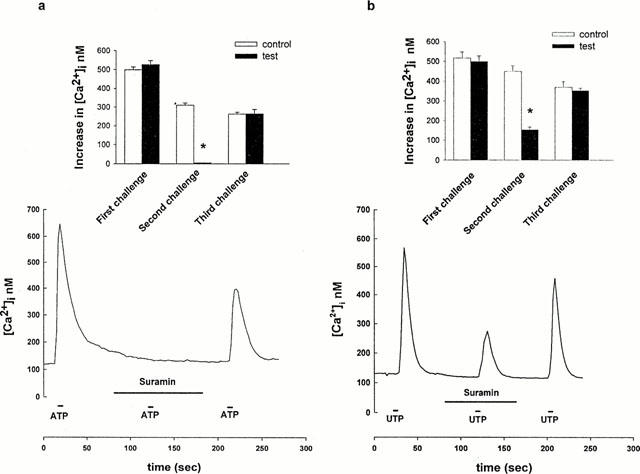
Effect of Suramin on the ATP- and UTP-evoked [Ca2+]i increase. (a) ATP or (b) UTP (100 μM, 10 s, respectively) was first tested alone, then the agonist was added again in the presence of Suramin (100 μM, 100 s). After removing the antagonist, (a) ATP (100 μM, 10 s) or (b) UTP (100 μM, 10 s) was applied again. Each trace is representative of 40–50 cells from three separate experiments. The insets show average changes in [Ca2+]i (difference between peak and resting [Ca2+]i level) for controls and for experiments with ATP(a) or UTP(b). Means±s.e.mean are shown, * significantly different from the respective controls in the absence of the antagonist (P<0.05).
Adenosine receptor characterization
To investigate which adenosine receptor subtype is present on RBCEC we challenged the cells with subtype-specific adenosine receptor agonists. 2-p-(2-Carboxyethyl) phenethylamino-5′-N-ethylcarboxamidoadenosine (CGS-21680), the A2A receptor specific agonist (Poulsen & Quinn, 1998) failed to induce [Ca2+]i changes at 100 nM concentration (data not shown). N-ethylcarboxamidoadenosine (NECA), the A2B receptor agonist (Dubey et al., 2000) at 50 nM concentration also caused no change in [Ca2+]i (data not shown). The A1 receptor agonist N6 - Cyclopentyladenosine (CPA) at 100 nM concentration caused a ∼100 nM change in [Ca2+]i in 40% of the cells tested (n=37, Figure 7). This [Ca2+]i elevation is about 25% of the ATP-induced [Ca2+]i change, which is similar to the response to adenosine (Figure 1a).
Figure 7.
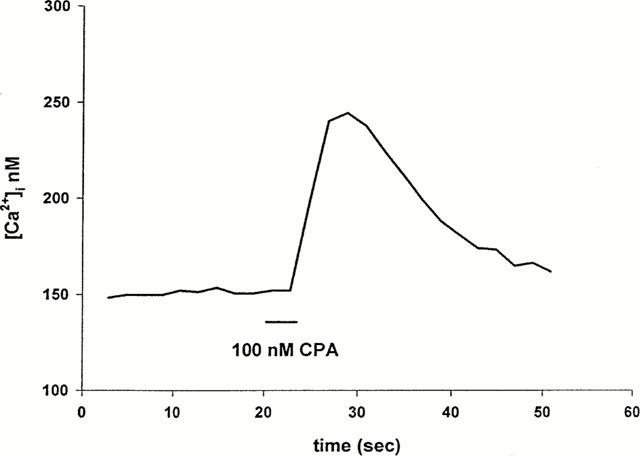
Activation of A1 type adenosine receptor. Fifty per cent of the RBCE cells tested (n=38) responded to the selective A1 agonist CPA (100 nM, 10 s), while other subtype selective agonists (NECA, CGS 21680) failed to increase [Ca2+]i.
Comparison with RBCE cells grown on rat tail collagen
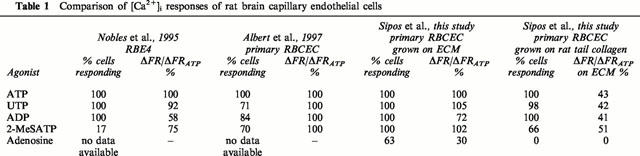
Growth of cells on ECM appeared to give more uniform responses within the endothelial cell population (percentage cells responding to each agonist) than previously observed with the RBE4 cell line (Nobles et al., 1995) or with primary cultured rat brain endothelial cells grown on collagen (Albert et al., 1997). In order to test whether the difference could be due to the differentiating effect of ECM, selected agonists were tested on RBCE cells grown on rat tail collagen instead of ECM (Table 1). Cells grown on rat tail collagen gave smaller responses than those grown on ECM, moreover, only 66% responded to 2-MeSATP, and none responded to adenosine (compared with 100 and 63% respectively from cells grown on ECM).
Table 1.
Comparison of [Ca2+]i responses of rat brain capillary endothelial cells
Discussion
A number of different experimental models have been used to examine the nucleotide sensitivity of endothelial cells, both in vivo and in vitro. They include the isolated mesenteric arterial bed of the rat (Ralevic & Burnstock, 1996), the in situ mesenteric microvessels of the frog (He et al., 1996), human placental cotyledons (Ralevic et al., 1997), and well-studied endothelial culture preparations from pulmonary artery, HUVEC and adrenal microvessel. Preparations of brain endothelium investigated with nucleotides include in situ pial microvessels of the frog and rat (Olesen, 1989), isolated and cannulated penetrating arterioles of the brain surface (Janigro et al., 1996), and cell cultures using primary cells, clonal populations of passaged cells, and immortalized cell lines (Albert et al., 1997; Vigne et al., 1994a; Nobles et al., 1995). The studies in primary cultures have used cells between P0 to P10.
The present demonstration of P2U/P2Y2 receptors on primary cultured rat brain endothelial cells agrees with the findings of Nobles et al. (1995) and Albert et al. (1997), suggesting that these cells when isolated and grown in similar ways show reproducible expression of this receptor. However, although Boarder and co-workers observed Ca2+ responses to 2-MeSATP suggesting presence of a P2Y1-like receptor in primary cultured rat brain endothelial cells (Albert et al., 1997), they found no detectable P2Y1 mRNA using RT–PCR (Anwar et al., 1999). Vigne et al. (2000) provided functional evidence for P2Y1 receptors on B7 and B10 clones of passaged rat brain endothelial cells. The present observation of additive responses to 2-MeSATP and ATP, the response to 2-MeSADP and inhibition of the response to 2-MeSADP by PAPS, provide clear pharmacological evidence for a P2Y1-like receptor, which is separate from the P2Y2-like receptor. It remains to be established whether this corresponds to the cloned P2Y1 receptor, or represents a novel variant.
The unpassaged primary brain endothelial cultures are derived from freshly isolated microvessels, and are hence likely to reflect rather closely the in situ condition. The present study shows that these cells grown on ECM gave greater and more uniform agonist-induced Ca2+-response than cells grown on a collagen-coated surface (Table 1); the responses were also more uniform than those reported by Albert et al. (1997) from primary cells grown on collagen (Table 1). The introduction of a biological ECM as growth substrate in the present study followed evidence that ECM components can improve the expression of a number of differentiated properties in cell culture (Lin & Bissell, 1993). Specific blood-brain barrier characteristics that are upregulated by ECM include tight junctions (Arthur et al., 1987; Tilling et al., 1998; Hoheisel et al., 1998), P-glycoprotein (Tatsuta et al., 1994) and enzymes including alkaline phosphatase and γ-glutamyl transpeptidase (Mischeck et al., 1989; Mizuguchi et al., 1994; 1997). As far as we have been able to establish, the present study is the first to show that the differentiation-promoting function of ECM extends to pharmacological phenotype.
In comparison with cells grown on the biological ECM, cells grown on a collagen-coated surface appeared not to express adenosine-sensitive receptors, and a smaller percentage of cells responded to 2-MeSATP. Furthermore, the agonist-induced [Ca2+]i changes were significantly smaller in cells grown on collagen (Table 1), possibly due to a lower receptor density or altered signal transduction mechanisms. The differences suggest that the complex ECM generated by growing cells acts as a more complete source of local differentiating signals for the brain endothelial cells than simpler ECM preparations such as rat tail collagen or purified Type I collagen (see also Tagami et al., 1992).
Co-existence of P2Y and P2U purinoceptors has been reported on primary bovine aortic endothelial cells (Brown et al., 1995), bovine pulmonary endothelium (Chen et al., 1996) and in primary endothelial cells from adrenal medulla (Mateo et al., 1996). The last study showed that the P2Y purinoceptor activity in primary adrenomedullary endothelial cells was lost after a single passage. Vigne and co-workers showed that both P2U- and P2Y1-like receptors were present in the B7 clone of RBEC (Frelin et al., 1993; Vigne et al. 1994a). However, those studies used assays monitoring the behaviour of populations of cells, so gave no information on the percentage of cells expressing a particular receptor type. As the B10 clone of RBEC lacks P2U receptors, it was used for further study of the unusual P2Y1-like receptors which appeared to couple to mobilization of intracellular Ca2+ and to inhibition of adenylate cyclase (Feolde et al., 1995; Webb et al., 1996; Vigne et al., 1998a). However, the latter effect was not observed in primary cultured RBEC (Albert et al., 1997).
As shown in Table 1, the response to ATP and UTP of the RBCEC is similar to that previously reported for the RBE4 cell line (Nobles et al., 1995), suggesting similar expression of P2U receptors, but a smaller percentage of RBE4 cells responded to 2-MeSATP, likely to indicate lower expression of a P2Y1-like receptor. Forty per cent of the RBE4 cells also responded to α,β-MeATP, while none of the primary cells responded; Albert et al. (1997) showed that all first passage (P1) RBCEC responded to α,β-MeATP, while unpassaged (P0) cells did not. This comparison suggests that some of the RBE4 cells were expressing the phenotype of early passaged cells.
Adenosine caused a Ca2+-response in RBCEC. ATP is readily converted to ADP, AMP and adenosine in vivo and in vitro through a sequential reaction catalyzed by ecto-ATPases and 5′ nucleotidase (Marcus et al., 1997). However, it is likely that the short application of the drugs (8–10 s) and the large volume of the diluting solution mean that breakdown of ATP or ADP to adenosine made no contribution to the agonist-induced [Ca2+]i signal under our experimental conditions (see also Vigne et al. (1998b)). The small [Ca2+]i rise in response to adenosine could have resulted from activation of the P2Y1-like receptor, but the fact that the A1-selective agonist gave a similar sized calcium response suggests that both adenosine and CPA exerted their actions via an A1 adenosine receptor.
The presence of several types of nucleotide receptors on brain endothelial cells supports the hypothesis that the receptors are designed to respond differently to the natural ligands in vivo. P2Y1 and P2Y2 receptors appear to exert their effector actions (such as production and release of PGI2 and NO) by overlapping pathways, including the calcium-mediated activation of endothelial nitric oxide synthase (ecNOS, NO) and activation of MAPK and phospholipase A2 (PGI2). However, the different G proteins involved allow for subtle differences in feedback control and modulation (Boarder > Hourani, 1998). Reports of an atypical nucleotide receptor coupled to inhibition of adenylate cyclase (Webb et al., 1996; see also Vigne et al., 1998a,1998b), and an unidentified ATP receptor activating adenylate cyclase (Albert et al., 1997), raise the possibility of additional and even ‘brain-specific' signal transduction pathways. They would also be expected to contribute to the growing evidence for ‘cross-talk' between second messenger signalling systems in the brain endothelium (Vigne et al., 1994b, Nobles & Abbott, 1998). The identification of brain endothelial specific receptors/transduction pathways capable of regulating tight junctional permeability, acting through calcium, cyclic AMP and tyrosine kinase/MAPK cascades would prepare the way for targeted therapies, such as deliberate blood-brain barrier opening for drug delivery, and blood-brain barrier tightening to reverse the permeability increase associated with some neuropathologies including inflammatory conditions, hypoxia and ischaemia (Abbruscato & Davis, 1999; Abbott, 2000).
Acknowledgments
We thank Katalin Takács for her excellent technical assistance. This work was supported by grants from OTKA, ETT, MTA, MKM to V. Adam-Vizi and a joint British Council Hungarian Government exchange grant to N.J. Abbott and V. Adam-Vizi.
Abbreviations
- α, β-MeATP
α,β-methyleneATP
- CGS-21680
2-p-(2-carboxyethyl) phenethylamino-5′-N-ethylcarboxamido adenosine
- CPA
N6-cyclopentyladenosine
- 2-MeSADP
2-methylthio ADP
- 2-MeSATP
2-methylthio ATP
- NECA
N ethylcarboxamidoadenosine
- PAPS
adenosine 3′-phosphate 5′-phosphosulphate
References
- ABBOTT N.J. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol. Neurobiology. 2000;20:131–147. doi: 10.1023/a:1007074420772. [DOI] [PubMed] [Google Scholar]
- ABBOTT N.J., HUGHES C.C.W., REVEST P.A., GREENWOOD J. Development and characterization of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J. Cell Sci. 1992;103:23–38. doi: 10.1242/jcs.103.1.23. [DOI] [PubMed] [Google Scholar]
- ABBOTT N.J., REVEST P.A. Control of brain endothelial permeability. Cerebr. Brain Metab. Rev. 1991;3:39–72. [PubMed] [Google Scholar]
- ABBOTT N.J., ROMERO I.A. Transporting therapeutics across the blood-brain barrier. Mol. Med. Today. 1996;2:106–113. doi: 10.1016/1357-4310(96)88720-x. [DOI] [PubMed] [Google Scholar]
- ABBRACCHIO M.P., BRAMBILLA R., CERUTI S., CATTABENI I. Signalling mechanism involved in P2Y receptor-mediated reactive astrogliosis. Prog. Brain Res. 1999;120:333–342. doi: 10.1016/s0079-6123(08)63567-0. [DOI] [PubMed] [Google Scholar]
- ABBRUSCATO T.J., DAVIS T.P. Combination of hypoxia/aglycemia compromises in vitro blood-brain barrier integrity. J. Pharmacol. Exp. Ther. 1999;289:668–675. [PubMed] [Google Scholar]
- ALBERT J.L., BOYLE J.P., ROBERTS J.A., CHALLISS R.A.J., GUBBY S.E., BOARDER M.R. Regulation of brain capillary endothelial cells by P2Y receptors coupled to Ca2+, phospholipase C and mitogen-activated protein kinase. Br. J. Pharmacol. 1997;122:935–941. doi: 10.1038/sj.bjp.0701453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANWAR Z., ALBERT J.L., GUBBY S.E., BOYLE J.P., ROBERTS J.A., WEBB T.E., BOARDER M.R. Regulation of cyclic AMP by extracellular ATP in cultured brain capillary endothelial cells. Br. J. Pharmacol. 1999;128:465–471. doi: 10.1038/sj.bjp.0702792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARTHUR F.E., SHIVERS R.R., BOWMAN P.D. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Developmental Brain. Res. 1987;36:155–159. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- BARNARD E.A., WEBB T.E., SIMON J., KANAPULI S.P. Ciba Foundation Symposium 198. Chichester: Wiley; 1996. P2 purinoceptors, localization and transduction mechanism; pp. 166–188. [DOI] [PubMed] [Google Scholar]
- BOARDER M.R., HOURANI S.M.O. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. TiPS. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BOGDANOV Y.D., WILDMAN S.S., CLEMENTS M.P., KING B.F., BURNSTOCK G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br. J. Pharmacol. 1998;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN C., TANNA B., BOARDER M.R. PPADS: an antagonist at endothelial P2Y-purinoceptors but not P2U-purinoceptors. Br. J. Pharmacol. 1995;116:2413–2416. doi: 10.1111/j.1476-5381.1995.tb15088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÜLTMANN R., TULUC F., STARKE K. On the suitability of adenosine 3′-phosphate 5′- phosphosulphate as a selective P2Y receptor antagonist in intact tissues. Eur. J. Pharmacol. 1998;351:209–215. doi: 10.1016/s0014-2999(98)00309-4. [DOI] [PubMed] [Google Scholar]
- CHEN B.C., LEE C.-M., LIN W.-W. Inhibition of ecto-ATPase by PPADS, suramin and reactive blue in endothelial cells, C6-glioma cells and RAW 267.7 macrophages. Br. J. Pharmacol. 1996;119:1628–1634. doi: 10.1111/j.1476-5381.1996.tb16082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DÖMÖTÖR E., ABBOTT N.J., ADAM-VIZI V. Na+ -Ca2+ exchange and its implications for calcium homeostasis in primary cultured rat brain microvascular endothelial cells. J. Physiol. (Lond.) 1999;515:147–155. doi: 10.1111/j.1469-7793.1999.147ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DÖMÖTÖR E., SIPOS I., KITTEL A., ABBOTT N.J., ADAM-VIZI V. Improved growth of cultured brain microvascular endothelial cells on glass coated with a biological matrix. Neurochem. Int. 1998;33:473–478. doi: 10.1016/s0197-0186(98)00057-6. [DOI] [PubMed] [Google Scholar]
- DUBEY R.K., GILLESPIE D.G., SHUE H., JACKSON E.K. A2B receptors mediate antimitogenesis in vascular smooth muscle cells. Hypertension. 2000;35:267–272. doi: 10.1161/01.hyp.35.1.267. [DOI] [PubMed] [Google Scholar]
- FEOLDE E., VIGNE P., BREITTMAYER J.P., FRELIN C. ATP, a partial agonist of atypical P2Y purinoceptors in rat brain microvascular endothelial cells. Br. J. Pharmacol. 1995;121:1121–1126. doi: 10.1111/j.1476-5381.1995.tb15025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILIPPOV A.K., BROWN D.A., BARNARD E.A. The P2Y1 receptor closes the N-type 2+ channel in neurones, with both adenosine triphosphates and diphosphates as potent agonist. Br. J. Pharmacol. 2000;129:1063–1066. doi: 10.1038/sj.bjp.0703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DUBYAK G.R., HARDEN T.K., JACOBSON K.A., SCHWABE U., WILLIAMS M. Towards a revised nomenclature for P1 and P2 receptors. TiPS. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRELIN C., BREITTMAYER J.P., VIGNE P. ADP induces inositol phosphate-independent intracellular [Ca2+] mobilization in brain capillary endothelial cells. J. Biol. Chem. 1993;268:8787–8792. [PubMed] [Google Scholar]
- GORDON J.L. Extracellular ATP: effects, sources and fate. Biochem. J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSPODAROWICZ D.Preparation of extracellular matrices produced by cultured bovine corneal endothelial cells and PF-HR-9 endodermal cells: their use in cell culture Methods for Preparation of Media Supplements and Substrata for Serum-free Animal Cell Culture 1984New York: Alan R. Liss; 275–293.ed. Barnes, D.A., pp [Google Scholar]
- GRANT G.A., ABBOTT N.J., JANIGRO D. Understanding the physiology of the blood-brain barrier: in vitro models. News Physiol. Sci. 1998;13:287–293. doi: 10.1152/physiologyonline.1998.13.6.287. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of calcium indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HE P., ZHANG X., CURRY F.E. Ca2+-entry through conductive pathway modulates receptor-mediated increase in microvessel permeability. Am. J. Physiol. 1996;271:H2377–H2387. doi: 10.1152/ajpheart.1996.271.6.H2377. [DOI] [PubMed] [Google Scholar]
- HOHEISEL D., NITZ T., FRANKE H., WEGENER J., HAKVOORT A., TILLING T., GALLA H.J. Hydrocortisone reinforces the blood-brain barrier properties in a serum free culture system. Biochem. Biophys. Res. Com. 1998;244:312–316. doi: 10.1006/bbrc.1997.8051. [DOI] [PubMed] [Google Scholar]
- JANIGRO D., NGUYEN T.S., GORDON E.L., WINN H.R. Physiological properties of ATP-activated cation channel in rat brain microvascular endothelial cells. Am. J. Phys. 1996;270:H1423–H1434. doi: 10.1152/ajpheart.1996.270.4.H1423. [DOI] [PubMed] [Google Scholar]
- KING B.F., TOWNSEND-NICHOLSON A., BURNSTOCK G. Metabotropic receptors for ATP and UTP: exploring the correspondence between native and recombinant nucleotide receptors. TiPS. 1998;19:506–514. doi: 10.1016/s0165-6147(98)01271-1. [DOI] [PubMed] [Google Scholar]
- LIN C.Q., BISSELL M.J. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;9:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- MARCUS A.J., BROEKMAN M.J., DROSOPOULOS J.H.F., ISLAM N., ALYONYCHEVA T.N., SAFIER L.B., HAJJAR K.A., POSNETT D.N., SCHOENEBORN M.A., SCHOOLEY K.A., GAYLE R.B., MALISZEWSKI C.R. The endothelial cell ecto-ATPase responsible for inhibition of platelet function in CD39. J. Clin. Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATEO J., MIRAS-PORTUGAL M.T., CASTRO E. Co-existence of P2Y- and PPADS-insensitive P2U-purinoceptors in endothelial cells from adrenal medulla. Br. J. Pharmacol. 1996;119:1223–1232. doi: 10.1111/j.1476-5381.1996.tb16026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISCHECK U., MEYER J., GALLA H.J. Characterization of γ-glutamyl transpeptidase activity of cultured endothelial cells from porcine brain capillaries. Cell Tissue Res. 1989;256:221–226. doi: 10.1007/BF00224737. [DOI] [PubMed] [Google Scholar]
- MIZUGUCHI H., HASHIOKA Y., FUYII A., UTOGUCHI N., KUBO K., NAGAKAWA S., BABA A., MAYUMI T. Glial extracellular matrix modulates γ-glutamyl transpeptidase activity in cultured bovine brain capillary and bovine aortic endothelial cells. Brain Res. 1994;651:155–159. doi: 10.1016/0006-8993(94)90692-0. [DOI] [PubMed] [Google Scholar]
- MIZUGUCHI H., UTOGUCHI N., MAYUMI T. Preparation of glial extracellular matrix: a novel method to analyze glial-endothelial cell interaction. Brain Res. Prot. 1997;1:339–343. doi: 10.1016/s1385-299x(97)00008-1. [DOI] [PubMed] [Google Scholar]
- NOBLES M., ABBOTT N.J. Modulation of the effects of extracellular ATP on [Ca2+]I in rat brain microvascular endothelial cells. Eur. J. Pharmacol. 1998;361:119–127. doi: 10.1016/s0014-2999(98)00671-2. [DOI] [PubMed] [Google Scholar]
- NOBLES M., REVEST P.A., COURAUD P.O., ABBOTT N.J. Characteristics of nucleotide receptors that cause elevation of cytoplasmic calcium in immortalized rat brain endothelial cells (RBE4) and in primary cultures. Br. J. Pharmacol. 1995;115:1245–1252. doi: 10.1111/j.1476-5381.1995.tb15032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'DONNELL M.E., MARTINEZ M., SUN D. Cerebral microvascular endothelial cell Na-K-Cl co-transport: regulation by astrocyte-conditioned medium. Am. J. Physiol. 1995;268:C747–C754. doi: 10.1152/ajpcell.1995.268.3.C747. [DOI] [PubMed] [Google Scholar]
- OLESEN S.-P. An electrophysiological study of microvascular permeability and its modulation by chemical mediators. Acta Physiol. Scand. 1989;136:S1–S28. [PubMed] [Google Scholar]
- POENIE M. Alteration of intracellular fura-2 fluorescence by viscosity: a simple correction. Cell Calcium. 1994;11:85–91. doi: 10.1016/0143-4160(90)90062-y. [DOI] [PubMed] [Google Scholar]
- POULSEN S.A., QUINN R.J. Adenosine receptors: new opportunities for future drugs. Bioorg. Med. Chem. 1998;6:619–641. doi: 10.1016/s0968-0896(98)00038-8. [DOI] [PubMed] [Google Scholar]
- PURKISS J.R., WEST D., WILKES L.C., SCOTT C., YARROW P., WILKINSON G.F., BOARDER M.R. Stimulation of phospholipase C in cultured microvascular endothelial cells from human frontal lobe by histamine, endothelin and purinoreceptor agonists. Br. J. Pharmacol. 1994;111:1041–1046. doi: 10.1111/j.1476-5381.1994.tb14849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Discrimination by PPADS between endothelial P2Y- and P2U-purinoceptors in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 1996;118:428–434. doi: 10.1111/j.1476-5381.1996.tb15420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURRELL S., KINGDOM J., BURNSTOCK G. Characterization of P2 receptors for purine and pyrimidine nucleotides in human placental cotyledons. Br. J. Pharmacol. 1997;121:1121–1126. doi: 10.1038/sj.bjp.0701262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REVEST P.A., ABBOTT N.J., GILLESPIE J.I. Receptor mediated changes in intracellular [Ca2+] in cultured rat brain capillary endothelial cells. Brain Res. 1991;549:159–161. doi: 10.1016/0006-8993(91)90614-2. [DOI] [PubMed] [Google Scholar]
- TAGAMI M., YAMAGATA K., FUJINO H., KUBOTA A., NARA Y., YAMORI Y. Morphological differences of endothelial cells co-cultured with astrocytes on type I. and type IV. Collagen. Cell Tissue Res. 1992;268:225–232. doi: 10.1007/BF00318790. [DOI] [PubMed] [Google Scholar]
- TAMAI I., TSUJI A. Drug delivery through the blood-barrier barrier. Adv. Drug. Delivery Rev. 1996;19:401–424. [Google Scholar]
- TATSUTA T., NAITO M., MIAKAMI K., TSURUO T. Enhanced expression by the brain matrix of P-glycoprotein in brain capillary endothelial cells. Cell Growth Differ. 1994;5:1145–1152. [PubMed] [Google Scholar]
- TILLING T., KORTE D., HOHEISEL D., GALLA H.J. Basement membrane proteins influence brain capíllary endothelial barrier function in vitro. J.Neurochem. 1998;71:1151–1157. doi: 10.1046/j.1471-4159.1998.71031151.x. [DOI] [PubMed] [Google Scholar]
- VIGNE P., BREITTMAYER J.P., FRELIN C. Diadenosine polyphosphates as agonists of the endogenous P2Y1 receptor in rat brain capillary endothelial cells of the B7 and B10 clones. Br. J. Pharmacol. 2000;129:1506–1512. doi: 10.1038/sj.bjp.0703228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIGNE P., CHAMPIGNY G., MARSAULT R., BARBRY P., FRELIN C., LAZDUNSKI M. A new type of amiloride sensitive cationic channel in endothelial cells of brain microvessels. J. Biol. Chem. 1989;264:7663–7668. [PubMed] [Google Scholar]
- VIGNE P., FEOLDE E., BREITTMAYER J.P., FRELIN C. Characterization of the effects of 2-methylthio-ATP and 2-chloro-ATP on brain capillary endothelial cells: similarities to ADP and differences from ATP. Br. J. Pharmacol. 1994a;112:775–780. doi: 10.1111/j.1476-5381.1994.tb13146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIGNE P., FEOLDE E., BREITTMAYER J.P., FRELIN C. Analysis of the influence of nucleotidases on the apparent activity of exogenous ATP and ADP at P2Y1 receptors. Br. J. Pharmacol. 1998a;125:675–680. doi: 10.1038/sj.bjp.0702115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIGNE P., LUND L., FRELIN C. Cross-talk among Cyclic AMP, cyclic GMP, and Ca2+-dependent intracellular signalling mechanisms in brain capillary endothelial cells. J. Neurochem. 1994b;62:2269–2274. doi: 10.1046/j.1471-4159.1994.62062269.x. [DOI] [PubMed] [Google Scholar]
- VIGNE P., PACAUD P., LIORAND G., BREITTMAYER J.P. PPADS inhibits P2Y1 purinoreceptors in rat brain capillary endothelial cells and in rat ileal myocytes by an indirect mechanism. Biochem. Biophys. Res. Com. 1998b;244:332–335. doi: 10.1006/bbrc.1998.8262. [DOI] [PubMed] [Google Scholar]
- WEBB T.E., FEOLDE E., VIGNE P., NEARY J.T., RUNBERG A., FRELIN C., BARNARD E.A. The P2Y purinoceptor in rat brain microvascular endothelial cells couple to inhibition of adenylate cyclase. Br. J. Pharmacol. 1996;119:1385–1392. doi: 10.1111/j.1476-5381.1996.tb16050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


