Abstract
The pharmacological characteristics of muscarinic receptors mediating contraction of dog isolated ciliary muscle were determined and compared to those mediating contraction of dog urinary bladder smooth muscle.
(+)-Cis-dioxolane induced concentration-dependent contractions of ciliary muscle (pEC50=7.18±0.07, Emax=453±64 mg, n=19) and urinary bladder isolated smooth muscle (pEC50=6.55±0.07, Emax=11±1 g, n=19). These responses were antagonized by several muscarinic receptor antagonists (pKb values for the ciliary muscle and the bladder smooth muscle, respectively): atropine (8.25±0.14 and 9.21±0.09), pirenzepine (6.31±0.13 and 6.70±0.25), tolterodine (7.97±0.14 and 8.68±0.12), oxybutynin (7.40±0.08 and 7.88±0.12), zamifenacin (6.46±0.19 and 7.69±0.11), S-secoverine (6.66±0.14 and 8.13±0.07), AQ-RA 741 (6.16±0.15 and 7.08±0.23), p-F-HHSiD (7.10±0.27 and 7.35±0.07) and responses were not antagonized by PD 102807 (up to 100 nM).
In urinary bladder smooth muscle, the profile of antagonist pKB values correlated significantly with pKi values at human recombinant m3 muscarinic receptors, suggesting that M3 muscarinic receptors mediated the response. In the ciliary muscle, a significant (P<0.01) correlation was obtained with human recombinant m3 and m5 receptors.
Darifenacin displayed insurmountable antagonism at receptors in the bladder. At receptors in the ciliary muscle, it exhibited two phases of antagonism, comprising an initial low affinity (pKB<6) component and a high affinity phase (pKB>8).
The role of pigmentation in the atypical behaviour of darifenacin was examined. In blue coloured eyes, darifenacin produced apparent surmountable, competitive antagonism of the responses to (+)-cis-dioxolane (pKB=8.76±0.07). The antagonist profile obtained in this tissue suggested the involvement of a site which has the pharmacological attributes of the M5 receptor.
We suggest that the dog urinary bladder contracts in response to M3 muscarinic receptor activation. Contraction of the brown-eyed dog ciliary muscle is more complex and may include involvement of at least two receptors, possibly the M5 and M3 receptor, whereas blue-eyed dog ciliary muscle may involve a single population of M5 muscarinic receptors.
Keywords: M3 muscarinic receptors, M5 receptors, atypical receptor, ciliary muscle, melanin, urinary bladder smooth muscle
Introduction
Muscarinic receptors in the iris sphincter muscle regulate the diameter of the pupilliary aperture and thus control the level of light admitted to the eye. The pharmacological identity of muscarinic receptors mediating this response in rabbit is most consistent with activation of M3 muscarinic receptors (Fuder et al., 1989; Bognar et al., 1992; Choppin et al., 1998). Isolated ciliary smooth muscle of cow (Honkanen et al., 1990), canine (McIntyre & Quinn, 1995), sheep (German et al., 1998) and human (Woldemussie et al., 1993; Gil et al., 1997) express M3 muscarinic receptors, and these are thought to mediate the contractile response. A limitation of previous classification studies is that those ligands used to define the subtypes had limited selectivity, particularly in differentiating the M3 over M5 receptor (see Eglan & Nahorski (2000) for review). Indeed Gil et al. (1997) have reported the presence of other subtypes, including the M5 receptor, in ciliary smooth muscle using immunoprecipitation. Using the selective M3 antagonist, zamifenacin, McIntyre & Quinn (1995) argued that the functional M3 receptor in the dog ciliary muscle differed from the M3 receptor in gastrointestinal smooth muscle, due to the very low pA2 value (<6). However, there are alternative explanations for the low value. In the iris, high levels of melanin pigmentation induce muscarinic antagonist sequestration, leading to an underestimation of antagonist concentration in the receptor vicinity (Salazar et al., 1976) and ambiguous antagonist affinities.
To our knowledge, a comprehensive study of dog ciliary muscle muscarinic receptors has not been reported. The objective of the present study was, therefore, to examine the pharmacological characteristics of muscarinic receptor(s) mediating contraction of dog isolated ciliary muscle using several novel, selective antagonists. This was undertaken using eyes with blue and brown colouration, in order to assess the effect of pigmentation. The antagonists employed included darifenacin (M3-selective; Smith & Wallis, 1997), AQ-RA 741 (M2-selective; Doods et al., 1991), PD 102807 (M4 selective) and S-secoverine (discriminating M5 receptors from M3: Choppin et al., 1999b). The data were correlated with two series of affinity estimates. First, affinity estimates were compared to those determined at recombinant m1 – m5 human muscarinic receptors expressed in CHO cells (Nilvebrant et al., 1996; Hegde et al., 1997; Loury et al., 1999) and secondly, with those mediating contraction of dog urinary bladder. Previous studies have shown that contractions in this latter tissue are mediated by M3 muscarinic receptors (Choppin et al., 1999a).
Preliminary accounts of the findings have been presented previously at several meetings of the British Pharmacology Society (Choppin et al., 1999a,1999b,1999c; Choppin & Eglen, 2000).
Methods
In vitro contractile studies
Beagle or Mongrel dogs (male or female) were euthanized by an overdose of pentobarbital (tissue from dogs having a different colour pigment in each eye were used when assessing the role of pigmentation). The eyes were removed and placed in oxygenated Tyrode's solution (composition in mM: NaCl 137.0, KCl 2.7, CaCl2 1.8, MgCl:6H2O 1.0, KH2PO4 0.4, NaHCO3 11.9 and dextrose 5.6). From the same animal, urinary bladder was isolated, cleared of adhering adipose tissue and placed in oxygenated Krebs' solution (composition in mM: NaCl 118.2, KCl 4.6, CaCl2 2.5, MgSO4:7H2O 1.2, KH2PO4 1.2, NaHCO3 24.8 and dextrose 10.0). Both physiological solutions contained indomethacin (10 μM) in order to reduce prostaglandin-induced spontaneous activity of the tissues. Four strips of ciliary muscle were cut from each eye and eight strips of urinary bladder smooth muscle were cut from the supratrigonal portion of the bladder (longitudinal section, mucosa removed). The tissues were mounted in 10 ml organ baths containing Tyrode's or Krebs' solution, maintained at 37°C and constantly aerated with 95% O2/5% CO2 (pH=7.4). Grass FT03 transducers were used to measure changes in isometric tension of the tissues which were displayed on a Grass 7E polygraph. The tissues were maintained at a resting tension of 150 mg and 2 g for the ciliary muscle and the bladder, respectively, during an equilibration period of 60 min. Tension adjustments were made as necessary. The tissues were washed every 15 min.
After washing, cumulative concentration-effect curves to (+)-cis-dioxolane, a non-selective muscarinic agonist, (1 nM – 0.3 mM) were then constructed in each tissue. Thereafter, tissues were equilibrated in either the absence (time control) or presence of antagonist for a 90 min period during which tissues were washed every 10 min. Subsequently, a second concentration-effect curve to (+)-cis-dioxolane was constructed. Each strip was used to test only one concentration of antagonist or vehicle.
Melanin content determination
Quantitative determination of the melanin content in the dog ciliary muscle from brown and blue eyes was performed using the method described by Aravind Menon et al. (1992). Tissue samples (0.05 – 0.20 mg) were weighed, mixed with 10 ml of 0.5 N KOH, and incubated at 60°C for 4 h. Samples were centrifuged and aliquots of the supernatant were diluted 4 fold with 0.5 N KOH. Absorption at 420 nm was determined for the diluted samples and concentrations of melanin in these solutions were calculated from a standard curve generated from the absorption of solutions containing known amounts of melanin in 0.5 N KOH. The melanin content of each tissue was expressed in terms of μg melanin/mg tissue (mean±s.e.mean).
In addition, sections of eyes were stained using Masson's trichrome method described by Luna (1968) to highlight the different ocular tissue content of melanin.
Data anlysis
Contractions were recorded as changes in tension from baseline and expressed as a percentage of the maximum response of the first agonist concentration-effect curve. Agonist concentration-response curves were fitted using a nonlinear iterative fitting programme (Origin, Microcal Software, Inc., Northampton, MA, U.S.A.) using the relationship of Parker and Waud (1971). Agonist potencies and maximum response are expressed as pEC50 (−logarithm of the molar concentration of agonist producing 50% of the maximum response) and Emax, respectively. Concentration ratios (CRs) were determined from EC50 values in the presence and absence of antagonist. Antagonist affinity estimates (pKB values) were determined with the equation described by Furchgott (1972; pKB=−log ([antagonist]/CR-1)).
Correlation plots were drawn, showing the relationship of binding affinity data generated at human m1 – m5 recombinant muscarinic receptors with functional affinity data. Pearson correlation coefficients (r) and associated P-values were calculated using the method described by Dixon & Massey (1983). The sum of squares of differences in affinity estimates for each plot (Σ (y−x)2, noted ssq) defines the proximity of the data points to the line of identity (y=x). All data are expressed as means±s.e.mean.
The statistical significance was determined by using the Students t-test.
Compounds used
Atropine sulphate, indomethacin and oxybutynin chloride were obtained from Sigma Chemical Co (MO, U.S.A.). (+)-Cis-dioxolane, pirenzepine dihydrochloride and p-F-HHSiD (para-fluoro-hexahydrosiladifenidol) hydrochloride were obtained from Research Biochemicals Inc. (MA, U.S.A.). Tolterodine and s-secoverine were synthesized at Roche Bioscience (Palo Alto, CA, U.S.A.). Darifenacin hydrobromide and zamifenacin fumarate were generously provided by Dr Wallis (Pfizer Central Research, Sandwich, Kent, U.K.). AQ-RA 741 (11-({4-[4-(diethylamino)butyl]-1-piperidinyl}acetyl)-5,11-dihydro-6H-pyrido(2,3-b) (1,4)benzodiazepine-6-one) was donated by Boehringer Ingelheim Pharmaceuticals, Inc. (Ridgefield, CT, U.S.A.). PD 102807 (3,6a,11,14-Tetrahdro-9-methoxy-2-methyl-12H-isoquino [1,2-b] pyrrolo[3,2-f] [1,3] benzoxazine-1-carboxylic acid ethyl ester) was generously provided by Dr R. Schwarz (Parke-Davis Pharmaceutical Research, Ann Arbor, MI, U.S.A.).
Results
Characterization of muscarinic receptors mediating contractions of the dog urinary bladder smooth muscle
(+)-Cis-dioxolane induced concentration-dependent contractions of the dog urinary bladder smooth muscle (pEC50=6.55±0.07, Emax=11±1 g, n=19). Time-control experiments showed that two consecutive concentration-effect curves to this agonist could be constructed in the same tissue without any significant temporal change in the agonist potency and maximum response (data not shown).
Several antagonists (atropine, AQ-RA 741, zamifenacin, methoctramine, oxybutynin, tolterodine, pirenzepine and PD 102807) were tested for their ability to inhibit (+)-cis-dioxolane-induced responses and their functional affinity estimates (pKB) are summarized in Table 1. Cumulative agonist concentration-response curves were surmountably antagonized by these compounds in a concentration-dependent fashion, with parallel rightward displacements. The rank order of antagonist affinities (pKB) was: atropine (9.21±0.09), tolterodine (8.68±0.12), s-secoverine (8.13±0.07), oxybutynin (7.88±0.12), zamifenacin (7.69±0.11), p-F-HHSiD (7.35±0.07), AQ-RA 741 (7.08±0.23), pirenzepine (6.70±0.25) and PD 102807 (<7.00). However, darifenacin behaved insurmountably in the dog bladder (Figure 1), as described in the rat bladder (Hegde et al., 1997).
Table 1.
Affinity estimates for muscarinic antagonists in dog ciliary muscle and urinary bladder smooth muscle
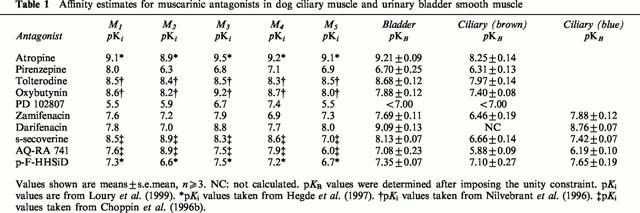
Figure 1.
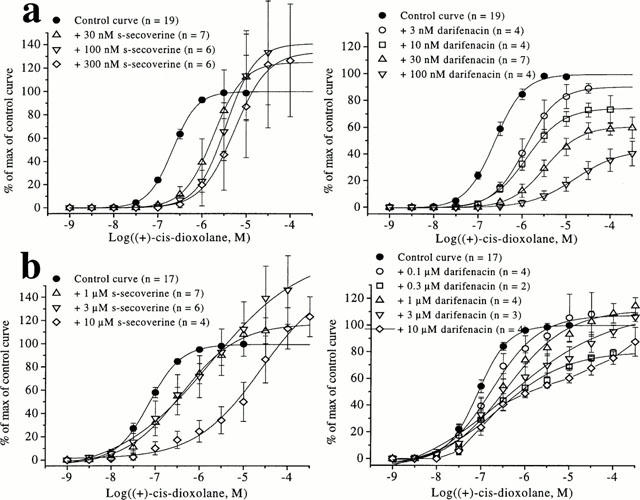
Effects of s-secoverine and darifenacin on the cumulative concentration-response curves of (+)-cis-dioxolane (a) on the dog urinary bladder smooth muscle and (b) on the brown-eyed dog ciliary muscle. Contractile effects were expressed as percentages of the maximum response of the control curve. The values shown are means±s.e.mean, n=2 – 7 animals. A single concentration of antagonist was applied to each tissue.
Comparison of functional data for dog urinary bladder smooth muscle with binding data at human recombinant muscarinic receptors
The best correlation between the affinities of antagonists at the muscarinic receptor(s) in dog urinary bladder smooth muscle and the affinities at human recombinant receptors was obtained at m3 receptors (r=0.86, P=0.003, ssq=2.22). However, a significant correlation was also found with the m5 receptor (r=0.84, P=0.004, ssq=4.30). In contrast, the correlation was less favourable at the other subtypes (r=0.58, ssq=4.33; r=0.39, ssq=9.51; r=0.61, ssq=4.45 at m1, m2 and m4, respectively (Figure 2)).
Figure 2.
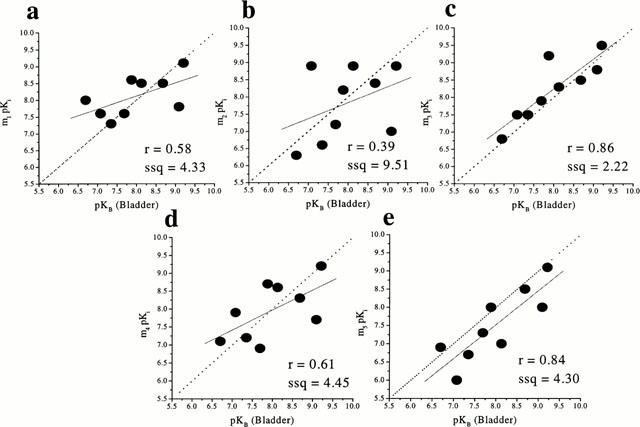
Correlation between the functional affinities (pKB values) of muscarinic antagonists at muscarinic receptor(s) in dog urinary bladder smooth muscle and binding affinities (pKi) at human recombinant muscarinic receptors (m1 – m5; a – e respectively). The binding data were taken from Nilvebrant et al., 1996; Eglen et al., 1997; Hegde et al., 1997; Loury et al., 1999. The broken line is the line of identity (x=y) while the solid line is the correlation plot (the inserts give the correlation factors (r) and the sum of squares values (ssq)).
Characterization of muscarinic receptors mediating contractions of the dog ciliary muscle
(+)-Cis-dioxolane produced concentration-dependent contractions of the dog ciliary muscle (pEC50=7.18±0.07, Emax=453±64 mg, n=19 brown eyes; pEC50=6.83±0.05, Emax=233±21 mg, n=20 blue eyes). No time-dependent changes in agonist sensitivity were observed during the construction of the second curve (data not shown). Pharmacological characterization of the muscarinic receptor involved was done by determination of antagonist affinities. In the ciliary muscle of the brown eye, concentration-effect curves to (+)-cis-dioxolane were surmountably antagonized by atropine, pirenzepine, tolterodine, oxybutynin, PD 102807, zamifenacin, s-secoverine (Figure 1), AQ-RA 741 and p-F-HHSiD (pKB values summarized in Table 1). The antagonism produced by darifenacin exhibited two phases (Figure 1): a darifenacin-resistant (pKB<6) and a darifenacin-sensitive (pKB>8) component.
Comparison of functional data for dog ciliary muscle with binding data at human recombinant muscarinic receptors
Correlation analysis between the affinities of the antagonists at muscarinic receptors in the dog ciliary muscle (brown eye) and the affinities at human recombinant muscarinic receptors showed a significant correlation (r=0.92, P=0.001, ssq=2.71) with m5. In contrast, poor correlations were observed at m1, m2, m3 and m4 (r=0.70, ssq=12.98; r=0.28, ssq=16.18; r=0.78, ssq=12.87; r=0.63, ssq=11.37) respectively (Figure 3).
Figure 3.
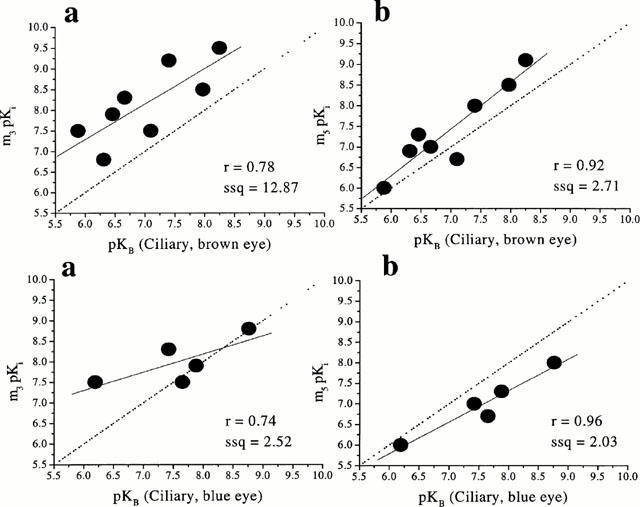
Correlation between the functional affinities (pKB values) of muscarinic antagonists at muscarinic receptor(s) in dog ciliary muscle (brown and blue eyes; top and bottom respectively) and binding affinities (pKi values) at human recombinant muscarinic receptors (m3 and m5; a and b, respectively). The binding data were taken from Dörje et al., 1991; Eglen et al., 1997; Hegde et al., 1997; Nilvebrant et al., 1996). The broken line is the line of identity (x=y) while the solid line is the correlation plot (the inserts give the correlation factors (r) and the sum of squares values (ssq)).
Similar results were obtained with blue-eyed dog ciliary muscle: when the affinities of antagonists (Table 1) in the dog ciliary muscle (blue eye) were compared with the affinities at human recombinant muscarinic receptors, a highly significant correlation (r=0.96, P=0.009) was obtained at m5 (Figure 3), close to the line of identity (ssq=2.03). However, it should be noted that, in the ciliary muscle from the blue eye, darifenacin produced apparent surmountable, competitive antagonism of the response to (+)-cis-dioxolane (pKB=8.76±0.07).
Quantitative determination of the melanin contents in the dog ciliary muscle from brown and blue eyes
A comparison of the amounts of melanin in dog ciliary muscle of brown and blue eyes demonstrated that the melanin content in the ciliary muscle from brown eyes (26±3 μg mg−1 of tissue, n=4) was marginally greater as compared to the ciliary muscle from the blue eyes (15±3 μg mg−1 of tissue, n=4). However, the difference was not significant (P>0.05).
Histology
Using selective dies, histology experiments highlighted the presence of melanin within the smooth muscle layers of the brown eye but not in the blue eye (Figure 4).
Figure 4.
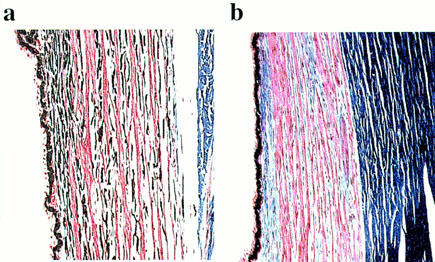
Histology (a) on brown-eyed dog ciliary muscle and (b) on blue-eyed dog ciliary muscle (Masson's trichrome method). Muscle fibres are shown in red, collagen in blue and melanin in black.
Discussion
On the basis of differential affinities for zamifenacin, the muscarinic receptor subtype(s) mediating contraction of the dog isolated ciliary muscle has been suggested to differ from that in ileum (McIntyre & Quinn, 1995; see Eglen et al., 1996, for review). The present study has assessed the pharmacological characteristics of ciliary muscle and compared it with a second canine smooth muscle tissue – the isolated urinary bladder.
Dog urinary bladder smooth muscle
(+)-Cis-dioxolane produced concentration-dependent contractions that were blocked in a concentration-dependent and surmountable fashion by several muscarinic antagonists. The apparent affinity estimates of these antagonists correlated most closely with the binding affinities of the antagonists at recombinant m3 muscarinic receptors and are consistent with the involvement of M3 muscarinic receptors in the contractile response. This supports previous findings in rabbit (Tobin, 1995; Tobin & Sjogren, 1995; Choppin et al., 1998), rat (Longhust et al., 1995; Hegde et al., 1997) and human (Newgreen & Naylor, 1996) urinary bladder. It should be noted, however, that a good correlation (r=0.84, ssq=4.30) was also obtained with the binding affinities of the antagonists at the m5 recombinant muscarinic receptor. This is unsurprising given that most antagonists discriminate poorly between M3 and M5 receptors, and highlights the difficulty of excluding a role for the latter in M3-mediated responses. However, the high affinity values obtained with s-secoverine and AQ-RA 741 (pKB=8.13±0.07 and 7.08±0.23, respectively) support the involvement of a single M3 muscarinic receptor population.
Darifenacin, which has been reported to behave surmountably in rabbit bladder (Choppin et al., 1998) but insurmountably in rat bladder (Hegde et al., 1997) produced insurmountable antagonism in dog bladder (present study).
Dog ciliary muscle (brown)
McIntyre & Quinn (1995) suggest that the M3 muscarinic receptor in the dog ciliary muscle may differ from the M3 receptors in canine ileum. In particular, the very low affinity of the M3 selective antagonist, zamifenacin (pA2<6; McIntyre & Quinn, 1995; Wallis, 1995) raised doubts equating this receptor with M3. The intermediate affinity of pirenzepine (pA2=6.7; McIntyre & Quinn, 1995) is compatible with either M2 or M3 receptors, but the low affinity of methoctramine (pA2<5.5; McIntyre & Quinn, 1995) would argue against the involvement of an M2 receptor. In the present study, the low affinities of pirenzepine (6.31) and PD 102807 (<7.0) excluded involvement of M1 and M4 receptors respectively.
Furthermore, the affinity (pKB) of zamifenacin (6.46±0.19) supported the low affinity reported for this compound by McIntyre & Quinn (1995). Experimental differences may account for these minor disparities, including the agonist employed (carbachol; McIntyre & Quinn, 1995) versus (+)-cis-dioxolane (present study). However, these estimates and the biphasic antagonism produced by darifenacin, a structurally related M3 selective antagonist (Wallis, 1995), were inconsistent with a simple interaction at a singular M3 muscarinic receptor. The atypical profile of darifenacin in the ciliary muscle of the brown-eyed dog is not fully understood.
Our group (Choppin et al., 1999b) has previously demonstrated that AQ-RA 741 and s-secoverine are two defining ligands in M5 receptor characterization, as they display a low affinity for this subtype over the remaining four (>19 fold lower when compared to other subtypes). Interestingly, the low affinities found in the present study using these compounds (6.66 and 5.88 for s-secoverine and AQ-RA 741, respectively) suggest the presence of functional M5 receptors. Furthermore, among the five recombinant muscarinic receptors, the best correlation of the functional pKB estimates in this tissue was obtained with affinities at the recombinant human m5 receptor.
We suggest, therefore, that the muscarinic receptor mediating contraction of the dog ciliary smooth muscle equates most closely with m5/M5 receptor, although we cannot exclude the concurrent involvement of the M3 muscarinic receptor. It should be noted that the human ciliary muscle is one of the few examples of the localization of the m5 subtype outside the CNS (Zhang et al., 1995), the others being in human macrophages and, interestingly, iris muscle (Ferrari-Dileo & Flynn, 1995; Gil et al., 1997). Our group confirmed functionally this result in human but also feline tissue (Choppin & Eglen, 2000).
Dog ciliary muscle (blue)
The affinities of muscarinic antagonists obtained from both brown and blue ciliary muscles were similar, with the exception of darifenacin, and suggested that the muscarinic receptors mediating contraction of brown and blue ciliary muscle have the pharmacological attributes of the M5 muscarinic receptors.
Drug (darifenacin) – melanin interaction
Muscarinic ligands appeared to bind to a high molecular weight pigment called melanin or, more specifically eumelanin (Akesson et al., 1983; Ito, 1986). Non-saturable binding, as well as internal redistribution of the drug affects the agonist – antagonist interactions and could explain the marked differences between individuals in ocular responses to anti-muscarinic compounds. From a clinical standpoint, association of the compound with melanin may reduce the concentration of drug available to take effect at the muscarinic receptors (melanin acting as a ‘drug-reservoir'; German et al., 1999). Aravind Meon et al. (1992) have shown that the melanin content in the ciliary muscle of human brown eyes is statistically higher than that of blue eyes. Consequently, human brown eyes possess a larger capacity to bind certain drugs, possibly serving as a depot for storage and subsequent release of these drugs. In order to explore this melanin hypothesis in the context of modulating muscarinic antagonist affinity, experiments were carried out using canine blue eyes. The hypothesized drug (darifenacin) – melanin in the brown eye could not be reproduced in the blue eye by addition of free melanin (Choppin et al., 1999c): incubation of blue-eyed dog ciliary muscle with melanin (5, 10 or 50 μg mg−1 of tissue for 1.5 or 18 h) did not alter the competitive behaviour of darifenacin in this tissue. This protocol probably underestimated the concentration of melanin within the muscle layer, unable to reach the physiological concentration.
Conclusions
The present study demonstrates that the muscarinic antagonist profile in the dog ciliary muscle and bladder are different. Muscarinic receptors in dog bladder urinary smooth muscle equate closely with the m3 muscarinic receptor, whereas m5 receptors seem to be involved in the ciliary muscle. These results therefore support an earlier proposal for the involvement of a muscarinic receptor different from the ‘classical' ileal M3 receptor in dog ciliary smooth muscle (McIntyre & Quinn, 1995). However, it should be noted that postulation of the involvement of M5 receptors in canine ciliary muscles relies on very few ligands which discriminate between M5 and M3 receptors. Additional studies need to be performed to support our conclusion. An implication of this study is that the presence of functional M5 receptors in peripheral tissues may have been overlooked, given its similarity in pharmacology to the M3 receptor.
Abbreviations
- AQ-RA 741
(11-({4-[4-(diethylamino)butyl]-1-piperidinyl}acetyl)-5,11-dihydro-6H-pyrido(2,3-b)(1,4)benzodiazepine-6-one)
- PD 102807
(3,6a,11,14-Tetrahydro-9-methoxy-2-methyl-12H-isoquino[1,2-b]pyrrolo[3,2-f][1,3]benzoxazine-1-carboxylic acid ethyl ester)
- p-F-HHSiD
para fluoro hexahydrosiladifenidol
References
- AKESSON C., SWANSON C., PATIL P.N. Muscarinic receptors of rabbit irises. Naunyn-Schmiedeberg's Arch. Pharmacol. 1983;322:104–110. doi: 10.1007/BF00512382. [DOI] [PubMed] [Google Scholar]
- ARAVIND MENON I., WAKEHAM D.C., PERSAD S.D., AVARIA M., TROPE G.E., BASU P.K. Quantitative determination of the melanin contents in ocular tissues from human blue and brown eyes. J. Ocular Pharmacol. 1992;8:35–42. doi: 10.1089/jop.1992.8.35. [DOI] [PubMed] [Google Scholar]
- BOGNAR I.T., ALTES U., BEINHAUER C., KESSLER I., FUDER H. A muscarinic receptor different from the M1, M2, M3 and M4 subtypes mediates the contraction of the rabbit iris sphincter. Naunyn-Schmiedeberg's Arch. Pharmacol. 1992;345:611–618. doi: 10.1007/BF00164573. [DOI] [PubMed] [Google Scholar]
- CHOPPIN A., EGLEN R.M. Pharmacological characterisation of muscarinic receptors in feline and human isolated ciliary smooth muscle. Br. J. Pharmacol. 2000;129:206P. doi: 10.1038/sj.bjp.0703901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOPPIN A., EGLEN R.M., HEGDE S.S. Pharmacological characterisation of muscarinic receptors in rabbit isolated iris sphincter muscle and urinary bladder smooth muscle. Br. J. Pharmacol. 1998;124:883–888. doi: 10.1038/sj.bjp.0701920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOPPIN A., HEGDE S.S., EGLEN R.M. Pharmacological characterisation of muscarinic receptors in dog ciliary smooth muscle. Br. J. Pharmacol. 1999a;126:93P. doi: 10.1038/sj.bjp.0703901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOPPIN A., LOURY D.N., WATSON N., HEGDE S.S., EGLEN R.M. S-secoverine: a defining ligand in muscarinic M5 receptors characterization. Br. J. Pharmacol. 1999b;128:33P. doi: 10.1038/sj.bjp.0702696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOPPIN A., SMITH S., HEGDE S.S., EGLEN R.M. Role of pigmentation in the atypical behaviour of darifenacin in dog ciliary smooth muscle. Br. J. Pharmacol. 1999c;128:90P. [Google Scholar]
- DIXON W.J., MASSEY F.J. Introduction to statistical analysis 1983New York: McGraw-Hill Publishing Company; 4th edition [Google Scholar]
- DOODS H., ENTZEROTH M., MAYER N. Cardioselectivity of AQ-RA 741, a novel tricyclic antimuscarinic drug. Eur. J. Pharmacol. 1991;192:147–152. doi: 10.1016/0014-2999(91)90081-z. [DOI] [PubMed] [Google Scholar]
- DÖRJE F., WESS J., LAMBRECHT G., TACKE R., MUTSCHLER E., BRANN M.R. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J. Pharmacol. Exp. Ther. 1991;256:727–733. [PubMed] [Google Scholar]
- EGLEN R.M., BONHAUS D.W., CALIXTO J.J., CHOPPIN A., LEUNG E., LOEB M., LOURY D., MOY T., WILDA M., HEGDE S.S. Characterization of the interaction of tolterodine at muscarinic receptor subtypes in vitro and in vivo. Br. J. Pharmacol. 1997;120:63P. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., HEGDE S.S., WATSON N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- EGLEN R.M., NAHORSKI S.R. The muscarinic M5 receptor: a silent or emerging subtype. Br. J. Pharmacol. 2000;130:13–21. doi: 10.1038/sj.bjp.0703276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRARI-DILEO G., FLYNN D.D.Characterization of muscarinic receptors on cultured microglial cells Life Sci. 1995561037(abstract) [Google Scholar]
- FUDER H., SCHÖPF J., UNCKELL J., WESNER M. TH., MELCHIORRE C., TACKE R., MUTSCHLER E., LAMBRECHT G. Different muscarinic receptors mediate the prejunctional inhibition of [3H]-noradrenaline release in rat or guinea-pig iris and the contraction of the rabbit iris sphincter muscle. Naunyn-Schmiedeberg's Arch. Pharmacol. 1989;345:611–618. doi: 10.1007/BF00717733. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F.The classificiation of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory Catecholamines, Handbook of Experimental Pharmacology 197233Berlin, Heidelberg, New York: Springer; 283–335.Ed. Blaschko, H. & Muscholl, E. pp [Google Scholar]
- GERMAN E.J., WOOD D., HURST M.A. Comparison of muscarinic receptor subtypes in the sheep ciliary muscle and iris sphincter using a radioligand binding aproach. Br. J. Pharmacol. 1998;125:62P. [Google Scholar]
- GERMAN E.J., WOOD D., HURST M.A. Ocular effects of antimuscarinic compounds: is clinical effect determined by binding affinity for muscarinic receptors or melanin pigment. J. Ocular Pharmacol. Ther. 1999;15:257–269. doi: 10.1089/jop.1999.15.257. [DOI] [PubMed] [Google Scholar]
- GIL D.W., KRAUSS H.A., BOGARDUS A.M., WOLDEMUSSIE E. Muscarinic receptor subtypes in human iris-ciliary body measured by immunoprecipitation. Invest. Ophthalmol. Vis. Sci. 1997;38:1434–1442. [PubMed] [Google Scholar]
- HEGDE S.S., CHOPPIN A., BONHAUS D., BRIAUD S., LOEB M., MOY T.M., LOURY D., EGLEN R.M. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br. J. Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONKANEN R.E., HOWARD E.F., ABDEL-LATIF A.A. M3-muscarinic receptor subtype predominates in the bovine iris sphincter smooth muscle and ciliary processees. Invest. Ophthalmol. Vis. Sci. 1990;31:590–593. [PubMed] [Google Scholar]
- ITO S. Re-examination of the structure of eumelanin. Biochim. Biophys. Acta. 1986;883:155–161. doi: 10.1016/0304-4165(86)90146-7. [DOI] [PubMed] [Google Scholar]
- LONGHURST P.A., LEGGETT R.E., BRISCOE J.A.K. Characterization of functional muscarinic receptors in the rat urinary bladder. Br. J. Pharmacol. 1995;116:2279–2285. doi: 10.1111/j.1476-5381.1995.tb15065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOURY D.N., HEGDE S.S., BONHAUS D.W., EGLEN R.M. Ionic strength of assay buffers influences antagonist binding affinity estimates at muscarinic M1-M5 cholinoceptors. Life Sci. 1999;64:6P. [Google Scholar]
- LUNA L.G. Manual of histologic staining methods of the Armed Forces Institute of Pathology 1968New York: McGraw-Hill Book Company; 3rd edition [Google Scholar]
- MCINTYRE P., QUINN P. Characterisation and comparison of muscarinic receptors in the dog ciliary muscle with ileum. Br. J. Pharmacol. 1995;115:139P. [Google Scholar]
- NEWGREEN D.T., NAYLOR A.M. Characterization of functional muscarinic receptors in human bladder. Br. J. Pharmacol. 1996;119:45P. [Google Scholar]
- NILVEBRANT L., SUNDQUIST S., GILLBERG P.-G.Tolterodine is not subtype (m1-m5) selective but exhibits functional bladder selectivity in vivo Neurourol. Urodyn. 19961534(abstract) [Google Scholar]
- PARKER R.B., WAUD D.R. Pharmacological estimation of drug-receptor dissociation constants. Statistical evaluation. I. Agonists. J. Pharmacol. Exp. Ther. 1971;177:1–12. [PubMed] [Google Scholar]
- SALAZAR M., SHIMADA K., PATIL P.N. Iris pigmentation and atropine mydriasis. J. Pharmacol. Exp. Ther. 1976;197:79–88. [PubMed] [Google Scholar]
- SMITH C.M., WALLIS R.M. Characterisation of [3H]-darifenacin as a novel radioligand for the study of muscarinic M3 receptors. J. Receptor Signal transduction Res. 1997;17:177–184. doi: 10.3109/10799899709036602. [DOI] [PubMed] [Google Scholar]
- TOBIN G. Muscarinic receptor subtypes in the submandibular gland and the urinary bladder of the rabbit: in vivo and in vitro functional comparisons of receptor antagonists. J. Auton. Pharmacol. 1995;15:451–463. doi: 10.1111/j.1474-8673.1995.tb00410.x. [DOI] [PubMed] [Google Scholar]
- TOBIN G., SJOGREN C. In vivo and in vitro effects of muscarinic receptor antagonists on contractions and release of [3H]-acetylcholine in the rabbit urinary bladder. Eur. J. Pharmacol. 1995;281:1–8. doi: 10.1016/0014-2999(95)00221-6. [DOI] [PubMed] [Google Scholar]
- WALLIS R.M. Pre-clinical and clinical pharmacology of selective muscarinic M3 receptor antagonists. Life Sci. 1995;56:861–868. doi: 10.1016/0024-3205(94)00021-j. [DOI] [PubMed] [Google Scholar]
- WOLDEMUSSIE E., FELDMANN B.J., CHEN J. Sphincter and ciliary smooth muscle cells. Exp. Eye Res. 1993;56:385–392. doi: 10.1006/exer.1993.1052. [DOI] [PubMed] [Google Scholar]
- ZHANG X., HERNANDEZ M.R., YANG H., ERICKSON K. Expression of muscarinic receptor subtype mRNa in the human ciliary muscle. Invest. Ophthalmol. Vis. Sci. 1995;36:1645–1657. [PubMed] [Google Scholar]


