Abstract
Sulphamethoxazole has been associated with the occurrence of hypersensitivity reactions. There is controversy as to whether the immune response is metabolism-dependent or -independent. We have therefore investigated the site of antigen formation and the nature of the drug signal presented to the immune system in vivo.
Male Wistar rats were dosed with sulphamethoxazole, sulphamethoxazole hydroxylamine or nitroso sulphamethoxazole. Antigen formation on cell surfaces was determined by flow cytometry using a specific anti-sulphamethoxazole antibody. Immunogenicity was determined by assessment of ex vivo T-cell proliferation.
Administration of nitroso sulphamethoxazole, but not sulphamethoxazole or sulphamethoxazole hydroxylamine, resulted in antigen formation on the surface of lymphocytes, splenocytes and epidermal keratinocytes, and a strong proliferative response of splenocytes on re-stimulation with nitroso sulphamethoxazole. Rats dosed with sulphamethoxazole or sulphamethoxazole hydroxylamine did not respond to any of the test compounds.
CD4+ or CD8+ depleted cells responded equally to nitroso sulphamethoxazole. The proliferative response to nitroso sulphamethoxazole was seen even after pulsing for only 5 min, and was not inhibited by glutathione. Responding cells produced IFN-γ, but not IL-4.
Haptenation of cells by sulphamethoxazole hydroxylamine was seen after depletion of glutathione by pre-treating the rats with diethyl maleate. Splenocytes from the glutathione-depleted sulphamethoxazole hydroxylamine-treated rats responded weakly to nitroso sulphamethoxazole, but not to sulphamethoxazole or sulphamethoxazole hydroxylamine.
Dosing of rats with sulphamethoxazole produced a cellular response to nitroso sulphamethoxazole (but not to sulphamethoxazole or its hydroxylamine) when the animals were primed with complete Freund's adjuvant.
These studies demonstrate the antigenicity of nitroso sulphamethoxazole in vivo and provide evidence for the role of drug metabolism and cell surface haptenation in the induction of a cellular immune response in the rat.
Keywords: Delayed type hypersensitivity, skin, T-lymphocytes, antigen presentation, in vivo animal model, sulphamethoxazole
Introduction
Co-trimoxazole, a combination of sulphamethoxazole and trimethoprim, is the preferred treatment for Pnuemocystis carinii pneumonia in patients with HIV-infection. Administration of co-trimoxazole is frequently associated with skin rashes (Naldi et al., 1999). Cutaneous manifestations vary in the type of clinical presentation; co-trimoxazole and allergenic drugs in general cause both mild urticarial rashes and severe reactions such as toxic epidermal necrolysis (Barranco & Lopez-Serrano, 1998; Becker, 1998). Immunohistochemical analyses of skin at the time of a reaction have revealed a dermal and epidermal infiltrate of CD4+ and CD8+ cells, respectively (Miyauchi et al., 1991; Correia et al., 1993). The incidence of cutaneous adverse reactions in HIV-infected patients is much higher than in the general population; greater than 30% develop allergic symptoms (Coopman et al., 1993; Koopmans et al., 1995).
Skin rashes are presumed to involve the formation of a drug-protein conjugate. Conjugation may alter protein structure necessary for the maintenance of cell integrity leading to apoptosis or necrosis (Rieder et al., 1988; 1995; Hess et al., 1999). However, the most widely accepted view is that covalent modification leads to perturbation of the host's own immune system. Following antigen recognition, the drug-modified protein is processed and presented in association with MHC class I and/or II molecules to T-cells (signal 1) (Coleman & Blanca, 1998; Griem et al., 1998). Signal 1 governs the nature of the immune response (i.e., CD4+ or CD8+) (Kalish & Askenase, 1999), while co-stimulatory signals (signal 2) and/or cytokine synthesis (signal 3) determine whether antigen presentation results in tissue damage or tolerance (Curtsinger et al., 1999).
Central to this hypothesis is the formation of a chemically reactive metabolite (Figure 1). In the liver, sulphamethoxazole is metabolized to a hydroxylamine (Cribb & Spielberg, 1990; 1992). Sulphamethoxazole hydroxylamine circulates in blood and tissues and is excreted in human (Gill et al., 1996; Mitra et al., 1996) and rat urine (Gill et al., 1997). Further (auto)oxidation yields a nitroso compound (Cribb et al., 1991; Naisbitt et al., 1996) that can haptenate proteins, including the surface of viable lymphocytes (Naisbitt et al., 1999). These observations and identification of (a) sulphamethoxazole-substituted hepatic proteins in vitro (Cribb et al., 1996), (b) sulphamethoxazole-substituted serum proteins and anti-SMX antibodies in patient sera (Meekins et al., 1994; Daftarian et al., 1995; Gruchalla et al., 1998), and (c) CD8+ dermal T-cells that proliferate in response to microsome-generated sulphamethoxazole metabolites (Hertl et al., 1995) are consistent with the involvement of both drug metabolism and the immune system in the pathogenesis of drug allergy.
Figure 1.
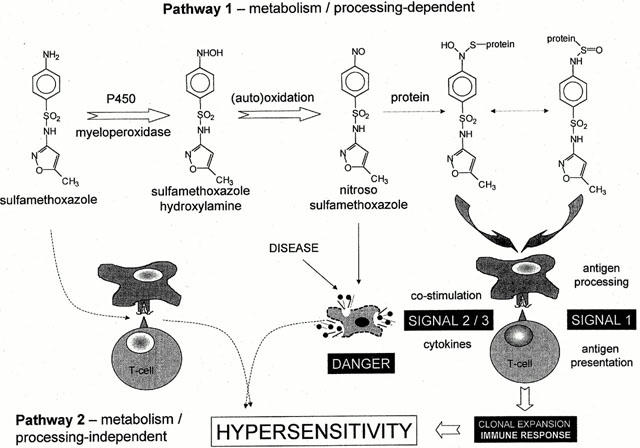
Scheme illustrating the proposed mechanisms of sulphamethoxazole hypersensitivity reactions. Pathway 1 involves sequential oxidation to the reactive nitroso metabolite that can bind irreversibly to thiol groups of either circulating or cell surface proteins. Antigen presenting cells process and present the antigenic peptide fragment to T-cells. T-cells proliferate and in the presence of the appropriate co-stimulatory signals may cause hypersensitivity. Pathway 2, proposed by Pichler et al. (Mauri-Hellweg et al., 1995; Schnyder et al., 1997; Zanni et al., 1998; von Greyerz et al., 1999), suggests that sulphamethoxazole stimulates T-cell proliferation, in the absence of drug metabolism and antigen processing, via a direct, reversible interaction between the parent drug and an antigenic peptide embedded within the MHC binding groove of antigen presenting cells.
Although drug metabolism is thought to be essential for immune recognition, recent studies have demonstrated an alternative (or additive) mechanism that occurs in the absence of protein conjugation and antigen processing (Mauri-Hellweg et al., 1995; Schnyder et al., 1997; Zanni et al., 1998; Von greyerz et al., 1999). It has been termed the HLA-restricted, processing- and metabolism-independent pathway of immune recognition by drugs (Figure 1) (Zanni et al., 1998). In these experiments, the large majority of T-cell clones, generated from two sulphamethoxazole-hypersensitive patients, proliferated in response to sulphamethoxazole, but not sulphamethoxazole hydroxylamine or nitroso sulphamethoxazole (Schnyder et al., 2000; Burkhart et al., 2001). Stimulation of sulphamethoxazole required the continuous presence of the drug (i.e., pre-incubation of sulphamethoxazole and then washing did not cause a response). These observations are consistent with an unstable, non-covalent presentation of sulphamethoxazole. It is of course possible that both pathways of antigen presentation and/or recognition are involved in the complex mechanisms of drug allergy.
The aim of these studies was to investigate the role of drug metabolism in the antigenicity and T-cell reactivity of sulphamethoxazole in vivo in an experimental model of immunogenicity. Critical questions to be addressed were: first, what is the site of antigen formation?; secondly, what is the nature of the drug signal (i.e., parent drug or protein reactive metabolite) presented to the immune system?; and thirdly, what is the role of T-cells, their phenotype and cytokine profile in the immune response? The rat was chosen as an appropriate animal model for two reasons: first, metabolism of sulphamethoxazole and the fate of its oxidative metabolites have been described previously and shown to be similar to that of man (Gill et al., 1996; 1997; Mitra et al., 1996); and secondly, specific IgG antibodies have been identified from rats administered sulphamethoxazole (Gill et al., 1997).
Methods
Materials
Bromobimane, complete Freund's adjuvant (CFA), diethyl maleate, dimethyl sulphoxide (DMSO), dispase, Dulbecco's modified Eagles medium (DMEM), EDTA, fluorescein isothiocyanate (FITC)-conjugated anti-rabbit antibody, Hanks balanced salt solution (HBSS), L-glutamine, HEPES, mercaptoethanol, penicillin, sulphamethoxazole, streptomycin, [3H]-thymidine and trypsin were obtained from Sigma Chemical Co. (Poole, U.K.). Sulphamethoxazole-conjugated human serum albumin was kindly donated by Prof E. Gleichmann (Heinrich Heine University, Dusseldorf, Germany). Anti-sulphamethoxazole IgG antibody was raised as described by Cribb et al. (1996) Fluorescent labelled anti CD3+, CD4+, CD8+ and CD25+ antibodies were obtained from Beckman Coulter (Luton, U.K.). Pre-coated ELISA plates for the determination of IFN-γ and IL-4 were from IDS Ltd. (Tyne and Wear, U.K.). MACS anti-rat IgG microbeads for indirect cell sorting were from Miltenyi Biotec Ltd. (Surrey, U.K.). Sulphamethoxazole hydroxylamine and nitroso sulphamethoxazole were synthesised according to the method of Naisbitt et al. (1996) and found to be >99% pure by LC-MS and NMR. Lymphoprep (1.077 g ml−1) was obtained from Nycomed (Birmingham, U.K.).
Animals and immunizing protocols
Male Wistar rats between 8 – 12 weeks old (175 – 225 g) were purchased from Charles River U.K. Ltd (Kent, U.K.). For antigenicity studies (n=4, for each group), sulphamethoxazole (50 – 100 mg kg−1), sulphamethoxazole hydroxylamine (50 – 100 mg kg−1) and nitroso sulphamethoxazole (50 – 100 mg kg−1) in DMSO (100 μl) were administered by a single i.p. or i.v. injection. For the immunogenicity studies (n=4, for each group), sulphamethoxazole (10 – 250 mg kg−1), sulphamethoxaozle hydroxylamine (1 – 10 mg kg−1) and nitroso sulphamethoxaozle (0.01 – 10 mg kg−1) were administered in DMSO (100 μl; i.p.) four times weekly for 2 weeks. In separate experiments, rats were administered test compounds by a single i.p. injection in CFA (200 μl) containing Mycobacterium tuberculosis. To cause a depletion of glutathione, certain rats were pre-dosed with diethyl maleate (4 mmol kg−1 i.p.) 1 h prior to the administration of sulphamethoxazole or sulphamethoxazole hydroxylamine. Hepatic and extra-hepatic [peripheral blood mononuclear cells (PBMC) and splenocyte] glutathione levels were determined by high performance liquid chromatography (HPLC) using the fluorescent probe bromobimane (Hamel et al., 1992).
Antigenicity of sulphamethoxazole, sulphamethoxazole hydroxylamine and nitroso sulphamethoxazole (in vivo haptenation)
In preliminary experiments, freshly isolated rat PBMC (0.5×106 ml−1) were incubated with DMSO, sulphamethoxazole, sulphamethoxazole hydroxylamine or nitroso sulphamethoxazole (1 – 250 μg ml−1) in HBSS for 1 h at 37°C. Antigen formation on cell surfaces was determined by flow cytometry using a specific anti-sulphamethoxazole IgG antibody (1 : 500; 40 μl) and a FITC-conjugated anti-IgG secondary antibody (Naisbitt et al., 1999). Forward and side scatter were measured simultaneously and the FITC fluorescence of cells was acquired on fluorescence channel FL1. The majority of cells were gated for analysis, and the forward threshold was raised to exclude cell debris. At least 5000 cells were analysed from each incubation. Cells incubated with an anti-IgG isotype control were analysed first and used to set the parameters for the test samples. The percentage of cells staining positive for covalently bound sulphamethoxazole was calculated as the difference in fluorescence intensity when drug-treated cells were compared with incubations containing DMSO alone.
In vivo antigen formation on cell surfaces by sulphamethoxazole and its metabolites was determined by collecting blood (4 ml), spleen and skin (4 cm×2 cm) from rats dosed with the test compounds. PBMC and red cell depleted splenocytes were isolated by density centrifugation. Epidermal keratinocytes were harvested by incubation of skin with dispase (0.5%, in phosphate buffered saline; 4 h), followed by trypsin / EDTA (0.05% / 0.02%, in phosphate buffered saline; 7 min). DMEM containing foetal calf serum (10%) was used to stop the trypsin reaction. Cells found to be greater than 90% viable, as assessed by trypan blue dye exclusion, were washed with HBSS (PBMC and splenocytes) or DMEM (keratinocytes) (2×1 ml) and diluted to the concentration required for antibody staining. The percentage of cells staining positive for covalently bound sulphamethoxazole was calculated as the difference in fluorescence intensity between drug-treated and control animals.
Immunogenicity of sulphamethoxazole, sulphamethoxazole hydroxylamine and nitroso sulphamethoxazole (ex vivo proliferative response)
Blood and spleen were collected from drug- and control-treated rats. Single cell splenocyte suspensions were prepared by perfusion with HBSS. Red cells were removed by density centrifugation with Lymphoprep (600 g; 30 min). Mononuclear cells were washed and viability was assessed by trypan blue dye exclusion. Cells found to be greater than 95% viable were resuspended in culture medium (RPMI 1640 supplemented with heat-inactivated FCS [10%], HEPES [25 mM], L-glutamine [2 mM], 2-mercaptoethanol [50 μM], streptomycin [100 μg ml−1] and penicillin [100 U ml−1]) and incubated (1.5×105/well) with sulphamethoxazole (10 – 250 μg ml−1), sulphamethoxazole N4-acetate (10 – 250 μg ml−1) sulphamethoxazole hydroxylamine (1 – 25 μg ml−1), nitroso sulphamethoxazole (1 – 25 μg ml−1), nitroso benzene, (1 – 25 μg ml−1), or sulphamethoxazole-conjugated human serum albumin (1 – 250 μg ml−1) in U-bottomed 96-well plates for 72 h (37°C, 5% CO2). To measure proliferation, [3H]-thymidine (0.5 μCi) was added to the cultures for 8 h. Cells were harvested and [3H]-thymidine incorporation was measured as counts per min on a β-counter (PerkinElmer Life Sciences, Cambridge, U.K.). Proliferative responses were calculated as stimulation index (SI; counts per min in drug-treated cultures/counts per min in cultures with DMSO alone).
Assessment of the mechanism(s) of antigen presentation
To determine the role of cellular haptenation in antigen presentation, red cell depleted splenocytes (1.5×105/well) from nitroso sulphamethoxazole treated rats (1 mg kg−1) were pulsed with nitroso sulphamethoxazole (10 μg ml−1) for 0.1 – 72 h. To remove unbound drug, cells were washed with HBSS (2×1 ml) and resuspended in culture medium for the remainder of the incubation period (72 h). Alternatively, glutathione (300 μg ml−1; 0 – 72 h) was added to reduce nitroso sulphamethoxazole (Naisbitt et al., 1996). To measure proliferation, [3H]-thymidine (0.5 μCi) was added to the cultures for 8 h. Cells were harvested and [3H]-thymidine incorporation was measured as counts per min on a β-counter. Cell surface antigen formation, within the proliferation assay, was also determined by flow cytometry using a specific anti-sulphamethoxazole antibody (Naisbitt et al., 1999).
Assessment of the phenotype of responding T-lymphocytes
Splenocytes from DMSO and nitroso sulphamethoxazole-treated (1 mg kg−1) rats, and T-cells exposed to nitroso sulphamethoxazole (10 μg ml−1; 72 h) in culture were assayed for CD4+, CD8+ and CD25+ expression by dual colour immunofluorescence using specific monoclonal antibodies. In addition, T-cell proliferation was determined with CD4+ and CD8+ depleted splenocytes (1.5×105/well). Depletion was performed using immunomagnetic microbeads. Proliferation was measured by [3H]-thymidine incorporation as described.
Assessment of cytokine synthesis by responding T-lymphocytes
Supernatants were collected from DMSO and nitroso sulphamethoxazole-treated (10 μg ml−1) splenocytes on day 2 of culture. Cytokine production (IL-4, and IFN-γ) was quantified by ELISA using commercially available kits (IDS Ltd; Tyne and Wear, U.K.). The limit of detection was 5 pg ml−1.
Statistical analysis
All data are expressed as mean±s.d. Values to be compared were analysed for non-normality (Shapiro-Wilk test). Values were often found to be non-normally distributed, and therefore, the Mann – Whitney test was used for comparison of the two groups, accepting P<0.05 as significant.
Results
Cell surface antigen formation by sulphamethoxazole and its metabolites
In preliminary experiments, using freshly isolated rat PBMC in vitro, sulphamethoxazole hydroxylamine and nitroso sulphamethoxazole, but not the parent amine, caused a concentration-dependent increase in cell surface antigen formation (Figure 2).
Figure 2.
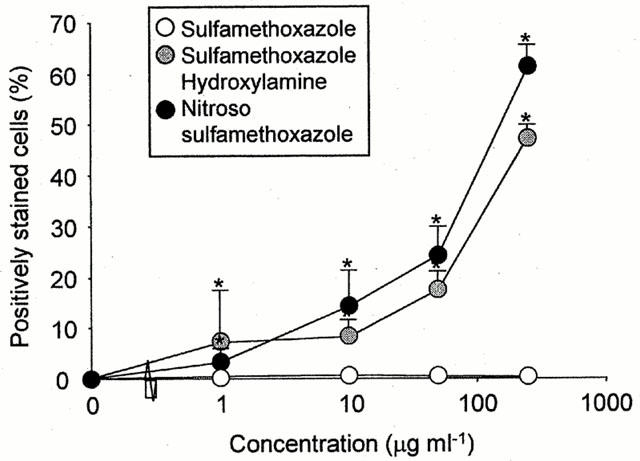
Cell surface haptenation of rat PBMC after incubation with sulphamethoxazole hydroxylamine or nitroso sulphamethoxazole, but not sulphamethoxazole, in vitro for 1 h. Fluorescence intensity of viable cells was analysed by flow cytometry using an anti-sulphamethoxazole antibody and a FITC-conjugated anti-IgG secondary antibody. The majority of cells were gated for analysis and the forward threshold was raised to exclude debris. The results are expressed as the percentage of positively stained cells from three experiments carried out in triplicate. Statistical analysis compares the ability of drugs to haptenate cells with that of the control values (*P<0.05).
Consequently, PBMC were used as a peripheral marker to investigate the antigenicity of sulphamethoxazole and its metabolites in vivo. Administration of nitroso sulphamethoxazole to male Wistar rats caused a concentration-dependent increase in antigen formation, while no detectable haptenation was observed with sulphamethoxazole hydroxylamine or sulphamethoxazole (Figure 3). A significant increase in antigen formation on the surface of red cell depleted splenocytes and epidermal keratinocytes was also observed when nitroso sulphamethoxazole-treated rats were compared to those that received DMSO (vehicle) alone (Figure 4). Binding of nitroso sulphamethoxazole to splenocytes (10.2±4.1%; 100 mg kg−1) and keratinocytes (11.9±4.0%; 100 mg kg−1) was similar to that obtained with PBMC (9.5±0.6%; 100 mg kg−1). However, approximately 5% of the keratinocytes gave an intense fluorescent signal indicative of a cell population particularly susceptible to haptenation (Figure 4b).
Figure 3.
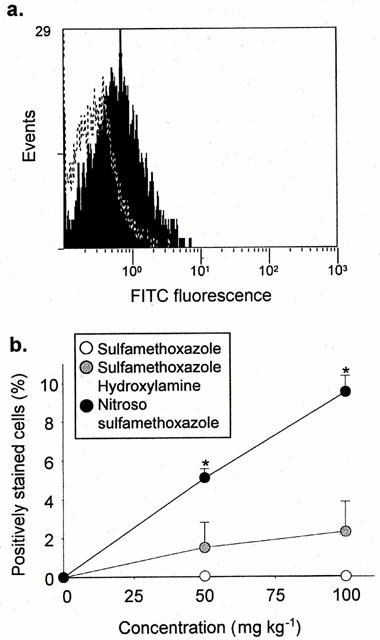
In vivo cell surface haptenation of rat PBMC after administration of sulphamethoxazole and its metabolites for 1 h. PBMC were isolated and haptenation was determination by flow cytometry using a specific anti-sulphamethoxazole antibody. The majority of cells were gated for analysis and the forward threshold was raised to exclude debris. (a) Flow cytometric traces showing differential staining of PBMC populations from animals administered DMSO (dotted line) and nitroso sulphamethoxazole (shaded; 100 mg kg−1). (b) Graph showing cell surface haptenation of rat PBMC after administration of nitroso sulphamethoxazole, but not sulphamethoxazole hydroxylamine or sulphamethoxazole. The results are presented as the percentage of positively stained cells from four rats, incubations carried out in triplicate. Statistical analysis compares drug-treated rats with animals administered DMSO (*P<0.05).
Figure 4.
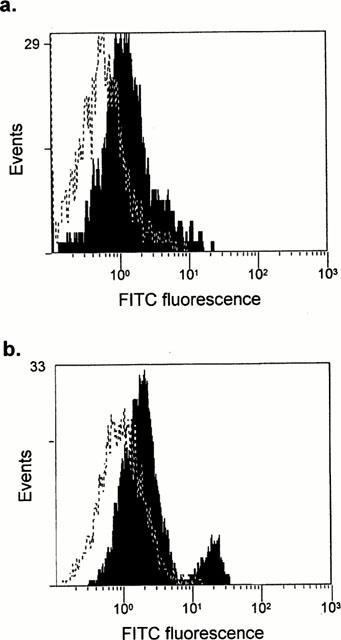
In vivo cell surface haptenation of rat splenocyes (a) and epidermal keratinocytes (b) following the administration of nitroso sulphamethoxazole for 1 h. Viable cells were isolated using standard procedures (see Methods) and haptenation was determination by flow cytometry using a specific anti-sulphamethoxazole antibody. Flow cytometric traces show differential staining of cells from animals administered DMSO (dotted line) or nitroso sulphamethoxazole (shaded; 100 mg kg−1). The results are representive of one out of four rats.
Diethyl maleate binds irreversibly to glutathione and is frequently used to deplete intracellular glutathione (Boyland & Chasseaud, 1967; Kubal et al., 1995). In these studies, diethyl maleate caused a significant depletion of hepatic (14.6±9.6% of control levels; P<0.05) and extrahepatic [PBMC (55.0±22.2% of control levels; P<0.05); splenocytes (51.4±17.6% of control levels; P<0.05)] glutathione. Administration of sulphamethoxazole hydroxylamine to diethyl maleate-treated rats caused a significant increase in antigen formation (9.7±3.8%; 100 mg kg−1; P<0.05), when compared with rats administered with sulphamethoxazole hydroxylamine alone (2.2±1.3% 100 mg kg−1). Nitroso sulphamethoxazole-modified PBMC were detected in two out of four diethyl maleate-treated animals administered with sulphamethoxazole (4.4±1.0 and 5.8±1.5%; 100 mg kg−1).
Nitroso sulphamethoxazole stimulates a T-cell response in naïve rats
The immunogenicity of sulphamethoxazole, sulphamethoxazole hydroxylamine and nitroso sulphamethoxazole was determined by assessment of ex vivo T-cell proliferation. Splenocytes, but not PBMC, from animals administered with nitroso sulphamethoxazole (0.01 – 10 mg kg−1) displayed a concentration-dependent proliferative response on restimulation with nitroso sulphamethoxazole (1 – 25 μg ml−1; Figures 5 and 6). Concentrations of nitroso sulphamethoxazole above 25 μg ml−1 inhibited proliferation; however, these cells retained their capacity to exclude typan blue. Restimulation with the structurally related compound nitroso benzene produced a weak response [stimulation index (SI) 4.2±1.0; P<0.05]. There was no discernible proliferation on restimulation with sulphamethoxazole, sulphamethoxazole hydroxylamine, N-acetyl sulphamethoxazole or sulphamethoxazole-conjugated human serum albumin. Animals administered sulphamethoxazole, sulphamethoxazole hydroxylamine or DMSO did not respond to any of the test compounds (P>0.05).
Figure 5.
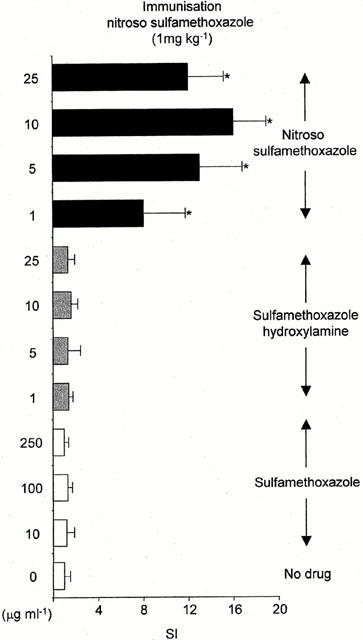
Proliferative response of splenocytes from nitroso sulphamethoxazole-treated rats (1 mg kg−1) cultured with sulphamethoxazole, sulphamethoxazole hydroxylamine or nitroso sulphamethoxazole for 72 h. Proliferation was determined by incorporation of [3H]-thymidine over an additional 8 h. The cpm in the control cultures (DMSO alone) did not exceed 600. Results are presented as mean±s.d. from four rats, incubations carried out in triplicate. Statistical analysis compares drug-treated splenocytes with cells containing DMSO (*P<0.05).
Figure 6.
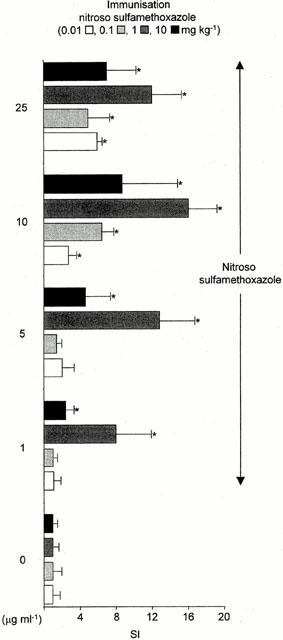
Dose-dependent proliferative response of splenocytes from nitroso sulphamethoxazole-treated rats (0.01 – 10 mg kg−1) cultured with nitroso sulphamethoxazole for 72 h. Proliferation was determined by incorporation of [3H]-thymidine over an additional 8 h. The c.p.m. in the control cultures (DMSO alone) did not exceed 600. Results are presented as mean±s.d. from four rats, incubations carried out in triplicate. Statistical analysis compares drug-treated splenocytes with cells containing DMSO (*P<0.05).
Certain animals were dosed with diethyl maleate prior to administration of sulphamethoxazole or sulphamethoxazole hydroxylamine. Diethyl maleate caused a significant decrease in glutathione (see above). Glutathione levels were allowed to recover before the animals were sacrificed. Splenocyte cultures from sulphamethoxazole hydroxylamine-treated rats (SI 2.8±1.3; P<0.05; Table 1), but not sulphamethoxazole (P>0.05), responded weakly to nitroso sulphamethoxazole (10 μg ml−1). No proliferation was observed with sulphamethoxazole or sulphamethoxazole hydroxylamine.
Table 1.
T-cell reactivity of sulphamethoxazole and its metabolites in vivo in the rat
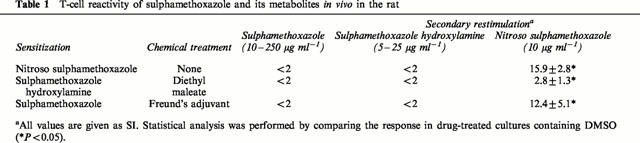
Sulphamethoxazole stimulates a T-cell response in adjuvant-primed rats
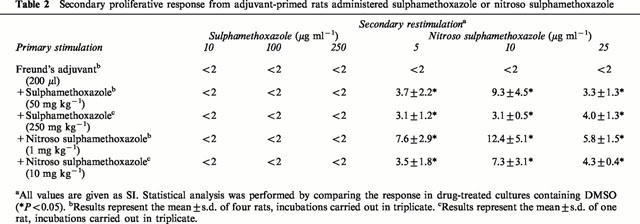
Adjuvants (both endogenous and exogenous) regulate the quantity and quality of a cellular response to known antigens by providing signal 2 and/or signal 3 (Vogel, 1998). To investigate the role of co-stimulation in the immunogenicity of sulphamethoxazole, rats were administered test compounds in CFA. Splenocytes from nitroso sulphamethoxazole-treated rats proliferated in response to nitroso sulphamethoxazole (Table 2). Interestingly, adjuvant-primed rats dosed with sulphamethoxazole did produce a response, but only to nitroso sulphamethoxazole (Table 2). Cells from all rats responded weakly to sulphamethoxazole hydroxylamine, but not to sulphamethoxazole.
Table 2.
Secondary proliferative response from adjuvant-primed rats administered sulphamethoxazole or nitroso sulphamethoxazole
T-cell recognition of nitroso sulphamethoxazole requires cellular conjugation
The role of cellular conjugation in T-cell proliferation to nitroso sulphamethoxazole was determined by: firstly, pulsing splenocytes from nitroso sulphamethoxazole-treated rats with nitroso sulphamethoxazole (10 μg ml−1; 0.1 – 72 h); secondly, the addition of glutathione (300 μg ml−1; 0.1 – 72 h); and thirdly, flow cytometric analysis of nitroso sulphamethoxazole-pulsed cells. Pulsing cultures with nitroso sulphamethoxazole and the addition of glutathione, which reduces nitroso sulphamethoxazole (Naisbitt et al., 1999), did not prevent proliferation (Figure 7a, b). These data demonstrate that T-cell proliferation does not require the continuous presence of the drug (metabolite). Consistent with these observations, nitroso sulphamethoxazole bound to the surface of pulsed slenocytes (Figure 7c).
Figure 7.
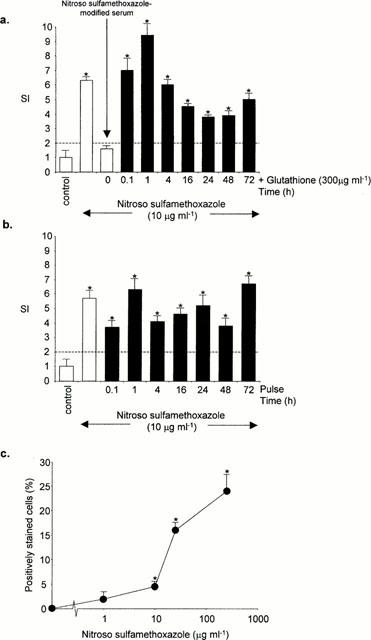
Role of cell surface haptenation in the proliferation of nitroso sulphamethoxazole-specific splenocytes. (a) Splenocytes from nitroso sulphamethoxazole-treated rats (1 mg kg−1) were incubated with nitroso sulphamethoxazole (10 μg ml−1) and glutathione (300 μg ml−1; 0.1 – 72 h). (b) Splenocytes from nitroso sulphamethoxazole-treated rats (1 mg kg−1) were pulsed with nitroso sulphamethoxazole (10 μg ml−1; 0.1 – 72 h), washed and resuspended in drug-free medium for the remainder of the incubation period. After 72 h, proliferation was determined by incorporation of [3H]-thymidine over an additional 8 h. The results are presented as mean±s.d. of one representative rat out of four. Statistical analysis compares drug-treated splenocytes with cells containing DMSO alone (*P<0.05). (c) Cell surface haptenation of rat splenocytes after incubation with nitroso sulphamethoxazole in culture medium for 1 h. Fluorescence intensity of viable cells was analysed by flow cytometry using an anti-sulphamethoxazole antibody. The results are expressed as the percentage of positively stained cells from three experiments carried out in triplicate. Statistical analysis compares the ability of nitroso sulphamethoxazole to haptenate cells with that of control values (*P<0.05).
Splenocytes from nitroso sulphamethoxazole-treated rats did not respond to nitroso sulphamethoxazole-modified serum protein (i.e., medium incubated with nitroso sulphamethoxazole for 5 min, followed by the addition of glutathione) (Figure 7a).
Phenotypic analysis of nitroso sulphamethoxazole-specific T-lymphocytes
CD4+ and CD8+ depletion experiments were performed using immunomagnetic microbeads. Depleted cells were greater than 99.0% CD4+ and 99.5% CD8+, as determined by dual colour flow cytometry (results not shown). Splenocyte cultures from nitroso sulphamethoxazole-treated (1 mg kg−1) rats, containing either CD4+ or CD8+ depleted cells, proliferated in response to nitroso sulphamethoxazole (Figure 8). No proliferation was observed with sulphamethoxazole or sulphamethoxazole hydroxylamine.
Figure 8.
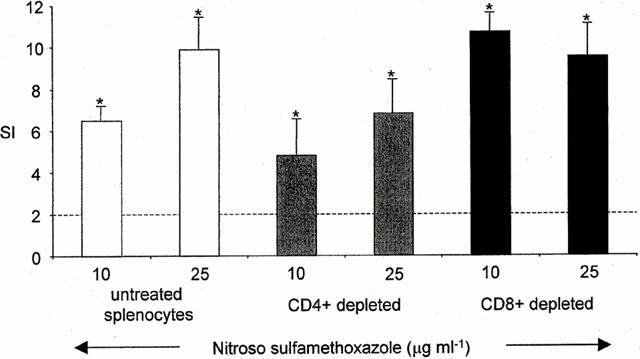
Proliferative response of CD4+ and CD8+ depleted splenocytes from nitroso sulphamethoxazole-treated rats (1 mg kg−1) cultured with nitroso sulphamethoxazole for 72 h. Proliferation was determined by incorporation of [3H]-thymidine over an additional 8 h. CD4+ and CD8+ depletion was performed using immunomagnetic microbeads. The results are presented as mean±s.d. from four rats, incubations carried out in triplicate. Statistical analysis compares drug-treated splenocytes with cells containing DMSO (*P<0.05).
T-cells isolated from nitroso sulphamethoxazole-treated rat spleen were 70.8±3.6% CD4+ and 29.2±3.3% CD8+, as determined by flow cytometry. Of these cells, 5.0±1.4% CD4+ cells expressed the activation marker CD25+, whereas 11.3±4.2% CD8+ cells were CD25+ positive. Splenocytes incubated with nitroso sulphamethoxazole ex vivo for 72 h were 78.5±2.9% CD4+ and 21.5±2.8% CD8+. Of these, 43.0±12.9% of the CD4+ cells and 7.8±2.8% of the CD8+ expressed CD25+.
Nitroso sulphamethoxazole-specific T-lymphocytes secrete IFN-γ, but not IL-4
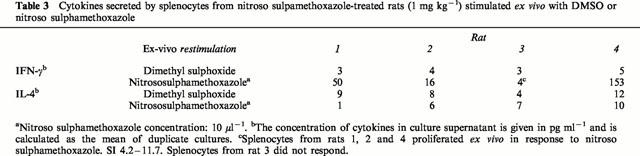
Splenocytes from nitroso sulphamethoxazole-treated rats (1 mg kg−1) produced IFN-γ, but not IL-4, on restimulation with nitroso sulphamethoxazole (Table 3). Neither IFN-γ nor IL-4 were detectable in cultures containing DMSO or from DMSO-treated rats (restimulated with DMSO or nitroso sulphamethoxazole).
Table 3.
Cytokines secreted by splenocytes from nitroso sulpamethoxazole-treated rats (1 mg kg−1) stimulated ex vivo with DMSO or nitroso sulphamethoxazole
Discussion
Sulphamethoxazole is metabolised to sulphamethoxazole hydroxylamine – a pro-hapten – in human subjects and laboratory animals (Cribb et al., 1990; 1992; Gill et al., 1997). Further non-enzymatic oxidation yields nitroso sulphamethoxazole (Cribb et al., 1991; Naisbitt et al., 1996), an electrophilic intermediate that, in the absence of glutathione and/or other antioxidants, binds to sulfhydryl-containing cell surface proteins (Naisbitt et al., 1999) (Figure 1). Thus, haptenation is likely to occur only when the critical balance between bioactivation and detoxication is disturbed. Such a process has been postulated to be involved in the pathogenesis of sulphamethoxazole hypersensitivity reactions, and would be in line with the classical hapten hypothesis of drug hypersensitivity. However, this basic concept has recently been questioned (Mauri-Hellweg et al., 1995; Schnyder et al., 1997; Zanni et al., 1998; Von greyerz et al., 1999): it has been proposed that sulphamethoxazole binds directly – via a reversible, non-covalent interaction – to the MHC complex (or a peptide embedded in the MHC cleft) in the absence of drug metabolism and antigen processing. In order to further investigate this, we have developed an animal model of sulphamethoxazole immunogenicity in order to delineate (a) the distribution of cell surface antigen formation; and (b) the role of drug metabolism in antigen presentation to T-cells.
Previous in vivo studies to characterize hapten formation in the liver were unsuccessful (Cribb et al., 1996). This may have been due to the very efficient detoxication mechanisms that are present in the liver, which would have precluded covalent binding (Gill et al., 1997; Cribb et al., 1995). In this study, we have concentrated on the skin since it expresses high levels of specific CYP isozymes and low thiol levels (i.e. the balance is in favour of bioactivation), in relation to the liver (Caldwell et al., 1990; Roos & Merk, 2000), and may therefore represent an environment susceptible to haptenation by chemically reactive drug metabolites. In accordance with this, nitroso sulphamethoxazole, but not sulphamethoxazole hydroxylamine or sulphamethoxazole, was found to bind to the surface of keratinocytes, and to a lesser extent, to PBMC and splenocytes in vivo (Figures 3 and 4). Cell surface antigen formation was evaluated with a hapten inhibitable antibody that recognises sulphamethoxazole and its oxidative metabolites, but not other sulphonamides (Gill et al., 1997). Such binding to cells in organs distant from the site of injection shows that nitroso sulphamethoxazole is sufficiently stable to circulate in the periphery. However, it is also likely that in man, metabolism of sulphamethoxazole to the hydroxylamine may occur in target tissues, as recently demonstrated for keratinocytes (Reilly et al., 2000). Based on our results, and the demonstration that sulphamethoxazole hydroxylamine can bind to human keratinocytes (Reilly et al., 2000), it may be hypothesized that naïve T-cells recognise sulphamethoxazole-conjugated peptides derived from skin; however, this is unknown at present and requires further investigation.
In a previous study using the same rat model, we showed that administration of nitroso sulphamethoxazole resulted in the formation of anti-drug antibodies (Gill et al., 1997). Our present study extends those findings by showing that haptenation of the nitroso metabolite also leads to a cellular response (Table 3). Thus, splenocytes from rats administered with nitroso sulphamethoxazole, but not sulphamethoxazole hydroxylamine or the parent drug, responded to nitroso sulphamethoxazole on restimulation ex vivo (Figure 5). Both CD4+ and CD8+ T-cells responded, as shown by CD25+ expression and ex vivo proliferation of CD4+ and CD8+ depleted splenocytes. Simultaneous activation of both CD4+ and CD8+ T-cells shows that in our model sulphamethoxazole-conjugated peptides would have been presented by MHC class II and MHC class I, respectively. In accordance with animal models of contact sensitivity (Xu et al., 1996; Singh et al., 1999), the responding T-cells secreted IFN-γ, but not IL-4, indicating a Th1/Tc1 response. Consistent with our results, IFN-γ production has been demonstrated in PBMC from patients with anticonvulsant-induced toxic epidermal necrolysis (Leyva et al., 2000; Koga et al., 2000).
These in vivo observations provide unequivocal evidence that bioactivation of sulphamethoxazole to a chemically reactive nitroso metabolite is critical for the development of a primary cellular immune response. A recall response to sulphamethoxazole was not detected. This contrasts with previous in vitro studies using cells obtained from sensitized human subjects (Mauri-Hellweg et al., 1995; Schnyder et al., 1997; Zanni et al., 1998; von Greyerz et al., 1999). In these studies, sulphamethoxazole, in the absence of drug metabolism, could act as an antigen and stimulate T-cell proliferation. In collaboration with Pichler, we have demonstrated that, although the T-cell repertoire of hypersensitive patients is skewed towards responses against the parent drug, T-cell clones that proliferate in the presence of nitroso sulphamethoxazole, do exist (Schnyder et al., 2000; Burkhart et al., 2001). It is possible that both pathways of antigen presentation are important for drug hypersensitivity reactions. The critical question that remains to be addressed is, to what extent the two mechanisms contribute towards the initiation of a primary cellular immune response and to what extent each mechanism might contribute to the elicitation of tissue damage.
The response to covalently-bound sulphamethoxazole was seen after pulsing splenocytes for only 0.1 h (Figure 7b). These findings demonstrate that the antigenic species is generated rapidly and provides evidence for the role of drug metabolism and protein conjugation in recognition of sulphamethoxazole. The identity of the antigenic protein (i.e., serum, cellular or both) in the proliferation assay remains unresolved; however, stimulation of splenocytes with nitroso sulphamethoxazole, but not splenocytes incubated with a mixture of nitroso sulphamethoxazole and glutathione (i.e., sulphamethoxazole-modified serum protein; Figure 7a) or sulphamethoxazole-conjugated human albumin, suggests that a cellular protein is the ultimate antigenic species. Several lines of evidence support this hypothesis. First, nitroso sulphamethoxazole has been shown to haptenate the surface of viable PBMC (and splenocytes in the proliferation assay in these studies), whereas sulphamethoxazole-serum conjugates were identified only when albumin was chemically modified to increase the free thiol content (Naisbitt et al., 1999). Secondly, studies with the experimental immunogen dinitrophenol demonstrated that dinitrophenyl conjugated lymphocytes elicit hypersensitivity reactions to a greater extent than dinitrophenyl conjugated to serum proteins (Sjoeberg et al., 1978).
We have previously demonstrated that sulphamethoxazole hydroxylamine and nitroso sulphamethoxazole undergo extensive reduction in rats (Gill et al., 1997), suggesting that detoxication processes are predominant. This is probably the reason why administration of sulphamethoxazole hydroxylamine did not result in either haptenation or a cellular response, unless glutathione was depleted, i.e. the balance was changed in favour of bioactivation.There is a clinical parallel for this since HIV positive patients have a relative deficiency of circulating thiols (Eck et al., 1989; Walmsley et al., 1997; Naisbitt et al., 2000). Taken together with the fact that sulphamethoxazole hydroxylamine readily circulates in peripheral blood (van der Ven et al., 1995), this may be a major factor accounting for the high prevalence of sulphamethoxazole hypersensitivity reactions in HIV-positive patients.
The interaction between a MHC-restricted antigen and the T-cell receptor (signal 1) is insufficient to stimulate a full immune response and in the absence of secondary signals, tolerance supersedes. Secondary signals include pro-inflammatory cytokines that act indirectly on antigen presenting cells by upregulating the expression of co-stimulatory molecules and polarising cytokines, released from the innate immune system (Curtsinger et al., 1999). These basic concepts incorporate a major aspect of the danger hypothesis recently described by Matzinger (1994; 1998). Exogenous agents such as lipopolysacarides and teichoic acids of bacterial cell walls are potent, well-defined danger signals; however, recent studies show that danger can also be received in the absence of foreign substances from virally infected cells or cells killed by necrosis or apoptosis (Gallucci et al., 1999; Shi et al., 2000). In our model, it is possible that nitroso sulphamethoxazole provided signal one while danger signals were derived from the release of inflammatory cytokines produced by necrotic cells consequent upon the known cytotoxicity of nitroso sulphamethoxazole (Rieder et al., 1988; 1995). In these studies, the role of co-stimulation in the immunogenicity of sulphamethoxazole was determined by administering test compounds in CFA, one of the strongest adjuvants known (Gallucci et al., 1999). Interestingly, splenocytes from sulphamethoxazole-treated rats proliferated in response to nitroso sulphamethoxazole, but not sulphamethoxazole (Tables 1 and 3). These observations provide unequivocal evidence that (a) nitroso sulphamethoxazole is generated in vivo; and (b) co-stimulation is required to stimulate an efficient response. Flow cytometric analysis of cell surface antigen formation indicated that haptenation could not be detected after administration of sulphamethoxazole alone. This may be because the critical antigen is either inaccessible or that the immune system responds to very small amounts of highly localised antigen. From these observations, we reason that an imbalance between oxidation of sulphamethoxazole and the reduction of its reactive metabolites, may predispose to hypersensitivity. However, the decision as to whether antigen presentation stimulates an immune response, seems to be determined by the extent of co-stimulation (danger), and not hapten density. These data are consistent with recent case reports where hypersensitivity to sulphasalazine was associated with the reactivation of human herpesvirus 6 (Suzuki et al., 1998; Tohyama et al., 1998).
In conclusion, we have demonstrated in an in vivo model that drug metabolism and cell surface binding is required in the stimulation of a primary immune response after administration of sulphamethoxazole and its metabolites. A response to the proximate toxin (hydroxylamine) and the parent drug was only observed under non-physiological conditions; however, these conditions may exist in HIV positive patients, and may account for the high frequency of hypersensitivity. An animal model of sulphamethoxazole antigenicity and immunogenicity, coupled with techniques of T-cell transfer for models of immune-mediated tissue damage (Nishimura & Ohta, 1999; Ohta et al., 2000) and transgenics for effector systems (e.g., Fas, perforin, TNF-α and IFN-γ), provide an unprecedented opportunity to develop an animal model of drug hypersensitivity which has been a quest of workers in the field for more than 20 years.
Acknowledgments
The authors would like to express their thanks to Dr A.C. Stalford for assistance with the synthesis of nitroso sulphamethoxazole. We also acknowledge Dr J.W. Coleman for his contribution in the preparation of this manuscript. These studies were funded by the Wellcome Trust. D.J. Naisbitt holds a Wellcome Trust Research Career Development Fellowship. S.F. Gordon is a PhD student funded by AVERT. B.K. Park is a Wellcome Principal Fellow.
Abbreviations
- CFA
complete Freund's adjuvant
- DMSO
dimethyl sulphoxide
- DMEM
Dulbecco's modified Eagles medium
- FITC
fluorescein isothiocyanate
- HBSS
Hanks balanced salt solution
- HPLC
high performance liquid chromatography
- PBMC
peripheral blood mononuclear cells
- SI
stimulation index
References
- BARRANCO P., LOPEZ-SERRANO M.C. General and epidemiological aspects of allergic drug reactions. Clin. Exp. Allergy. 1998;28 Suppl 4:61–62. [PubMed] [Google Scholar]
- BECKER D.S. Toxic epidermal necrolysis. Lancet. 1998;351:1417–1420. doi: 10.1016/S0140-6736(97)11369-1. [DOI] [PubMed] [Google Scholar]
- BOYLAND E., CHASSEAUD L.F. Enzyme-catalysed conjugations of glutathione with unsaturated compounds. Biochem. J. 1967;104:95–102. doi: 10.1042/bj1040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKHART C., VON GREYERZ S., DEPTA J.P.H., NAISBITT D.J., BRITSCHGI M., PARK B.K., PICHLER W.J. Influence of reduced glutathione on the proliferative response of sulphamethoxazole-specific and sulphamethoxazole-metabolite-specific human CD4+ T cells. Br. J. Pharmacol. 2001;132:623–663. doi: 10.1038/sj.bjp.0703845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL J., HOWES A.J., HOTCHKISS S.A. The toxicological significance of xenobiotic metabolism. Food Addit. Contam. 1990;7:S116–S1126. doi: 10.1080/02652039009373862. [DOI] [PubMed] [Google Scholar]
- COLEMAN J.W., BLANCA M. Mechanisms of drug allergy. Immunol. Today. 1998;19:196–198. doi: 10.1016/s0167-5699(97)01231-0. [DOI] [PubMed] [Google Scholar]
- COOPMAN S.A., JOHNSON R.A., PLATT R., STERN R.S. Cutaneous disease and drug reactions in HIV infection [see comments] N. Engl. J. Med. 1993;328:1670–1674. doi: 10.1056/NEJM199306103282304. [DOI] [PubMed] [Google Scholar]
- CORREIA O., DELGADO L., RAMOS J.P., RESENDE C., TORRINHA J.A. Cutaneous T-cell recruitment in toxic epidermal necrolysis. Further evidence of CD8+ lymphocyte involvement [see comments] Arch. Dermatol. 1993;129:466–468. [PubMed] [Google Scholar]
- CRIBB A.E., MILLER M., LEEDER J.S., HILL J., SPIELBERG S.P. Reactions of the nitroso and hydroxylamine metabolites of sulphamethoxazole with reduced glutathione. Implications for idiosyncratic toxicity. Drug Metab. Dispos. 1991;19:900–906. [PubMed] [Google Scholar]
- CRIBB A.E., NUSS C.E., ALBERTS D.W., LAMPHERE D.B., GRANT D.M., GROSSMAN S.J., SPIELBERG S.P. Covalent binding of sulphamethoxazole reactive metabolites to human and rat liver subcellular fractions assessed by immunochemical detection. Chem. Res. Toxicol. 1996;9:500–507. doi: 10.1021/tx950167j. [DOI] [PubMed] [Google Scholar]
- CRIBB A.E., SPIELBERG S.P. Hepatic microsomal metabolism of sulphamethoxazole to the hydroxylamine. Drug Metab. Dispos. 1990;18:784–787. [PubMed] [Google Scholar]
- CRIBB A.E., SPIELBERG S.P. Sulphamethoxazole is metabolized to the hydroxylamine in humans. Clin. Pharmacol. Ther. 1992;51:522–526. doi: 10.1038/clpt.1992.57. [DOI] [PubMed] [Google Scholar]
- CRIBB A.E., SPIELBERG S.P., GRIFFIN G.P. N4-hydroxylation of sulphamethoxazole by cytochrome P450 of the cytochrome P4502C subfamily and reduction of sulphamethoxazole hydroxylamine in human and rat hepatic microsomes. Drug Metab. Dispos. 1995;23:406–414. [PubMed] [Google Scholar]
- CURTSINGER J.M., SCHMIDT C.S., MONDINO A., LINS D.C., KEDL R.M., JENKINS M.K., MESCHER M.F. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- DAFTARIAN M.P., FILION L.G., CAMERON W., CONWAY B., ROY R., TROPPER F., DIAZ-MITOMA F. Immune response to sulphamethoxazole in patients with AIDS. Clin. Diagn. Lab. Immunol. 1995;2:199–204. doi: 10.1128/cdli.2.2.199-204.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECK H.P., GMUNDER H., HARTMANN M., PETZOLDT D., DANIEL V., DROGE W. Low concentrations of acid-soluble thiol (cysteine) in the blood plasma of HIV-1-infected patients. Biol. Chem. Hoppe Seyler. 1989;370:101–108. doi: 10.1515/bchm3.1989.370.1.101. [DOI] [PubMed] [Google Scholar]
- GALLUCCI S., LOLKEMA M., MATZINGER P. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- GILL H.J., HOUGH S.J., NAISBITT D.J., MAGGS J.L., KITTERINGHAM N.R., PIRMOHAMED M., PARK B.K. The relationship between the disposition and immunogenicity of sulphamethoxazole in the rat. J. Pharmacol. Exp. Ther. 1997;282:795–801. [PubMed] [Google Scholar]
- GILL H.J., MAGGS J.L., MADDEN S., PIRMOHAMED M., PARK B.K. The effect of fluconazole and ketoconazole on the metabolism of sulphamethoxazole. Br. J. Clin. Pharmacol. 1996;42:347–353. doi: 10.1046/j.1365-2125.1996.40110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIEM P., WULFERINK M., SACHS B., GONZALEZ J.B., GLEICHMANN E. Allergic and autoimmune reactions to xenobiotics: how do they arise. Immunol. Today. 1998;19:133–141. doi: 10.1016/s0167-5699(97)01219-x. [DOI] [PubMed] [Google Scholar]
- GRUCHALLA R.S., PESENKO R.D., DO T.T., SKIEST D.J. Sulfonamide-induced reactions in desensitized patients with AIDS–the role of covalent protein haptenation by sulphamethoxazole. J. Allergy Clin. Immunol. 1998;101:371–378. doi: 10.1016/S0091-6749(98)70250-7. [DOI] [PubMed] [Google Scholar]
- HAMEL D., WHITE C., EATON D. Determination of γ-glutamylcysteine synthetase and glutathione synthetase activity by HPLC. Toxicology Methods. 1992;1:273–279. [Google Scholar]
- HERTL M., JUGERT F., MERK H.F. CD8+ dermal T cells from a sulphamethoxazole-induced bullous exanthem proliferate in response to drug-modified liver microsomes. Br. J. Dermatol. 1995;132:215–220. doi: 10.1111/j.1365-2133.1995.tb05016.x. [DOI] [PubMed] [Google Scholar]
- HESS D.A., SISSON M.E., SURIA H., WIJSMAN J., PUVANESASINGHAM R., MADRENAS J., RIEDER M.J. Cytotoxicity of sulfonamide reactive metabolites: apoptosis and selective toxicity of CD8(+) cells by the hydroxylamine of sulphamethoxazole. FASEB. J. 1999;13:1688–1698. doi: 10.1096/fasebj.13.13.1688. [DOI] [PubMed] [Google Scholar]
- KALISH R.S., ASKENASE P.W. Molecular mechanisms of CD8+ T cell-mediated delayed hypersensitivity: implications for allergies, asthma, and autoimmunity. J. Allergy Clin. Immunol. 1999;103:192–199. doi: 10.1016/s0091-6749(99)70489-6. [DOI] [PubMed] [Google Scholar]
- KOGA T., KUBOTA Y., NAKAYAMA J. Interferon-gamma production in the peripheral lymphocytes of a patient with carbamazepine hypersensitivity syndrome [letter] Acta. Derm. Venereol. 2000;80:73. doi: 10.1080/000155500750012702. [DOI] [PubMed] [Google Scholar]
- KOOPMANS P.P., VAN DER VEN A.J., VREE T.B., VAN DER MEER J.W. Pathogenesis of hypersensitivity reactions to drugs in patients with HIV infection: allergic or toxic? [editorial] AIDS. 1995;9:217–222. [PubMed] [Google Scholar]
- KUBAL G., MEYER D.J., NORMAN R.E., SADLER P.J. Investigations of glutathione conjugation in vitro by 1H NMR spectroscopy. Uncatalysed and glutathione transferase catalysed reactions. Chem. Res. Toxicol. 1995;8:780–791. doi: 10.1021/tx00047a019. [DOI] [PubMed] [Google Scholar]
- LEYVA L., TORRES M.J., POSADAS S., BLANCA M., BESSO G., O'VALLE F., DEL MORAL R.G., SANTAMARIA L.F., JUAREZ C. Anticonvulsant-induced toxic epidermal necrolysis: monitoring the immunologic response. J. Allergy Clin. Immunol. 2000;105:157–165. doi: 10.1016/s0091-6749(00)90191-x. [DOI] [PubMed] [Google Scholar]
- MATZINGER P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- MATZINGER P. An innate sense of danger. Semin. Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- MAURI-HELLWEG D., BETTENS F., MAURI D., BRANDER C., HUNZIKER T., PICHLER W.J. Activation of drug-specific CD4+ and CD8+ T cells in individuals allergic to sulfonamides, phenytoin, and carbamazepine. J. Immunol. 1995;155:462–472. [PubMed] [Google Scholar]
- MEEKINS C.V., SULLIVAN T.J., GRUCHALLA R.S. Immunochemical analysis of sulfonamide drug allergy: identification of sulphamethoxazole-substituted human serum proteins. J. Allergy Clin. Immunol. 1994;94:1017–1024. doi: 10.1016/0091-6749(94)90120-1. [DOI] [PubMed] [Google Scholar]
- MITRA A.K., THUMMEL K.E., KALHORN T.F., KHARASCH E.D., UNADKAT J.D., SLATTERY J.T. Inhibition of sulphamethoxazole hydroxylamine formation by fluconazole in human liver microsomes and healthy volunteers. Clin. Pharmacol. Ther. 1996;59:332–340. doi: 10.1016/S0009-9236(96)80011-7. [DOI] [PubMed] [Google Scholar]
- MIYAUCHI H., HOSOKAWA H., AKAEDA T., IBA H., ASADA Y. T-cell subsets in drug-induced toxic epidermal necrolysis. Possible pathogenic mechanism induced by CD8-positive T cells. Arch. Dermatol. 1991;127:851–855. [PubMed] [Google Scholar]
- NALDI L., CONFORTI A., VENEGONI M., TRONCON M.G., CAPUTI A., GHIOTTO E., COCCI A., MORETTI U., VELO G., LEONE R. Cutaneous reactions to drugs. An analysis of spontaneous reports in four Italian regions. Br. J. Clin. Pharmacol. 1999;48:839–846. doi: 10.1046/j.1365-2125.1999.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAISBITT D.J., HOUGH S.J., GILL H.J., PIRMOHAMED M., KITTERINGHAM N.R., PARK B.K. Cellular disposition of sulphamethoxazole and its metabolites: implications for hypersensitivity. Br. J. Pharmacol. 1999;126:1393–1407. doi: 10.1038/sj.bjp.0702453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAISBITT D.J., O'NEILL P.M., PIRMOHAMED M., PARK B.K. Synthesis and reactions of nitroso sulphamethoxazole with biological nucleophiles - implications for immune-mediated toxicity. Bioorg. Med. Chem. Let. 1996;6:1511–1516. [Google Scholar]
- NAISBITT D.J., VILAR F.J., STALFORD A.C., WILKINS E.G.L., PIRMOHAMED M., PARK B.K. Plasma cysteine deficiency and decreased reduction of nitroso sulphamethoxazole with HIV infection. AIDS Res. Hum. Retroviruses. 2000;16:1929–1938. doi: 10.1089/088922200750054657. [DOI] [PubMed] [Google Scholar]
- NISHIMURA T., OHTA A. A critical role for antigen-specific Th1 cells in acute liver injury in mice. J. Immunol. 1999;162:6503–6509. [PubMed] [Google Scholar]
- OHTA A., SEKIMOTO M., SATO M., KODA T., NISHIMURA S., IWAKURA Y., SEKIKAWA K., NISHIMURA T. Indispensable role for TNF-α and IFN-γ at the effector phase of liver injury mediated by Th1 cells specific to hepatitis B virus surface antigen. J. Immunol. 2000;165:956–961. doi: 10.4049/jimmunol.165.2.956. [DOI] [PubMed] [Google Scholar]
- REILLY T.P., LASH L.H., DOLL M.A., HEIN D.W., WOSTER P.M., SVENSSON C.K. A role for bioactivation and covalent binding within epidermal keratinocytes in sulfonamide-induced cutaneous drug reactions. J. Invest. Dermatol. 2000;114:1164–1173. doi: 10.1046/j.1523-1747.2000.00985.x. [DOI] [PubMed] [Google Scholar]
- RIEDER M.J., KRAUSE R., BIRD I.A., DEKABAN G.A. Toxicity of sulfonamide-reactive metabolites in HIV-infected, HTLV-infected, and noninfected cells. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 1995;8:134–140. [PubMed] [Google Scholar]
- RIEDER M.J., UETRECHT J., SHEAR N.H., SPIELBERG S.P. Synthesis and in vitro toxicity of hydroxylamine metabolites of sulfonamides. J. Pharmacol. Exp. Ther. 1988;244:724–728. [PubMed] [Google Scholar]
- ROOS T.C., MERK H.F. Important drug interactions in dermatology. Drugs. 2000;59:181–192. doi: 10.2165/00003495-200059020-00003. [DOI] [PubMed] [Google Scholar]
- SCHNYDER B., BURKHART C., SCHNYDER-FRUTIG K., VON GREYERZ S., NAISBITT D.J., PIRMOHAMED M., PARK B.K., PICHLER W.J. Recognition of sulphamethoxazole and its reactive metabolites by drug-specific CD4+ T cells from allergic individuals. J. Immunol. 2000;164:6647–6654. doi: 10.4049/jimmunol.164.12.6647. [DOI] [PubMed] [Google Scholar]
- SCHNYDER B., MAURI-HELLWEG D., ZANNI M., BETTENS F., PICHLER W.J. Direct, MHC-dependent presentation of the drug sulphamethoxazole to human alphabeta T cell clones. J. Clin. Invest. 1997;100:136–141. doi: 10.1172/JCI119505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHI Y., ZHENG W., ROCK K.L. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. PNAS. 2000;97:14590–14595. doi: 10.1073/pnas.260497597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH V.K., MEHROTRA S., AGARWAL S.S. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol. Res. 1999;20:147–161. doi: 10.1007/BF02786470. [DOI] [PubMed] [Google Scholar]
- SJOEBERG B., SUMERSKA T., BINNS R.M., BALFOUR B.M. Contact sensitivity in the pig. II. Induction by intralymphatic infusion of DNP-conjugated cells. Int. Arch. Allergy Appl. Immunol. 1978;57:114. [PubMed] [Google Scholar]
- SUZUKI Y., INAGI R., AONO T., YAMANISHI K., SHIOHARA T. Human herpesvirus 6 infection as a risk factor for the development of severe drug-induced hypersensitivity syndrome. Arch. Dermatol. 1998;134:1108–1112. doi: 10.1001/archderm.134.9.1108. [DOI] [PubMed] [Google Scholar]
- TOHYAMA M., YAHATA Y., YASUKAWA M., INAGI R., URANO Y., YAMANISHI K., HASHIMOTO K. Severe hypersensitivity syndrome due to sulfasalazine associated with reactivation of human herpesvirus 6. Arch. Dermatol. 1998;134:1113–1117. doi: 10.1001/archderm.134.9.1113. [DOI] [PubMed] [Google Scholar]
- VAN DER VEN A.J., VREE T.B., VAN EWIJK-BENEKEN KOLMER E.W., KOOPMANS P.P., VAN DER MEER J.W. Urinary recovery and kinetics of sulphamethoxazole and its metabolites in HIV-seropositive patients and healthy volunteers after a single oral dose of sulphamethoxazole. Br. J. Clin. Pharmacol. 1995;39:621–625. doi: 10.1111/j.1365-2125.1995.tb05721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL F.R. Adjuvants in perspective. Dev. Biol. Stand. 1998;92:241–248. [PubMed] [Google Scholar]
- VON GREYERZ S., ZANNI M.P., FRUTIG K., SCHNYDER B., BURKHART C., PICHLER W.J. Interaction of sulfonamide derivatives with the TCR of sulphamethoxazole-specific human alpha beta+ T cell clones. J. Immunol. 1999;162:595–602. [PubMed] [Google Scholar]
- WALMSLEY S.L., WINN L.M., HARRISON M.L., UETRECHT J.P., WELLS P.G. Oxidative stress and thiol depletion in plasma and peripheral blood lymphocytes from HIV-infected patients: toxicological and pathological implications. AIDS. 1997;11:1689–1697. doi: 10.1097/00002030-199714000-00005. [DOI] [PubMed] [Google Scholar]
- XU H., DIIULIO N.A., FAIRCHILD R.L. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (IL) 4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J. Exp. Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZANNI M.P., VON GREYERZ S., SCHNYDER B., BRANDER K.A., FRUTIG K., HARI Y., VALITUTTI S., PICHLER W.J. HLA-restricted, processing- and metabolism-independent pathway of drug recognition by human alpha beta T lymphocytes. J. Clin. Invest. 1998;102:1591–1598. doi: 10.1172/JCI3544. [DOI] [PMC free article] [PubMed] [Google Scholar]


