Abstract
The electrophysiological effects of dronedarone, a new nonionidated analogue of amiodarone were studied after chronic and acute administration in dog Purkinje fibres, papillary muscle and isolated ventricular myocytes, and compared with those of amiodarone by applying conventional microelectrode and patch-clamp techniques.
Chronic treatment with dronedarone (2×25 mg−1 kg−1 day p.o. for 4 weeks), unlike chronic administration of amiodarone (50 mg−1 kg−1 day p.o. for 4 weeks), did not lengthen significantly the QTc interval of the electrocardiogram or the action potential duration (APD) in papillary muscle. After chronic oral treatment with dronedarone a small, but significant use-dependent Vmax block was noticed, while after chronic amiodarone administration a strong use-dependent Vmax depression was observed.
Acute superfusion of dronedarone (10 μM), similar to that of amiodarone (10 μM), moderately lengthened APD in papillary muscle (at 1 Hz from 239.6±5.3 to 248.6±5.3 ms, n=13, P<0.05), but shortened it in Purkinje fibres (at 1 Hz from 309.6±11.8 to 287.1±10.8 ms, n=7, P<0.05).
Both dronedarone (10 μM) and amiodarone (10 μM) superfusion reduced the incidence of early and delayed afterdepolarizations evoked by 1 μM dofetilide and 0.2 μM strophantidine in Purkinje fibres.
In patch-clamp experiments 10 μM dronedarone markedly reduced the L-type calcium current (76.5±0.7 %, n=6, P<0.05) and the rapid component of the delayed rectifier potassium current (97±1.2 %, n=5, P<0.05) in ventricular myocytes.
It is concluded that after acute administration dronedarone exhibits effects on cardiac electrical activity similar to those of amiodarone, but it lacks the ‘amiodarone like' chronic electrophysiological characteristics.
Keywords: Action potentials, dronedarone, SR 33589, amiodarone, electrophysiology, antiarrhythmic drugs
Introduction
The CAST (CAST investigators, 1989) and the SWORD (Waldo et al., 1996) studies showed that some Class I/C antiarrhythmic drugs, such as flecainide and encainide, and a ‘pure' Class III drug, D-sotalol, significantly increased the proarrhythmia related postinfarction mortality. These failures in the antiarrhythmic therapy shifted interest more toward amiodarone, a drug which is a particularly effective antiarrhythmic agent (Link et al., 1995) and, in contrast with flecainide and D-sotalol, did not seem to increase (Julian et al., 1997) or even reduce (Pfisterer et al., 1992) postinfarction mortality in different multicenter clinical trials.
Amiodarone was first recognized to lengthen cardiac action potential duration after chronic treatment (Singh & Vaughan Williams, 1970) and was, therefore, classified as a Class III antiarrhythmic drug (Vaughan Williams, 1975). Later it became evident that amiodarone has multiple actions, including use-dependent block of the inward sodium (Mason et al., 1984; Varró et al., 1985; Follmer et al., 1987) and calcium (Nishimura et al., 1989) currents, block of the α and β adrenoceptors (Polster & Broekhuysen, 1976) and it may also have some other yet less well explored properties. Amiodarone, in contrast with pure Class III drugs, decreased dispersion of repolarization between ventricular epicardial, endocardial M cells (Sicouri et al., 1997) and Purkinje fibres (Papp et al., 1996). These features of amiodarone may, at least partly, explain the low proarrhythmic potential of the drug (Lazzara, 1989).
Amiodarone, however, has a relatively wide range of extracardiac side effects (Harris et al., 1983) which has been attributed to the iodine in its chemical structure and can significantly diminish its use in clinical practice. Therefore, a noniodinated benzofuran analogue of amiodarone, dronedarone (SR 33589) was developed and tested in in vitro (Moro et al., 1999; Sun et al., 1999) and in vivo (Manning et al., 1995b) electrophysiological experiments and in experimental arrhythmia models (Finance et al., 1995; Manning et al., 1995a). These latter studies revealed that dronedarone, like amiodarone, possesses strong antiarrhythmic potency. In a recent in vitro study performed in rabbit papillary muscle it was found that dronedarone, both after long-term treatment and after acute application, exerted cardiac electrophysiological effects similar to those of amiodarone (Sun et al., 1999). This led to the conclusion that the electrophysiological effects of dronedarone are similar to those of amiodarone but more potent, despite deletion of iodine from its molecular structure, a finding of importance for the development of future Class III antiarrhythmic compounds (Sun et al., 1999).
However, our results obtained in cardiac preparations isolated from dog hearts and presented in this study showed that the effect of chronic dronedarone treatment strikingly differs from that of long-term administration of amiodarone. Therefore we favour an opposite conclusion, i.e. the unique chronic cardiac electrophysiological effects of amiodarone might be due to the inhibition of the cardiac thyroid receptors (Kodama et al., 1999) which may relate to the iodine in the molecular structure of the compound.
Methods
All experiments were conducted in compliance with the Guide for the Care and Use of Laboratory Animals (USA NIH publication No 85-23, revised 1985). The protocols were approved by the review board of Committee on Animal Research of the University of Szeged (54/1999 OEj).
Conventional microelectrode technique
Adult mongrel dogs (8–14 kg) of either sex were used. Following anaesthesia (sodium pentobarbital, 30 mg kg−1 administered intravenously), the heart of each animal was rapidly removed through right lateral thoracotomy. The hearts were immediately rinsed in oxygenated Tyrode's solution containing (in mM): NaCl, 115; KCl, 4; CaCl2, 1.8; MgCl2, 1; NaHCO3, 20; and glucose, 11. The pH of this solution was 7.40 to 7.45 when gassed with 95% O2 and 5% CO2 at 37°C. Purkinje strands obtained from either ventricle or the tip of the papillary muscles from the right ventricle were individually mounted in a tissue chamber (volume ≈ 50 ml). Each ventricular preparation was initially stimulated (HSE (Hugo Sachs Elektronik) stimulator type 215/II, March-Hugstetten, Germany) at a basic cycle length of 1000 ms (frequency=1 Hz), Purkinje fibre preparations were stimulated at basic cycle length of 500 ms (2 Hz) using 2 ms long rectangular constant voltage pulses isolated from ground and delivered across bipolar platinum electrodes in contact with the preparation. At least 1 h was allowed for each preparation to equilibrate while they were continuously superfused with Tyrode's solution. Temperature of the superfusate was kept constant at 37°C. Transmembrane potentials were recorded using conventional microelectrode recording techniques. Microelectrodes filled with 3 M KCl and having tip resistances of 5–20 mΩ were connected to the input of a high impedance electrometer (HSE microelectrode amplifier type 309), which was referenced to the ground. The first derivative of transmembrane potentials (Vmax) was electronically derived by an HSE differentiator (type 309). The voltage outputs from all amplifiers were displayed on a dual beam memory oscilloscope (Tektronix 2230 100 MHz digital storage oscilloscope, Beaverton, OR, U.S.A.).
The maximum diastolic potential (MDP), action potential amplitude (APA), and APD measured at 50 and 90% repolarization (APD50–90) were obtained using a software developed in our department (HSE-APES) on an IBM 386 microprocessor base personal computer connected to the digital output of the oscilloscope. Dogs were orally treated with 50 mg kg−1 amiodarone or 2×25 mg kg−1 dronedarone every day for 4 weeks. Control dogs were kept in similar condition but they did not receive treatment. Electrocardiogram recordings (limb I–III leads) were taken in the conscious animals before and after the 4 weeks of treatment. In those experiments in which the acute direct effects of the drugs were studied, after the control measurements, the preparations had been superfused for 60 min with Tyrode's solution containing 10 μM of the compounds before the electrophysiologic measurements were commenced. Both amiodarone and dronedarone were dissolved in 100% DMSO to make 10 mM stock solutions. The stock solutions were further diluted in the tissue bath to obtain the desired final drug concentrations.
Whole cell configuration of the patch-clamp technique
Ventricular myocytes were enzymatically dissociated from hearts of mongrel dogs of either sex weighing 10–20 kg following anaesthesia (sodium pentobarbital, 30 mg·kg−1 i.v.) as described earlier in detail (Varró et al., 2000).
One drop of cell suspension was placed within a transparent recording chamber mounted on the stage of an inverted microscope (TMS, Nikon, Tokyo, Japan), and individual myocytes were allowed to settle and adhere to the chamber bottom for at least 5 min before superfusion was initiated. Only rod shape cells with clear striations were used. HEPES buffered Tyrode's solution served as the normal superfusate. This solution contained (mM): NaCl 144, NaH2PO4 0.33, KCl 4.0, CaCl2 1.8, MgCl2 0.53, Glucose 5.5, and HEPES 5.0 at pH of 7.4.
Patch-clamp micropipettes were fabricated from borosilicate glass capillaries (Clark, Reading, U.K.) using a P-97 Flaming/Brown micropipette puller (Sutter Co., Novato, CA, U.S.A.). These electrodes had resistances between 1.5–2.5 MΩ when filled with pipette solution containing (in mM): K-aspartate 100, KCl 45, ATP 3, MgCl2 1, EGTA 10 and HEPES 5. The pH of this solution was adjusted to 7.2 by KOH. In case of measuring K+ currents, nisoldipine (1 μM) (obtained as a gift from the Bayer AG, Leverkusen, Germany) was placed in the external solution to eliminate inward Ca2+ current (ICa). The rapid IKr and slow IKs components of the delayed rectifier potassium current were separated by using the selective IKr blocker E-4031 (1 μM) or the IKs blocker chromanol 293B (30 μM). When Ca2+ current was measured the pipette solution contained (in mM): CsCl 110, CsOH 40, EGTA 10, HEPES 10, TEACl 20, ATP 5 (pH was adjusted to 7.2 by CsOH). Membrane currents were recorded with an Axopatch-1D amplifier (Axon Instruments, Foster City, CA, U.S.A.) using the whole-cell configuration of the patch-clamp technique. After establishing a high (1–10 Gohm) resistance seal by gentle suction, the cell membrane beneath the tip of the electrode was disrupted by suction or by application of 1.5 V electrical pulses for 1–5 ms. The series resistance was typically 4–8 MΩ before compensation (50–80%, depending on the voltage protocols). Experiments where the series resistance was high, or substantially increased during measurement, were discarded. Membrane currents were digitised using a 333 kHz analogue-to-digital converter (Digidata 1200, Axon Instruments) under software control (pClamp 6.0, Axon Instruments). Analyses were performed using Axon (pClamp 6.0) software after low-pass filtering at 1 kHz. All patch-clamp data were collected at 37°C.
Determination of plasma and tissue levels of dronedarone and amiodarone
Plasma and cardiac tissue levels of dronedarone and amiodarone in the chronically treated dogs were measured by the Bioanalysis Laboratory, in the Preclinical Department for Pharmacokinetics and Metabolism, at Sanofi-Synthelabo Research in Montpellier (France). After a liquid/liquid extraction, dronedarone plasma levels were determined using High Performance Liquid Chromatography (HPLC) (ultra violet detection 290 nm). Plasma levels of dronedarone were calculated by applying a daily prepared calibration curve using untreated dog plasma. The regressions were least squares linear and not forced through the origin and weighted on 1·y−2. The following concentrations were investigated: 0.020, 0.050, 0.100, 0.200, 0.500, 0.800 and 1.000 mg l−1. Similarly (ultra violet detection at 254 nm) amiodarone and desethylamiodarone plasma levels were calculated utilizing a daily prepared calibration curve using untreated dog plasma. The regressions were least squares linear regression not forced through the origin. The concentrations investigated were: 0.050, 0.100, 0.200, 0.500, 1.0, 2.0, 3.0 and 4.0 mg l−1. Heart samples were homogenized in Tris Buffer pH=7.4 and tissue drug levels were determined using HLPC, after liquid/liquid extraction with ultra violet detection at 290 nm for dronedarone and 254 nm for amiodarone. Dronedarone cardiac tissue levels were calculated by applying a daily prepared calibration curve using untreated dog heart. The regressions were least squares linear regression not forced through the origin and weighted on 1·x−2. The following concentrations were investigated: 0.040, 0.080, 0.200, 0.400, 0.800, 1.6, 2.0, 3.0, 4.0 and 6 mg kg−1. Cardiac tissue levels of amiodarone and desethylamiodarone were calculated using a daily prepared calibration curve using untreated dog heart. The regressions used were least squares linear not forced through the origin and weighted on 1·x−2. The concentrations applied were as follows: 0.150, 0.300, 0.600, 1.6, 3.2, 6.3, and 12.6 mg kg−1.
Determination of plasma levels of thyroid hormones
Plasma thyroxine (T4), triiodothyronine (T3) and reverse triiodothyronine (rT3) were measured with conventional RIA kits (T4: GammaCoat™ M[125I] Total T4 RIA kit, DiaSorin; T3: GammaCoat™ [125I] T3 RIA kit, DiaSorin; rT3: Reverse T3, Biochem Immuno Systems).
Statistical analysis
Student's t-test for paired and unpaired data was used to compare values between groups statistically. All data are expressed as the mean±s.e.mean; differences were considered significant when P values were less than 0.05.
Results
Chronic treatment
ECG in conscious dogs
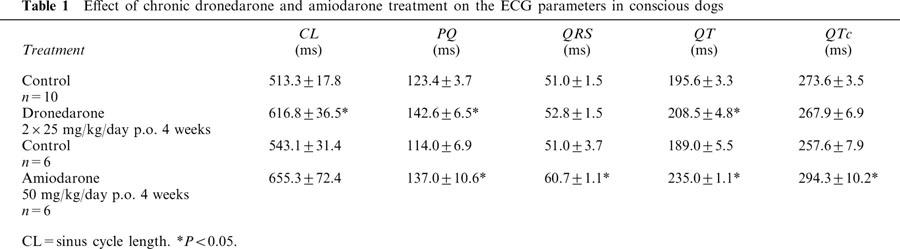
For 4 weeks dogs were given capsules filled with 25 mg kg−1 dronedarone twice a day (morning and afternoon) or capsules filled with 50 mg kg−1 amiodarone once a day (morning). Before the drug treatment ECG (limb leads I–III) records were taken in the conscious dogs. The recordings were repeated after 4 weeks of treatment 1–2 h before sacrificing the animals for the cellular electrophysiological measurements. The results are summarized in Table 1. After chronic treatment with amiodarone the PQ, QT, QRS and QTc intervals were significantly increased. After chronic dronedarone treatment the QRS and QTc intervals did not change considerably. The PQ interval and the sinus cycle length (CL) were, however, significantly prolonged. Obviously as a result of this latter effect, the QT interval was also lengthened by chronic treatment with dronedarone.
Table 1.
Effect of chronic dronedarone and amiodarone treatment on the ECG parameters in conscious dogs
Effects on action potential characteristics in dog ventricular muscle and Purkinje fibres
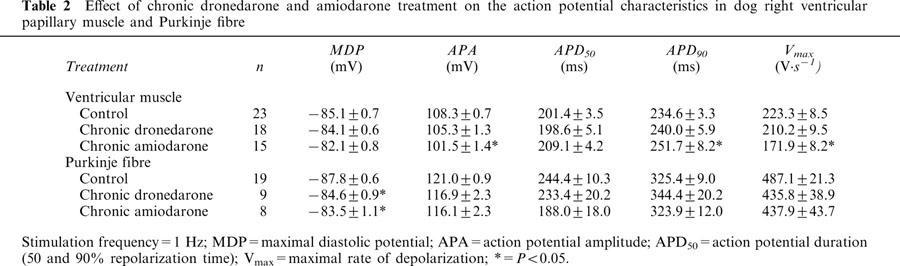
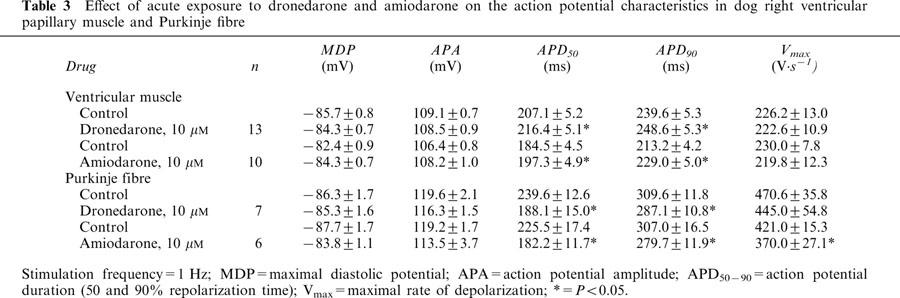
The effect of chronic dronedarone and amiodarone treatment on the action potential parameters in dog ventricular papillary muscle and Purkinje fibres at constant 1 Hz stimulation frequency is summarized in Table 2. Chronic dronedarone treatment did not alter the action potential parameters either in papillary muscle or in Purkinje fibres, except for a slight diminution of the maximal diastolic potential (MDP). In papillary muscle obtained from chronically amiodarone treated dogs APA and Vmax values were less, the APD90 was longer than those measured in muscles isolated from nontreated control dogs. In Purkinje fibre preparations none of the action potential parameters were different from those in the control fibres with the exception of MDP, which was slightly less negative in Purkinje fibres obtained from the amiodarone treated group compared with control.
Table 2.
Effect of chronic dronedarone and amiodarone treatment on the action potential characteristics in dog right ventricular papillary muscle and Purkinje fibre
Frequency dependent effects in dog ventricular muscle and Purkinje fibres
The frequency dependent effects of chronic dronedarone and amiodarone treatment on the APD and Vmax in ventricular papillary muscle and in Purkinje fibres are shown in Figure 1 and Figure 2, respectively. In papillary muscle chronic dronedarone treatment did not change significantly the APD in the physiological range of stimulation cycle lengths corresponding to the heart rate that may occur in vivo i.e. between 300 ms and 1000 ms, but at slow cycle lengths (1500–2000 ms) the APDs tended to be longer than those in the untreated control preparations. In contrast, after chronic amiodarone treatment, at all cycle lengths, the APD values were significantly longer than those in the control group. In papillary muscle chronic dronedarone treatment caused a weak but significant (P<0.05) decrease of Vmax at cycle lengths shorter than 1000 ms when compared to control. Chronic amiodarone treatment however resulted in a marked significant frequency dependent depression of Vmax, particularly at cycle lengths shorter than 1000 ms. The majority of the papillary muscles obtained from dogs chronically treated with amiodarone did not follow stimulation at cycle lengths of 300 and 400 ms presumably because of the marked change in the exitability due to depression of the sodium current at these high frequencies. APDs measured in Purkinje fibres obtained from dogs chronically treated with dronedarone and amiodarone did not significantly differ from those determined in Purkinje fibres isolated from untreated control animals. The Vmax values measured in the chronically amiodarone treated Purkinje fibres were significantly less than those in the control fibres especially at cycle lengths shorter than 1000 ms. No such change was obvious in the dronedarone treated Purkinje fibres.
Figure 1.
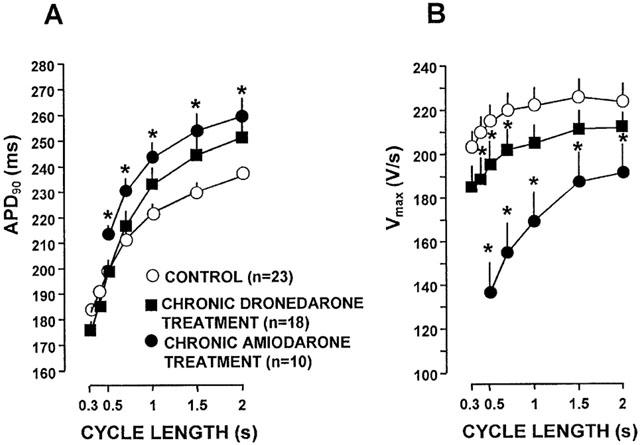
The frequency dependent effects of chronic dronedarone and amiodarone treatment on the action potential duration (APD) (A) and the maximal upstroke velocity (Vmax) (B) in dog right ventricular papillary muscles. Asterisks denote statistically significant (P<0.05) changes from control. Bars represent±s.e.mean.
Figure 2.
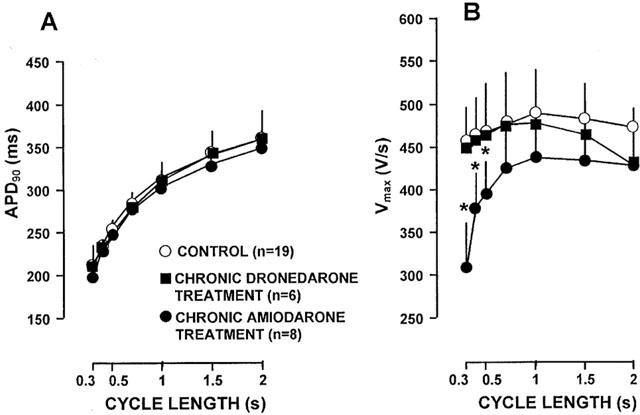
The frequency dependent effects of chronic dronedarone and amiodarone treatment on the action potential duration (APD) (A) and the maximal upstroke velocity (Vmax) (B) in dog cardiac Purkinje fibres. Asterisks denote statistically significant (P<0.05) changes from control. Bars represent±s.e.mean.
The use dependent Vmax block after chronic amiodarone and dronedarone treatment was further studied in papillary muscle by determining the offset (recovery) and onset kinetics of Vmax block. The offset kinetics of Vmax block was tested at the basic cycle length of 1000 ms. After every 20th basic stimulus an extra stimulus was delivered by gradually increasing coupling intervals after the end of the effective refractory period. Diastolic interval was defined as the time between the 90% repolarization of the basic action potential and the upstroke of the action potential evoked by the extra stimulus. As Figure 3A shows, in papillary muscles isolated from untreated control and chronically dronedarone treated dogs the Vmax almost completely recovered after 30–50 ms diastolic interval. In contrast, in papillary muscles obtained from chronically amiodarone treated animals (Figure 3A) the recovery of Vmax was considerably delayed, i.e., in early extrasystoles the amplitude of Vmax was less than that during late diastole. The corresponding recovery of Vmax time constant value in the muscle preparations isolated from amiodarone-treated dogs could be well fitted with single exponential, starting the procedure at 40 ms diastolic interval. The recovery of Vmax could be characterised by a time constant of 549.6±43.4 ms (n=5).
Figure 3.
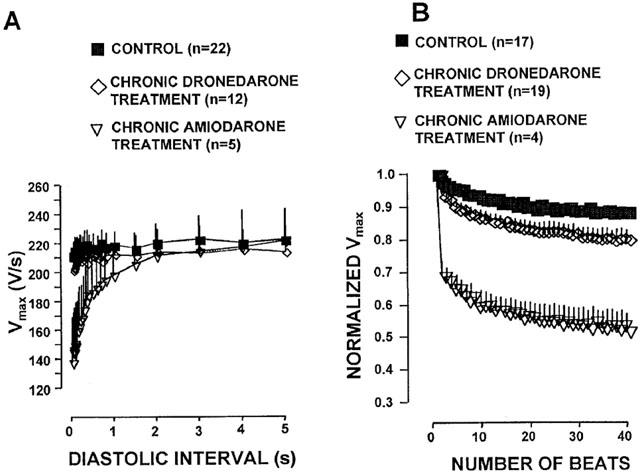
The effects of chronic dronedarone and amiodarone treatment on the offset (A) and onset (B) kinetics of Vmax block in dog right ventricular papillary muscle. In B, Vmax value is expressed as fraction of the first Vmax in the train. For the sake of clarity sign of significance was omitted. Bars represent±s.e.mean.
The onset kinetics of Vmax block was tested after 1 min stimulation free period, by applying a 40 beat train of stimuli with cycle length of 400 ms. Figure 3B shows that in chronically dronedarone treated preparations, as in untreated controls, there was only a moderate and relatively slow decrease of Vmax during the train, which was fitted with single exponential characterized by the rate constants of 8.9±0.5 ms (n=19) and 10.9±0.7 ms (n=17), respectively. In contrast, in papillary muscles obtained from chronically amiodarone treated dogs there was a marked Vmax change during the train. As Figure 3B indicates the onset kinetics of Vmax block could be best fitted with double exponential. In addition to the rate constant of the slow component (17.0±3.9, n=4) another fast rate constant (1.56±0.13, n=4) with a relatively large amplitude could also be determined.
Acute superfusion
Effects on action potential characteristics in dog ventricular muscle and Purkinje fibres
The effects of acute dronedarone and amiodarone superfusion on the action potential parameters in dog right ventricular papillary muscle and Purkinje fibres are shown in Figure 4 and summarised in Table 3. At stimulation cycle length of 1000 ms the effects of dronedarone and amiodarone were very similar to each other in papillary muscle, i.e. both drugs moderately lengthened repolarization measured as APD50 and APD90 without significantly changing other action potential parameters as MDP, APA and Vmax. However, dronedarone and amiodarone, presumably due to their I/B type sodium channel blocking activity, exerted some moderate but statistically significant (P<0.05) use dependent Vmax depression at stimulation cycle lengths shorter than 1000 ms in both papillary muscle (at cycle length of 300 ms, from 206.7±12.8 V·s−1 to 178.1±9.9 V·s−1 (n=12) for 10 μM dronedarone, and from 211.5±8.6 V·s−1 to 178.5±12.3 V·s−1 (n=8) for 10 μM amiodarone, and Purkinje fibre (at cycle length of 300 ms, from 437.4±37.5 V·s−1 to 351.6±53.1 V·s−1 (n=7) for 10 μM dronedarone and from 392.8±22.9 V·s−1 to 306.4±36.7 V·s−1 (n=5) for 10 μM amiodarone). In our previous experiments we observed that the solvent (DMSO), at concentrations corresponding to the concentration of DMSO applied as a solvent for 10 μM dronedarone, did not change significantly any of the action potential characteristics. In dog right papillary muscle both 10 μM dronedarone and 10 μM amiodarone moderately prolonged repolarisation (Table 3), but in Purkinje fibres both dronedarone and amiodarone superfusion significantly shortened repolarisation measured as APD50 and APD90 and reduced Vmax to various degrees, without significant change of the MDP and APA (Table 3).
Figure 4.
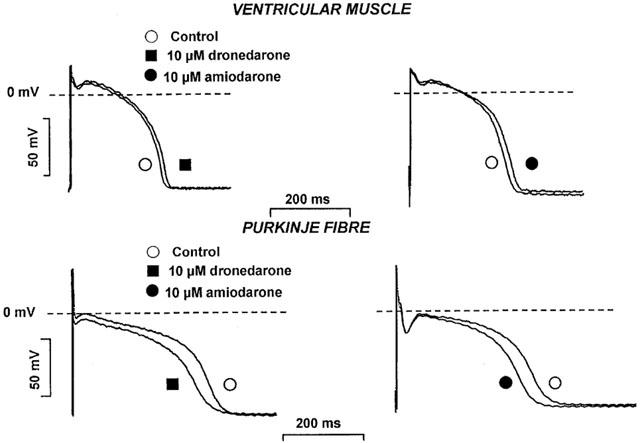
The effect of acute dronedarone (10 μM) and amiodarone (10 μM) superfusion on the action potentials in dog right ventricular papillary muscle and cardiac Purkinje fibre. The stimulation frequency: 1 Hz; and the incubation time: 60 min.
Table 3.
Effect of acute exposure to dronedarone and amiodarone on the action potential characteristics in dog right ventricular papillary muscle and Purkinje fibre
Effects on early and delayed afterdepolarization in dog cardiac Purkinje fibres
The effects of acute dronedarone and amiodarone superfusion on the early afterdepolarization (EAD) and delayed afterdepolarization (DAD) were studied in dog left ventricular Purkinje fibres. EADs were evoked by 20–40 min superfusion with 1 μM dofetilide. The stimulation frequency was gradually decreased from 1 to 0.08 Hz until EAD or spontaneous beat developed. Once EADs appeared, they persisted for more than 2 h as verified in separate control experiments. The incidence of EADs was calculated during a train of 30 beats before and after 40–60 min superfusions of 10 μM dronedarone or 10 μM amiodarone. In the presence of dronedarone and amiodarone the incidence, i.e. the ratio of the action potentials showing EADs versus normal beats without EADs, significantly decreased from 77.8±3.8% to 11.7±2.8% (n=6; P<0.05) and from 58.6±4.5% to 8.0±3.2% (n=5; P<0.05), respectively (Figure 5).
Figure 5.
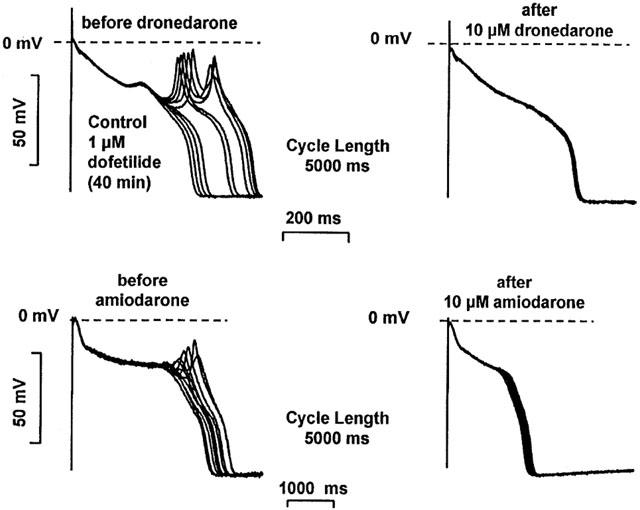
The effect of acute dronedarone and amiodarone superfusion on the early afterdepolarization (EAD) in dog cardiac left ventricular Purkinje fibres. EADs were evoked by 1 μM dofetilide at stimulation cycle length of 5000 ms. After superfusion with either 10 μM dronedarone or 10 μM amiodarone in the continuous presence of dofetilide, EADs were abolished.
To evoke delayed afterdepolarization (DAD) the preparations were driven by 2 Hz frequency interrupted by 2 min stimulation free periods. In the absence of ouabain, after cessation of the stimulation, DADs never occurred (Figure 6). In the presence of 0.2–0.3 μM ouabain for 40–60 min, after abrupt termination of the stimulation, DADs developed with amplitudes ranging from 4.7 to 13.7 mV (Figure 5). DADs were persistent in the presence of ouabain for more than 2 h as tested in separate experiments. After the DADs had been evoked, the preparations were superfused with 10 μM dronedarone or 10 μM amiodarone for 30–40 min. Both dronedarone and amiodarone abolished or significantly decreased the amplitudes of the ouabain induced DADs from 10.2±0.4 to 1.8±0.6 mV (n=5; P<0.05) and from 11.1±0.7 mV to 2.4±0.5 mV (n=5; P<0.05) respectively (Figure 6).
Figure 6.
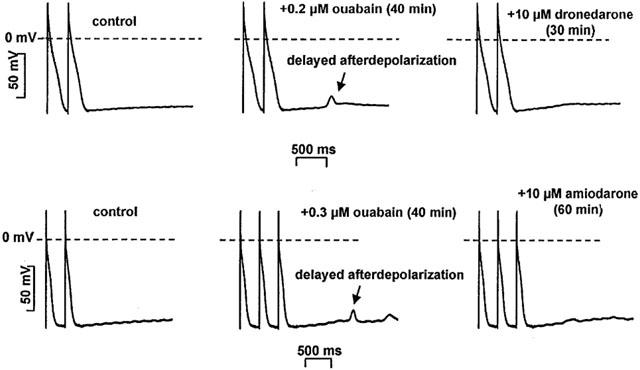
The effect of acute dronedarone and amiodarone superfusion on the delayed after depolarisation (DAD) in dog cardiac left ventricular Purkinje fibres. DADs were evoked by 0.2–0.3 μM ouabain at stimulation cycle length of 500 ms. After superfusion with either 10 μM dronedarone or amiodarone in the continuous presence of ouabain, DADs were abolished.
Effects on transmembrane ionic currents in dog ventricular myocytes
Since the ECG recordings showed lengthening of the PQ interval and the action potential measurements suggested inhibition of the repolarization after dronedarone application, the effect of 10 μM dronedarone on the various transmembrane ionic currents was also studied in single dog ventricular myocytes to elucidate the mechanisms of the dronedarone induced electrophysiological changes.
L-type inward calcium current (ICa) was evoked by 400 ms long depolarizing test pulses (ranging from −30 to +60 mV) from −40 mV holding potential. The amplitude of ICa was defined as the difference between the peak inward current at the beginning of the pulse and the current at the end of the pulse. In these measurements the KCl content of the pipette was replaced by CsCl to suppress potassium currents. As Figure 7 shows, 10 μM dronedarone, after 5 min superfusion, largely decreased ICa. The magnitude of the ICa depression at 0 mV was 76 % (from 1137.7±299.1 pA to 270.5±70.5 pA, n=6, P<0.05), which was only partially reversible even after 10 min washout.
Figure 7.
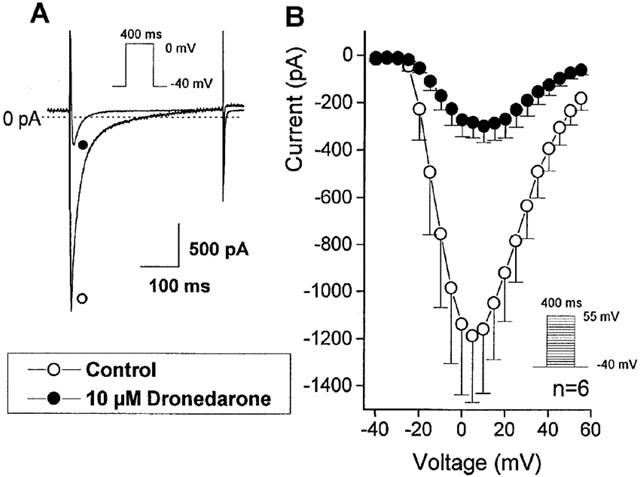
The effect of 10 μM dronedarone on the inward calcium current (ICa) in single dog ventricular myocyte. (A) shows original recordings from a representative experiment. (B) indicates the voltage current relation before (○) and after (•) dronedarone (10 μM for 3–5 min) superfusion in an average of six cells. Bars indicate±s.e.mean.
The possible effect of 10 μM dronedarone superfusion on the different potassium currents was also investigated. In these measurements the extracellular solution contained 1 μM nisoldipine to completely block ICa.
The transient outward potassium current (Ito) and the inward rectifier potassium current (Ikl) were activated from holding potential of −80 mV by 400 ms test pulses ranging from −120 to +60 mV. The amplitude of Ito was determined as the difference current between the peak outward current at the beginning and the steady-state current at the end of the pulse. Ikl was defined as the amplitude of the steady-state current at the end of the pulse. As Figure 8 shows, 10 μM dronedarone did not apparently and significantly influenced the amplitude of Ito and Ikl after 5–8 min superfusion.
Figure 8.
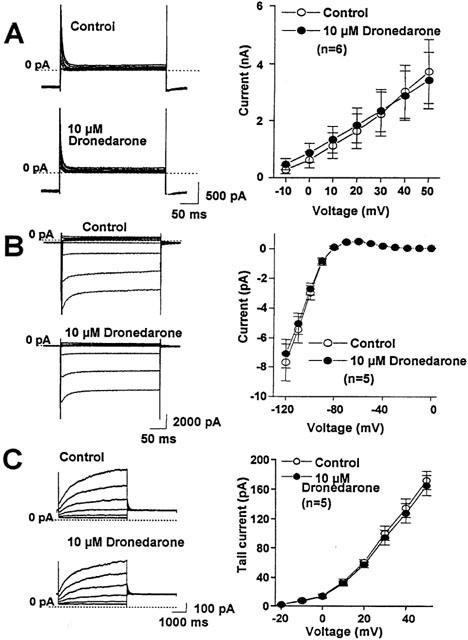
The effect of (A) 10 μM dronedarone on the transient outward potassium (Ito), (B) the inward rectifier potassium (Ikl), and (C) the slow component of the delayed rectifier outward potassium current (IKs) in single dog ventricular myocyte. The left side of each panel shows the original recording obtained in a representative experiment. On the right side the current voltage relations are illustrated before (○) and after the application of 10 μM dronedarone (•) superfusion in an average of 5–6 cells. Bars indicate±s.e.mean.
The slow (IKs) and rapid (IKr) components of the delayed rectifier outward potassium current were activated from −40 mV holding potential with 1 s (IKr) and 5 s (IKs) depolarizing test pulses of −20 to +60 mV at pulse frequency of 0.01 Hz. To define IKs and IKr, after returning the voltage to −40 mV, the amplitude of the tail current was determined. When the effect of dronedarone on the IKs was studied, the extracellular solution contained 1 μM E-4031 and when IKr was examined, the extracellular solution contained 30 μM chromanol 293B to block IKr and IKs selectively, thereby allowing proper separation of the two currents. As Figure 8 shows, 10 μM dronedarone did not significantly change the amplitude of the IKs tail current. In contrast, 10 μM dronedarone almost completely depressed the IKr tail current (Figure 9).
Figure 9.
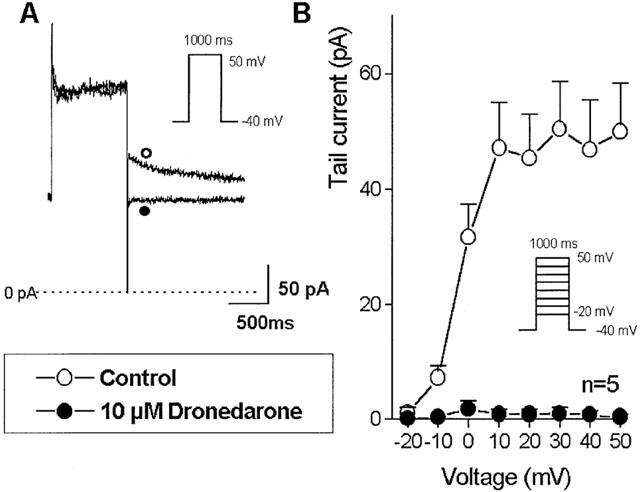
The effect of 10 μM dronedarone on the rapid component of the delayed rectifier outward potassium current (IKr) in single dog ventricular myocyte. (A) shows original recordings from a representative experiment. (B) indicates the voltage current relation before (○) and after (•) dronedarone (10 μM for 3–5 minutes) superfusion in an average of six cells. Bars indicate±s.e.mean.
Plasma and tissue concentrations
Dogs receiving daily 50 mg kg−1 amiodarone for 4 weeks had amiodarone and desethylamiodarone plasma levels of 7.87±1.74 μg ml−1 and 1.01±0.17 μg ml−1 respectively (n=6). The corresponding tissue (heart) concentrations were 6.71±1.5 μg mg−1 and 7.12±1.53 μg mg−1. Dogs receiving daily 2×25 mg−1 kg−1 day dronedarone had 1.01±0.32 μg ml−1 plasma and 10.68±4.51 μg mg−1 cardiac tissue concentration of the parent drug (n=7), and 0.09±0.03 μg ml−1 plasma and 2.04±0.69 μg ml−1 cardiac tissue concentration of the N-debuthyl metabolite.
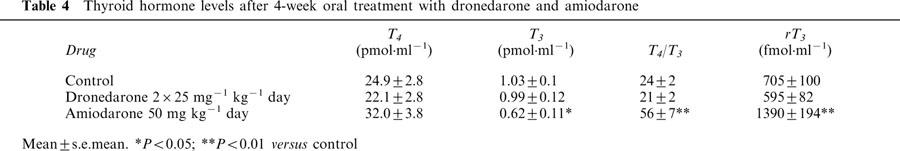
Plasma thyroid hormones concentrations
T4, T3 and reverse T3 (rT3) levels in the plasma were measured after 4-week treatment with amiodarone and dronedarone. In comparison with the control group, amiodarone induced a decrease in T3 and an increase in the T4·T3−1 ratio and rT3. Dronedarone did not modify significantly T4, T3 or rT3 levels (Table 4).
Table 4.
Thyroid hormone levels after 4-week oral treatment with dronedarone and amiodarone
Discussion
Major findings
The major findings of this study indicate that: (a) Dronedarone, unlike amiodarone, does not produce considerable repolarization lengthening in dog ventricular muscle after chronic treatment. (b) Acute dronedarone superfusion, like acute exposure to amiodarone, moderately shortens repolarization in the Purkinje fibres but only moderately lengthens it in the ventricular muscle. (c) Acute dronedarone application results in a mild use-dependent Vmax depressing and strong ICa and IKr inhibitory effects. (d) Acute dronedarone superfusion diminishes both types of triggered arrhythmogenic automaticity (EAD and DAD).
Comparison with previous reports
In our study the effects of both acute and chronic amiodarone application are similar to those published earlier by others (Sicouri et al., 1997; Kato et al., 1988; Mason et al., 1984; Anderson et al., 1989).
Our study shows that chronic dronedarone treatment does not lengthen the repolarization. These results are in good agreement with those of Finance et al. (1998) and Barthelemy et al., (1998a, 1998b) who observed that dronedarone, in a similar range of doses, did not change QTc. These authors, as well as Merot et al. (1999) found that amiodarone also does not induce a significant QTc prolongation, demonstrating that amiodarone-induced QTc lengthening is not always observed in dogs.
Recently Sun et al. (1999) reported that in the rabbit chronic dronedarone treatment caused similar and even more pronounced electrophysiological effects than those produced by long-term administration of amiodarone. Based on these results the authors speculated that iodine in the chemical structure–which was believed to be responsible for many of the unwanted side effects of amiodarone–was not a necessity to produce the ‘amiodarone like' chronic electrophysiological effects. Our present results obtained in dogs are different, and our conclusion is in sharp contrast with those of Sun et al. (1999), since in our hands, chronic dronedarone treatment did not result in convincing ‘amiodarone like' electrophysiological effects. Therefore, our data support previous speculations that iodine in the molecular structure is probably needed to cause the characteristic ‘amiodarone like' chronic electrophysiological changes.
Also, closer inspection of Table 2 in the study of Sun et al. (1999) reveals that the APD values in the control group of the chronic experiments were 130 ms, significantly shorter than those in the amiodarone (172 ms) and dronedarone (183 ms) pretreated groups. However, Figure 7 of the same report, in which the acute effect of dronedarone and amiodarone is presented on the APD, shows that in another control group (before dronedarone and amiodarone superfusion) APD was 193–205 ms, i.e. longer than that after chronic dronedarone and amiodarone treatment in Table 2. Furthermore, after chronic treatment we found strong use-dependent Vmax block with amiodarone but not with dronedarone. In contrast, Sun et al. (1999) observed a larger but apparently not use-dependent Vmax depression after chronic dronedarone than after chronic amiodarone treatment. Although the differences in dosage, experimental conditions and species may be of some significance, we can not fully explain this discrepancy between the results of Sun et al. (1999) and our own findings.
Despite the described differences between our observations and the results of Sun et al. (1999), our in vivo (ECG) findings with dronedarone virtually confirm the earlier results of Manning et al. (1995b) also obtained in dogs. It was also found (Verduyn et al., 1999) that in anaesthetized dogs with chronic A-V block i.v. dronedarone abolished almokalant induced EADs and torsade de pointes tachycardia, a finding which is consistent with the result of our similar in vitro measurement.
On the other hand our in vitro results with dronedarone are in good agreement with those of Moro et al. (1999) since we have also found only moderate or at cycle lengths shorter than 1000 ms use-dependent Vmax block after acute dronedarone superfusion.
Possible mechanism of the effect of dronedarone
The observed lack of considerable electrophysiological effect of chronic dronedarone treatment is an important finding. Insufficient absorption of the drug is not a cause, since the compound has good bioavailability and yielded significant concentration both in the plasma and in the heart. Dronedarone, unlike amiodarone, does not affect the level of thyroid hormones. Moreover, dronedarone and N-debutyldronedarone have weak affinity to human nuclear thyroid hormone receptors, slightly inferior to those of amiodarone (M. Delbruyère, 2001, unpublished results), i.e. it is much less than DEA affinity (Latham et al., 1987). These observations suggest that the inhibition of the thyroid hormone metabolism and/or the blockade of the thyroid hormone receptors during the chronic treatment with amiodarone are likely due to the accumulation of DEA in the tissues rather than to the presence are absence of iodine ions in the parent molecule. Furthermore, the decrease of T3 by DEA may affect the potassium channel protein synthesis leading to a decreased density of different potassium channels (Varró et al., 1996; Kodama et al., 1997; 1999; Bosch et al., 1999). It has to be mentioned that in the present study, after chronic dronedarone treatment, some APD lengthening was observed in the right ventricular papillary muscle, at stimulation cycle lengths longer than 1000 ms (Figure 1). However, at more physiological rates the APD lengthening was not present after chronic dronedarone treatment or it was too small to measure. In in vivo ECG measurements, where the spontaneous cycle length of the dogs was about 500–600 ms, no significant QTc prolongation was found. These findings suggest that the observed differences in the chronic electrophysiological effects between dronedarone and amiodarone are most probably due to the lack of iodine in the chemical structure of dronedarone.
On the basis of the high degree of IKr block observed in isolated ventricular myocytes after acute dronedarone application we could have expected more pronounced APD lengthening. It is not clear why the strong IKr block caused by dronedarone was not associated with more marked APD prolongation in papillary muscle. The conditions for drug diffusion are certainly better in the patch clamp than in the conventional microelectrode experiments. Also, one may speculate that dronedarone, like amiodarone, has multiple sites of action involving other ionic channels (Nattel, 1993; Kodama et al., 1997), such as the L-type calcium (Nishimura et al., 1989) and inward sodium channels which, by carrying depolarizing currents, may oppose the APD lengthening effect of the IKr block. This is definitely more pronounced in Purkinje fibres where these latter currents play more important role, but this mechanism is also likely to operate in the ventricular muscle.
The dronedarone induced sodium channel block is weaker than that evoked by amiodarone, but this may have an advantageous consequence. Dronedarone, like amiodarone and unlike the ‘pure' IKr blocker d-sotalol, may decrease the dispersion of repolarisation between ventricular muscle and Purkinje fibres. If this type of dispersion is augmented in pathophysiological situations (e.g. myocardial infarction, long QT syndrome, hypokalaemia), it may facilitate the onset of life-threatening arrhythmias, such as ‘torsade de pointes'; polymorphic ventricular tachycardia. Therefore drugs that decrease such an enhanced dispersion may have considerable advantage. Since the dronedarone induced sodium channel depression is not strong and does not result in substantial Vmax block and slowing of the impulse conduction, with this drug the likelihood of the development of the CAST type proarrhythmia, which is associated with drug induced conduction disturbances, is less likely with dronedarone than with other more potent sodium channel blockers, including even amiodarone. Furthermore, since the dronedarone induced reduction and suspension of DAD and EAD is supposedly due to the drug-related inhibition of the sodium and particularly the calcium channels, similar argument may be valid to interpret the beneficial effect of dronedarone on the DAD and EAD, when suppressing these afterdepolarizations.
Conclusion
It can be concluded that after acute administration the cardiac electrophysiological effects of dronedarone are similar to those of amiodarone, which may at least partly explain its in vivo antiarrhythmic effect. However, after long-term administration dronedarone, unlike amiodarone, does not produce marked electrophysiological changes. On chronic administration N-debutyl dronedarone, the main metabolite of dronedarone does not reach electrophysiologically active plasma and cardiac tissue levels, whereas N-desethylamiodarone, the highly potent principal metabolite of amiodarone, accumulates in cardiac tissue. One might therefore speculate that N-desethylamiodarone could be, at least in part, responsible for the difference in the chronic electrophysiological effects of dronedarone and amiodarone.
Acknowledgments
This study was supported by a grant-in-aid from Sanofi-Synthelabo Recherche (Montpellier, France), and by grants from the Hungarian National Research Foundation (OTKA T- 23455 and T- 032558), Hungarian Ministry of Health (ETT 532/2000 and 536/2000), from the Hungarian Ministry of Education (FKFP 1025/1997), from the Hungarian Academy of Sciences and by János Bolyai Research Scholarship (for L. Virág). Thanks are also extended to Mrs Zsuzsa Molnár, Ms Etelka Kiss and Ms Ágnes Krajevszky for the technical assistance.
Abbreviations
- APA
action potential amplitude
- APD
action potential duration
- APD50 and APD90
action potential durations at 50% and 90% of repolarization
- CL
cycle length
- EAD
early afterdepolarization
- DAD
delayed afterdepolarization
- ECG
electrocardiogram
- HPLC
High Performance Liquid Chromatography ICa, L-type calcium current
- Ik1
inward rectifier potassium current
- IKr and IKs
rapid and slow components of the delayed rectifier potassium current
- Ito
transient outward potassium current
- MDP
maximum diastolic potential
- Vmax
maximal rate of depolarization
References
- ANDERSON K.P., WALKER R., DUSTMAN T., LUX R.L., ERSHLER P.R., KATES R.E., URIE P.M. Rate-related electrophysiologic effects of long-term administration of amiodarone on canine ventricular myocardium in vivo. Circulation. 1989;79:948–958. doi: 10.1161/01.cir.79.4.948. [DOI] [PubMed] [Google Scholar]
- BARTHELEMY G., FAURE F., LIGNON S., RICHAUD J.P., BOUSSAC N., CAZAUBON C., GAUTIER P., NISATO D.Electrocardiographic, cardiovascular and sympatholytic action of dronedarone, a new antiarrhythmic agent, in conscious dogs J. Mol. Cell. Cardiol. 1998a30A32 [Google Scholar]
- BARTHELEMY G., FAURE F., LIGNON S., RICHAUD J.P., BOUSSAC N., CAZAUBON C., GAUTIER P., NISATO D.Hemodynamics, electrocardiographic and sympatholytic action of amiodarone in conscious dogs J. Mol. Cell. Cardiol. 1998b30A31 [Google Scholar]
- BOSCH R.F., LI G.R., GASPO R., NATTEL S. Electrophysiologic effects of chronic amiodarone therapy and hypothyroidism, alone and combination on guinea pig ventricular myocytes. J. Pharmacol. Exp. Ther. 1999;289:156–165. [PubMed] [Google Scholar]
- FINANCE O., MANNING A., CHATELAIN P. Effects of a new amiodarone - like agent, SR 33589, in comparison to amiodarone, D, L, -sotalol, and lignocaine, on ischemia - induced ventricular arrhythmias in anaesthetised pigs. J. Cardiovasc. Pharmacol. 1995;26:570–576. doi: 10.1097/00005344-199510000-00010. [DOI] [PubMed] [Google Scholar]
- FINANCE O., PLANCHENAULT J., BETHEGENIES S., RICHAUD J.P., GAUTIER P., NISATO D. Electrophysiological and haemodynamic effects of a new amiodarone like agent following acute and chronic oral treatment in anaesthetised dogs. J. Mol. Cell. Cardiol. 1998;30:A251. [Google Scholar]
- FOLLMER C.H., AOMINE M., YEH J.Z., SINGER D.H. Amiodarone-induced block of sodium current in isolated cardiac cells. J. Pharmacol. Exp. Ther. 1987;243:187–194. [PubMed] [Google Scholar]
- HARRIS L., MCKENNA W.J., ROWLAND E., HOLT D.W., STOREY G.C.A., KRIKLER D.M. Side effects of long-term amiodarone therapy. Circulation. 1983;67:45–51. doi: 10.1161/01.cir.67.1.45. [DOI] [PubMed] [Google Scholar]
- JULIAN D.G., CAMM A.J., FRANGIN G., JANSE M.J., MUNOZ A., SCHWARTZ P.J., SIMON P. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators. Lancet. 1997;349:667–674. doi: 10.1016/s0140-6736(96)09145-3. [DOI] [PubMed] [Google Scholar]
- KATO R., VENKATESH N., KAMIYA K., YABEK S., KANNAN R., SINGH B.N. Electrophysiologic effects of desethylamiodarone, an active metabolite of amiodarone: comparison with amiodarone during chronic administration in rabbits. Am. Heart J. 1988;115:351–359. doi: 10.1016/0002-8703(88)90481-4. [DOI] [PubMed] [Google Scholar]
- KODAMA I., KAMIYA K., TOYAMA J. Cellular electropharmacology of amiodarone. Cardiovasc. Res. 1997;35:13–29. doi: 10.1016/s0008-6363(97)00114-4. [DOI] [PubMed] [Google Scholar]
- KODAMA I., KAMIYA K., TOYAMA J. Amiodarone: ionic and cellular mechanisms of action of the most promising Class III agent. Am. J. Cardiol. 1999;84:20R–28R. doi: 10.1016/s0002-9149(99)00698-0. [DOI] [PubMed] [Google Scholar]
- LATHAM K.R., SELLITTI D.F., GOLDSTEIN R.E. Interaction of amiodarone and desethylamiodarone with solubilized nuclear thyroid hormone receptors. J. Am. Cell. Cardiol. 1987;9:872–876. doi: 10.1016/s0735-1097(87)80244-9. [DOI] [PubMed] [Google Scholar]
- LAZZARA R. Amiodarone and torsade de pointes. Ann. Intern. Med. 1989;111:549–551. doi: 10.7326/0003-4819-111-7-549. [DOI] [PubMed] [Google Scholar]
- LINK M.S., HOMOUD M., FOOTE C.B., ESTES N.A.M. Antiarrhythmic drug therapy for ventricular arrhythmias: current perspectives. J. Cardiovasc. Electrophysiol. 1995;6:880–886. doi: 10.1111/j.1540-8167.1996.tb00573.x. [DOI] [PubMed] [Google Scholar]
- MANNING A.S., BRUYNINCKX C., RAMBOUX J., CHATELAIN P. SR-33589, a new amiodarone-like agent: effect on ischaemia- and reperfusion-induced arrhythmias in anesthetized rats. J. Cardiovasc. Pharmacol. 1995a;26:453–461. [PubMed] [Google Scholar]
- MANNING A., THISSE V., HODEIGE D., RICHARD J., HEYNDRICKX J.P., CHATELAIN P. SR-33589, a new amiodarone like antiarrhythmic agent: electrophysiological effects in anesthetized dogs. J. Cardiovasc. Pharmacol. 1995b;25:252–261. doi: 10.1097/00005344-199502000-00010. [DOI] [PubMed] [Google Scholar]
- MASON J.W., HONDEGHEM L.M., KATZUNG B. Block of inactivated sodium channels and of depolarisation-induced automaticity in guinea pig papillary muscle by amiodarone. Circ. Res. 1984;55:277–285. doi: 10.1161/01.res.55.3.278. [DOI] [PubMed] [Google Scholar]
- MEROT J., CHARPENTIER F., POIRIER J.M., COUTRIS G., WEISSENBURGER J. Effects of chronic treatment by amiodarone on transmural heterogeneity of canine ventricular repolarisation in vivo: interaction with acute sotalol. Cardiovasc. Res. 1999;44:303–314. doi: 10.1016/s0008-6363(99)00232-1. [DOI] [PubMed] [Google Scholar]
- MORO S., CELESTINO D., ELIZARI M.V., SICOURI S. Acute amiodarone and dronedarone reduce transmural dispersion of repolarisation and abolished early after depolarisation (EADs) in the canine ventricle. Pace. 1999;22:786. [Google Scholar]
- NATTEL S. Comparative mechanisms of action of antiarrhythmic drugs. Am. J. Cardiol. 1993;72:13F–17F. doi: 10.1016/0002-9149(93)90959-g. [DOI] [PubMed] [Google Scholar]
- NISHIMURA M., FOLLMER C.H., SINGER D.H. Amiodarone blocks calcium current in single guinea pig ventricular myocytes. J. Pharmacol. Exp. Ther. 1989;251:650–659. [PubMed] [Google Scholar]
- PAPP J.G.Y., NÉMETH M., KRASSÓI I., MESTER L., HÁLA O., VARRÓ A. Differential electrophysiologic effects of chronically administered amiodarone on canine Purkinje fibres versus ventricular muscle. J. Cardiovasc. Pharmacol. Therapeut. 1996;1:287–296. doi: 10.1177/107424849600100404. [DOI] [PubMed] [Google Scholar]
- PFISTERER M., KIOWSKI W., BURCKHARDT D., FOLLATH F., BURKART F. Beneficial effect of amiodarone on cardiac mortality in patients with asymptomatic complex ventricular arrhythmias after acute myocardial infarction and preserved but not impaired left ventricular function. Am. J. Cardiol. 1992;69:1399–1402. doi: 10.1016/0002-9149(92)90889-7. [DOI] [PubMed] [Google Scholar]
- POLSTER P., BROEKHUYSEN J. The adrenergic antagonism of amiodarone. Biochem. Pharmacol. 1976;25:131–134. doi: 10.1016/0006-2952(76)90279-3. [DOI] [PubMed] [Google Scholar]
- SICOURI S., MORO S., LITOVSKY S., ELIZARI M.V., ANTZELEVITCH C. Chronic amiodarone reduces transmural dispersion of repolarisation in the canine heart. J. Cardiovasc. Electrophysiol. 1997;8:1269–1279. doi: 10.1111/j.1540-8167.1997.tb01018.x. [DOI] [PubMed] [Google Scholar]
- SINGH B.N., VAUGHAN WILLIAMS E.M. The effect of amiodarone, a new anti-anginal drug, on cardiac muscle. Br. J. Pharmacol. 1970;39:657–667. doi: 10.1111/j.1476-5381.1970.tb09891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN W., SARMA J.S.M., SINGH B.N. Electrophysiological effects of dronedarone (SR 33589), a noniodinated benzofuran derivative, in the rabbit heart. Comparison with amiodarone. Circulation. 1999;100:2276–2281. doi: 10.1161/01.cir.100.22.2276. [DOI] [PubMed] [Google Scholar]
- THE CAST INVESTIGATORS Preliminary report: effect of encainide and flecainide on mortality in randomised trial arrhythmia suppression after myocardial infarction. N. Engl. J. Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- VARRÓ A., BALÁTI B., IOST N., TAKÁCS J., VIRÁG L., LATHROP D.A., LENGYEL C., TÁLOSI L., PAPP J.G.Y. The role of the delayed component IKs in dog ventricular muscle and Purkinje fibre repolarization. J. Physiol. 2000;523:67–81. doi: 10.1111/j.1469-7793.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARRÓ A., NAKAYA Y., ELHARRAR V., SURAWICZ B. Use-dependent effects of amiodarone on Vmax in cardiac Purkinje fibres and ventricular muscle. Eur. J. Pharmacol. 1985;112:419–422. doi: 10.1016/0014-2999(85)90791-5. [DOI] [PubMed] [Google Scholar]
- VARRÓ A., VIRÁG L., PAPP J.G.Y. Comparison of the chronic and acute effects of amiodarone on the calcium and potassium currents in rabbit isolated cardiac myocytes. Br. J. Pharmacol. 1996;117:1181–1186. doi: 10.1111/j.1476-5381.1996.tb16713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHAN WILLIAMS E.M. Classification of anti-dysrhythmic drugs. Pharmacol. Ther. 1975;1:115–138. doi: 10.1016/0306-039x(75)90019-7. [DOI] [PubMed] [Google Scholar]
- VERDUYN S.C., VOS H.A., LEUNISSEN H.D.M., VAN OPSTAL J.M., WELLENS H.J.J. Evaluation of the acute electrophysiologic effects of intravenous dronedarone, an amiodarone-like agent, with special emphasis on ventricular repolarisation and acquired torsade de pointes arrhythmias. J. Cardiovasc. Pharmacol. 1999;33:212–222. doi: 10.1097/00005344-199902000-00006. [DOI] [PubMed] [Google Scholar]
- WALDO A.L., CAMM A.J., DERUYTER H., FRIEDMAN P.L., MACNEIL D.J., PAULS J.F., PITT B., PRATT C.M., SCHWARTZ P.J., VELTRI E.P. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]


