Abstract
The effects of anandamide on [3H]-acetylcholine release and muscle contraction were studied on the myenteric plexus-longitudinal muscle preparation of the guinea-pig ileum preincubated with [3H]-choline.
Anandamide increased both basal [3H]-acetylcholine release (pEC50 6.3) and muscle tone (pEC50 6.3). The concentration-response curves for anandamide were shifted to the right by 1 μM capsazepine (pKB 7.5 and 7.6), and by the combined blockade of NK1 and NK3 tachykinin receptors with the antagonists CP99994 plus SR142801 (each 0.1 μM). The CB1 and CB2 receptor antagonists, SR141716A (1 μM) and SR144528 (30 nM), did not modify the facilitatory effects of anandamide.
Anandamide inhibited the electrically-evoked release of [3H]-acetylcholine (pEC50 5.8) and contractions (pEC50 5.2). The contractile response to the muscarinic agonist methacholine was not significantly affected by 10 μM anandamide.
The inhibitory effects of anandamide were not changed by either capsazepine (1 μM), SR144528 (30 nM) or CP99994 plus SR142801 (each 0.1 μM). SR141716A (1 μM) produced rightward shifts in the inhibitory concentration-response curves for anandamide yielding pKB values of 6.6 and 6.2.
CP55940 inhibited the evoked [3H]-acetylcholine release and contractions, and SR141716A (0.1 μM) shifted the concentration-response curves of CP55940 to the right with pKB values of 8.4 and 8.9.
The experiments confirm the existence of release-inhibitory CB1 receptors on cholinergic myenteric neurones. We conclude that anandamide inhibits the evoked acetylcholine release via stimulation of a receptor that is different from the CB1 and CB2 receptor. Furthermore, anandamide increases basal acetylcholine release via stimulation of vanilloid receptors located at primary afferent fibres.
Keywords: Guinea-pig ileum, acetylcholine release, anandamide, vanilloid receptors, cannabinoid receptors, SR141716A, CP55940, capsazepine
Introduction
Anandamide (arachidonylethanolamide) is a naturally occurring compound which has been suggested to be the endogenous ligand for cannabinoid receptors (di Marzo et al., 1998). Stimulation of cannabinoid receptors by exogenous agonists, such as CP55940 or WIN 55,212-2, causes inhibition of the electrically-evoked release of acetylcholine and motor activity in the guinea-pig ileum (see review by Pertwee, 1999), and these effects are competitively antagonized by the specific CB1 receptor antagonist SR141716A (Pertwee et al., 1996; Coutts & Pertwee, 1997). The endogenous ligand, anandamide, produces an inhibition of the electrically-evoked cholinergic contractions of the guinea-pig ileum as well (Pertwee et al., 1995a), but it is not clear whether this effect is mediated by CB1 receptors since interaction experiments with a CB1 receptor antagonist were not reported. Data concerning the effect of anandamide on acetylcholine release from the guinea-pig ileum have also not been published so far.
Anandamide is, in addition, an agonist at the vanilloid receptor (Zygmunt et al., 1999; Smart et al., 2000). The vanilloid receptor is a cation channel which is expressed on primary afferent fibres of the guinea-pig ileum (for reviews see Holzer, 1991; Szallasi & Blumberg, 1999). Stimulation of the vanilloid receptor by capsaicin causes an increase in basal acetylcholine release from myenteric neurons and smooth muscle contraction (Bartho et al., 1999; Mang et al., 2000). These excitatory effects of capsaicin are inhibited by the combined blockade of NK1 and NK3 tachykinin receptors (Bartho et al., 1999; Mang et al., 2000), which suggests that the stimulation of vanilloid receptors induces a release of tachykinins which, in turn, cause release of acetylcholine via activation of NK1 and NK3 receptors on cholinergic myenteric motoneurones.
In the present study we have investigated the effects of anandamide on acetylcholine release and longitudinal muscle contraction of the guinea-pig ileum. If anandamide stimulates cannabinoid CB1 receptors it should inhibit the electrically-evoked release of acetylcholine, and this effect should be antagonized by SR141716A. Should anandamide behave as an agonist at vanilloid receptors, an increase in basal acetylcholine release and contraction in a manner similar to capsaicin would be expected. The effects of anandamide were studied in the absence and presence of capsazepine, an antagonist of the vanilloid receptor (Bevan et al., 1992), and of selective antagonists at CB1 (SR141716A; Rinaldi-Carmona et al., 1994) and CB2 (SR144528, Rinaldi-Carmona et al., 1998) receptors. In addition, interaction experiments between anandamide and selective antagonists at the NK1 (CP99994) and NK3 (SR142801) receptor (for literature see Holzer & Holzer-Petsche, 1997) were performed. For comparison, the effects of the non-selective cannabinoid receptor agonist CP55940 were also investigated. Release of acetylcholine was measured in the absence of cholinesterase inhibition as outflow of tritium evoked by field stimulation from preparations that had been preincubated with [3H]-choline (Kilbinger & Wessler, 1980). Longitudinal muscle contractions were measured simultaneously in the same experiment. Part of this work has been communicated to the German Pharmacological Society (Mang et al., 2001).
Methods
Male guinea-pigs (350 – 450 g) were stunned by a blow to the head and exsanguinated. Longitudinal muscle strips with the myenteric plexus attached were prepared from the proximal half of the small intestine. The strips were suspended isometrically under a tension of 5 mN in a 2 ml organ bath and superfused (2 ml min−1) with a physiological salt solution (composition in mM: NaCl 137; KCl 2.7; CaCl2 1.8; MgCl2 1.0; NaHCO3 11.9; NaH2PO4 0.42; D-glucose 5.6; choline 0.001). The solution was bubbled with carbogen (95% O2 and 5% CO2) and warmed to a constant temperature of 37°C. The strips were allowed to equilibrate during 30 min and were stimulated during this period electrically at a frequency of 0.1 Hz (monophasic square wave pulses of 1 ms duration at supramaximal voltage). Superfusion was then stopped and the preparation incubated for 30 min with [3H]-choline (2.5 μCi ml−1) under electrical stimulation (0.1 Hz). At the end of the incubation the strips were superfused with physiological salt solution which contained 10 μM hemicholinium-3. After 70 min of washout the superfusate was collected in 3-min fractions. In most experiments the strips were stimulated twice 51 min apart (S1, S2; each 10 min at 0.1 Hz), and anandamide and CP55940 were added to the medium 27 min before S2. Antagonists (capsazepine, SR141716A, SR144528, CP99994 plus SR142801) were added 35 min before S1. Tritium content of the samples was measured by liquid scintillation spectrometry and corrected for counting efficiency using external standardization. At the end of the experiment the tissue was immersed overnight in 3 ml of 0.4 μM HClO4 and the radioactivity contained in the extract determined. The outflow of tritium was calculated as per cent of the tritium content in the tissue at the onset of the respective collection period. The outflow elicited by electrical stimulation or by anandamide was calculated as the difference between the total tritium outflow and the spontaneous outflow during the first two and last two unstimulated samples. The effects of drugs on the electrically-evoked outflow were calculated by expressing the ratio S2/S1 as a percentage of the equivalent ratio obtained on control experiments in which no drug was added after S1. The increase in basal release caused by anandamide was expressed as percentage of the release elicited by electrical stimulation during S1.
Longitudinal muscle contractions were recorded simultaneously. The peak tension developed by all 60 twitches evoked during S1 were added up and this total was compared to that obtained by adding up the individual responses in S2. The increase by anandamide of the basal muscle tone was expressed as percentage of the mean peak tension developed by all twitches elicited during S1.
The effect of anandamide against the contractile responses to the muscarinic agonist methacholine was studied on longitudinal muscle strips incubated in a 2 ml organ bath. In order to prevent the excitatory effect of anandamide on longitudinal muscle tone, tetrodotoxin (0.3 μM) was present throughout the experiment. During a 45 min equilibration period the strips were first challenged four times with 1 μM methacholine. The preparation was then incubated for 20 min with 10 μM anandamide before methacholine was added in cumulatively increasing concentrations (1 nM – 10 μM). Parallel control experiments without anandamide were performed under otherwise identical conditions.
Drugs
[Methyl-3H]-choline chloride (80 Ci mmol−1; NEN); anandamide, capsazepine, hemicholinium-3; methacholine (acetyl-ß-methylcholine chloride) (all Sigma); CP55940 (Tocris); CP99994 [(+)-(2S,3S)-3-(2-methoxybenzylamino)-2-phenylpiperidine] (Pfizer); SR142801 [(S)-(N)-(1-(3-(1-benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl)propyl)-4-phenylpiperidin-4-yl)-N-methylacetamide]; SR141716A [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide)]; SR144528 [N-([1s]-endo-1.3.3-trimethylbicyclo[2.2.1]heptan-2-yl)-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide] (all Sanofi). Drugs were dissolved in distilled water or DMSO (anandamide, capsazepine, CP55940, SR142801, SR141716A, SR144528). DMSO at 0.3% (maximal concentration) did not affect basal or electrically-evoked outflow of 3H.
Statistical analysis
Values are given as means with s.e.mean or 95% confidence limits. n represents the number of guinea-pigs used. The significance of differences between two mean values was assessed by Student's t-test. For comparison of one control with several experimental groups, the significance of difference was estimated by one-way analysis of variance followed by Dunnett's test. Concentration-response data for agonists were evaluated by sigmoid curve fitting and, −log EC50 values (pEC50) with their 95% confidence limits were calculated from individual values by nonlinear regression analysis by GraphPad Prism (San Diego, U.S.A.). Apparent pKB values were determined as described by Furchgott (1972) using a single antagonist concentration: pKB=log (concentration ratio −1) −(log antagonist concentration). Differences between mean values were considered significant if P<0.05.
Results
Effects of anandamide on basal acetylcholine release and smooth muscle tone
Anandamide caused transient and concentration-dependent increases in basal [3H] release and resting tone of the longitudinal muscle (Figure 1). In some experiments the strips were superfused at the end of S1 for 30 min with a calcium-free medium (n=2), or with 0.3 μM tetrodotoxin (Figure 4) before 10 μM anandamide was added. Both procedures abolished the effects of anandamide. We therefore assume that the increase by anandamide in the [3H] release represents release of neuronal [3H]-acetylcholine. Figure 2 shows the concentration-response curves for the effects of anandamide on basal release and on muscle tone. The maximal effects were obtained with 10 μM and the pEC50 value of 6.3 (5.5 – 7.0, 95% confidence limits) for release was in accordance with that for contraction (6.3; 5.9 – 6.8). Capsazepine (1 μM) significantly inhibited the facilitatory effects of anandamide (Figure 2). From the shift to the right of the concentration-response curves apparent pKB values of 7.5 (6.1 – 8.9) for release and 7.6 (6.9 – 8.4) for muscle tone were calculated for capsazepine. The effect of anandamide was also investigated after the combined blockade of NK1 and NK3 receptors by CP99994 plus SR142801 (each 0.1 μM). Figure 2 shows that the tachykinin receptor antagonists inhibited the facilitatory effects of anandamide in a similar manner as capsazepine.
Figure 1.
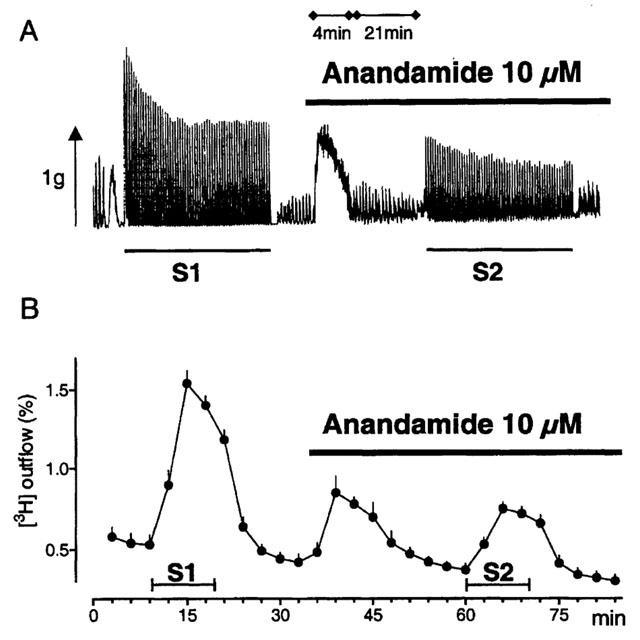
Effects of 10 μM anandamide on outflow of [3H] radioactivity and longitudinal muscle contraction. The strips were stimulated twice at 0.1 Hz (10 min) (S1, S2). Anandamide was present as indicated by the horizontal bar. Upper panel: Original recording of the effect of anandamide on basal muscle tone and on electrically-evoked contractions. Note that the chart speed was increased at the beginning of the anandamide application. Lower panel: Ordinate, outflow of [3H] as percentage of the tritium present in the tissue at the start of each fraction. Mean±s.e.mean (n=4).
Figure 4.
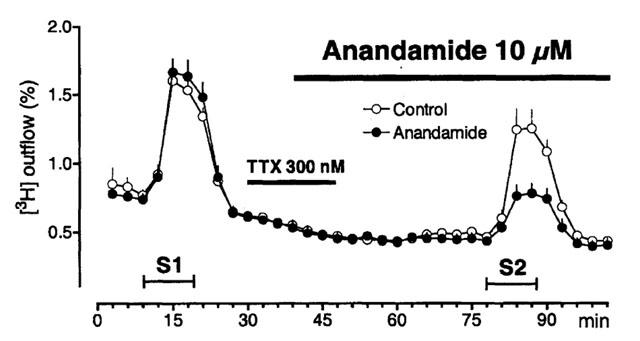
Effects of anandamide in the presence of tetrodotoxin on basal and electrically-evoked outflow of [3H]-acetylcholine. Electrical stimulation (0.1 Hz, 10 min) was performed 9 min (S1) and 78 min (S2) after the end of the washout period. Horizontal bars indicate superfusion with tetrodotoxin (TTX) and TTX plus anandamide. Control experiments, effect of a 18 min superfusion with tetrodotoxin (TTX) on the electrically-evoked outflow of [3H]-acetylcholine during S2. The S2/S1 ratio (0.89±0.06, n=4) was not different from that in experiments without TTX (see Table 1). Anandamide (n=4) was superfused first together with TTX (9 min), and then without TTX.
Figure 2.
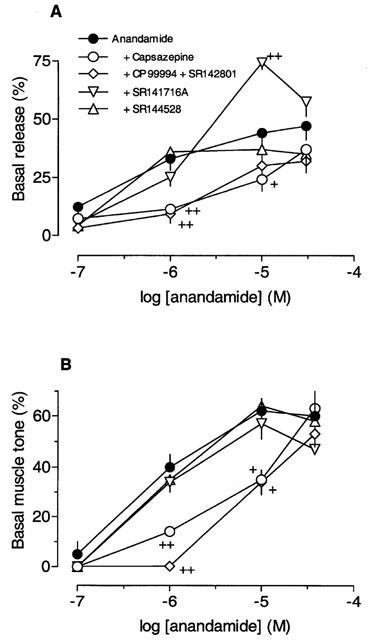
Increase by anandamide of basal [3H]-acetylcholine release (A) and longitudinal muscle tone (B) in the absence (n=4 – 11) and presence of 1 μM capsazepine (n=4 – 6), 1 μM SR141716A (n=4 – 8), 30 nM SR144528 (n=4 – 6), or CP99994 plus SR142801 (each 0.1 μM) (n=4 – 5). The antagonists were added 59 min before anandamide. The anandamide-induced increase in basal release is given as percentage of the [3H]-acetylcholine release caused by S1, and the increase in muscle tone is given as percentage of the mean peak tension developed by all twitches during S1. Each value is the mean of n individual observations; vertical bars indicate s.e.mean. Significance of difference from the effect of anandamide alone: +P<0.05, ++P<0.01.
The increase by anandamide of basal [3H]-acetylcholine release and muscle tone remained unchanged in the presence of 30 nM SR144528. Likewise, the increase in release elicited by 0.1, 1 and 30 μM anandamide was not significantly affected by 1 μM SR141716A (Figure 2). However, the facilitatory effect of 10 μM anandamide on basal [3H]-acetylcholine release was increased further in the presence of 1 μM SR141716A. The longitudinal muscle contractions elicited by anandamide were not modified by 1 μM SR141716A (Figure 2).
Effects of anandamide on electrically-evoked release and contractions
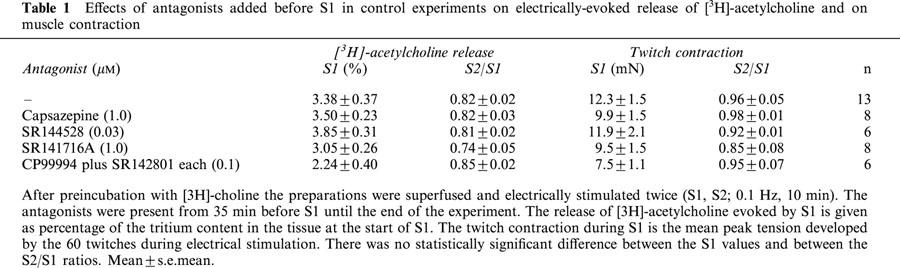
In control experiments the [3H]-acetylcholine release evoked by S2 was 82±2% (n=13) of that caused by S1. The antagonists, when added 35 min before S1, did not per se change either the evoked release and twitch contraction during S1, or the ratios S2/S1 for release and contraction (Table 1).
Table 1.
Effects of antagonists added before S1 in control experiments on electrically-evoked release of [3H]-acetylcholine and on muscle contraction
Anandamide concentration-dependently inhibited the electrically-evoked release and twitch contractions (Figures 1 and 3) with pEC50 values of 5.8 (5.4 – 6.1; release) and 5.2 (4.7 – 5.7; twitch). The inhibition might be due to depletion of the available store of tritiated transmitter by the preceding facilitatory effect of anandamide. In order to test this possibility, experiments were performed in which the excitatory effect of anandamide was prevented by tetrodotoxin. Figure 4 shows that during and after perfusion with 0.3 μM tetrodotoxin, anandamide (10 μM) no longer increased the outflow of [3H] radioactivity. However anandamide reduced the electrically-evoked release (to 41±6%; n=4) to the same extent as in the absence of tetrodotoxin (48±3%; n=5). Thus, the inhibition by anandamide is independent of the preceding excitatory action of anandamide.
Figure 3.
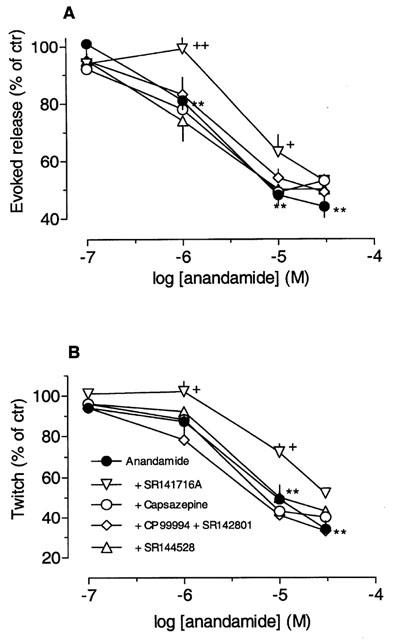
Inhibition by anandamide of the electrically-evoked release of [3H]-acetylcholine (A) and longitudinal muscle contractions (B). Strips were stimulated twice (S1, S2) and anandamide was added 27 min before S2. The antagonists, i.e. capsazepine (1 μM), SR141716A (1 μM), SR144528 (30 nM) or CP99994 plus SR142801 (each 0.1 μM), were added 35 min before S1. Control experiments without anandamide were run in parallel (Table 1), and the effects of anandamide are given as a percentage of the corresponding control value given in Table 1. Each value is the mean of n individual observations (see legend to Figure 2); vertical bars indicate s.e.mean. Significance of inhibition of the evoked release and of twitch contraction: **P<0.01 (ANOVA followed by Dunnett's test). Significance of difference from the effect of anandamide alone: +P<0.05, ++P<0.01 (t-test).
The inhibitory effects of anandamide were not modified either by 1 μM capsazepine or the combination of CP99994 plus SR142801 (each 0.1 μM; Figure 3). Likewise, the CB2 receptor antagonist SR144528 (30 nM) did not change the inhibitory effects of anandamide. On the other hand, a concentration of 1 μM SR141716A significantly attenuated the inhibitory effects of 1 and 10 μM anandamide on [3H]-acetylcholine release and twitch contraction (Figure 3), and apparent pKB values of 6.6 (5.9 – 7.5; release) and 6.2 (5.3 – 7.5; contraction) were calculated from the rightward shift of the concentration-response curves.
The effects of anandamide against the contractile responses to an exogenous muscarinic agonist were studied in separate experiments. We chose methacholine which, in contrast to acetylcholine, does not stimulate nicotinic receptors. Contractions caused by 10 and 100 nM methacholine (10 – 18 mN) which were similar in magnitude to twitch contractions elicited by electrical field stimulation (cf. S1 values in Table 1) were not affected by anandamide (Figure 5). Anandamide slightly but not significantly depressed the maximal effect of methacholine. The pEC50 value of methacholine (7.2; 6.4 – 8.0) was not significantly changed in the presence of anandamide (7.6; 6.1 – 9.1).
Figure 5.
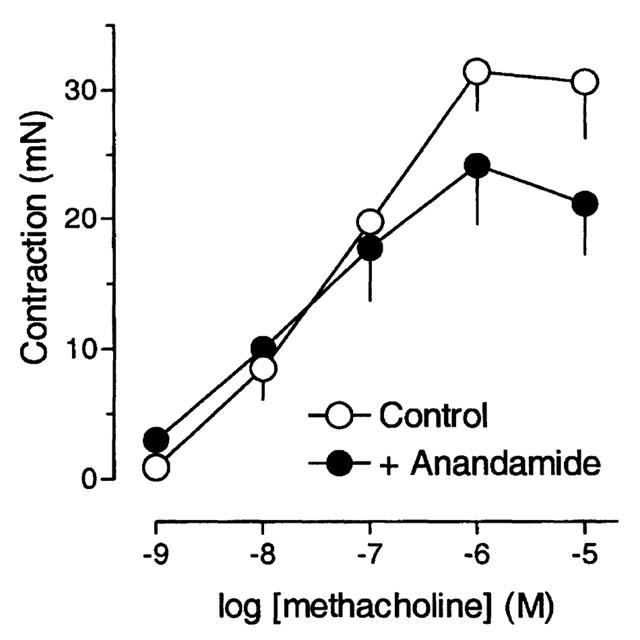
Concentration-response curves for methacholine in the absence and presence of 10 μM anandamide. Methacholine was applied in cumulatively increasing concentrations. Anandamide was added 20 min before methacholine. Ordinate, force of contraction (Newton). Each value is the mean of four individual observations. Vertical bars indicate s.e.mean.
Effects of CP55940 on electrically-evoked release and twitch contractions
CP55940 inhibited in a concentration-dependent manner the evoked release of [3H]-acetylcholine and twitch contractions with pEC50 values of 9.9 (9.5 – 10.3; release) and 9.9 (9.5 – 10.5; contraction) (Figure 6). Basal [3H] release and longitudinal muscle tone were not affected by CP55940. SR141716A (0.1 μM) shifted the concentration-inhibition curves to the right and apparent pKB values of 8.4 (7.9 – 9.0; release) and 8.9 (8.4 – 9.5; twitch) were calculated.
Figure 6.
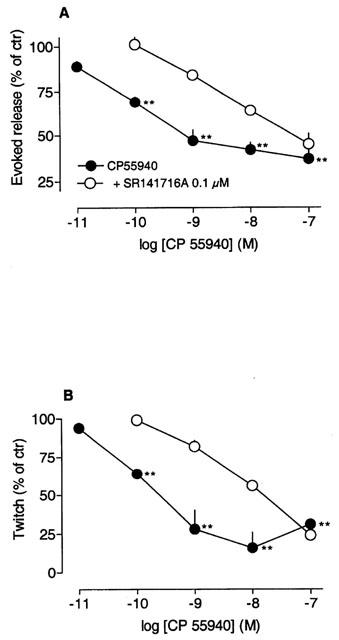
Inhibition by CP55940 of the electrically-evoked release of [3H]-acetylcholine (A) and longitudinal muscle contractions (B). Strips were stimulated twice (S1, S2) and CP55940 was added 27 min before S2. SR141716A (0.1 μM) was added 35 min before S1. Each value is the mean of 4 – 6 individual observations; vertical bars indicate s.e.mean. For further details see legend to Figure 3.
Effects of SR141716A on electrically-evoked release and twitch contractions
The CB1 receptor antagonist SR141716A has been found to enhance the electrically-evoked release of acetylcholine (Coutts & Pertwee, 1997) and longitudinal muscle contractions (Pertwee et al., 1996) of guinea-pig ileum. In the present experiments SR141716A, when added throughout superfusion, did not change the absolute values of [3H]-acetylcholine release and twitch contractions during S1 (Table 1). In order to study more rigorously the effects of SR141716A, the antagonist was added to the medium 27 min before S2. At a concentration of 0.1 μM SR141716A significantly enhanced both the evoked [3H]-acetylcholine release and twitch contractions to 162±15 and 161±13% (n=4) of the respective control values (100±5 and 100±8%, n=8). However, 1 μM SR141716A did not significantly change either the evoked release (101±11%) or the twitch contractions (109±18%, n=8).
Discussion
The present study shows that the endocannabinoid anandamide has opposite effects on basal and evoked release of acetylcholine which are mediated by different mechanisms.
Anandamide increased basal acetylcholine release and muscle tension and this effect was competitively antagonized by capsazepine but not by CB1 or CB2 receptor antagonists. The pKB values of capsazepine from our experiments (7.5 – 7.6) correlate well with the pKB values obtained for capsazepine at vanilloid receptors on sensory neurons (6.7 – 6.9; Bevan et al., 1992), and at recombinant rat (7.5; Jerman et al., 2000) and human vanilloid receptors (7.4; Smart et al., 2000). Furthermore, the present pEC50 value for anandamide (6.3) is similar to those reported for the vanilloid receptor-mediated vasorelaxation (6.0 – 6.5; Zygmunt et al., 1999). Thus, the anandamide-induced increase of acetylcholine release is probably mediated via vanilloid receptors. Stimulation by capsaicin of vanilloid receptors on primary afferent fibres in the guinea-pig ileum causes a release of tachykinins which, in turn, stimulate the release of acetylcholine via activation of NK1 and NK3 receptors, presumably located on the somadendritic region of cholinergic motoneurones (Bartho et al., 1999; Mang et al., 2000). The present study suggests that anandamide acts in the same way since the combined blockade of NK1 and NK3 receptors antagonized the facilitatory effect of anandamide.
An unexpected but consistent finding was the enhancement of the release-stimulating action by 10 μM anandamide in the presence of 1 μM SR141716A. We have not further investigated this effect, but it is conceivable that the endogenous tachykinins released by anandamide excite cholinergic neurones that are endowed with the receptor whose stimulation by anandamide causes inhibition of the electrically-evoked acetylcholine release. Blockade by SR141716A would then unmask this inhibition.
The mechanism through which anandamide inhibited the electrically-evoked release and contraction of the longitudinal muscle is not clear. Anandamide has previously been shown to decrease the twitch contraction of the myenteric plexus-longitudinal muscle preparation with an pEC50 value (5.05; Pertwee et al., 1995a) which is similar to the present value (5.2). However, the receptor mediating this inhibition has not been characterized. The contractile response to the muscarinic agonist methacholine was not significantly affected by anandamide which suggests that anandamide inhibits the electrically-evoked contraction by a prejunctional action.
It is generally accepted that the cholinergic myenteric neurones have release-inhibiting CB1 receptors (see Pertwee, 1999). The ability of CP55940 to inhibit the evoked acetylcholine release and muscle contraction, and of SR141716A to antagonize these inhibitions (Pertwee et al., 1996; Coutts & Pertwee, 1997) were confirmed under our experimental conditions (absence of cholinesterase inhibitor; measurement of release and contraction in the same experiment), and the pEC50 values of CP55940 (9.9) as well as the pA2 values for SR141716A (8.4 and 8.9) are in line with the values previously published (Pertwee et al., 1996; Coutts & Pertwee, 1997). Similarly, we confirmed the facilitatory effect of SR141716A alone on the electrically-evoked [3H]-acetylcholine release and twitch contraction. Thus, cholinergic neurotransmission in the myenteric plexus-longitudinal muscle preparation can be inhibited by cannabinoid CB1 receptors. However, the unexpectedly low potency of SR141716A in antagonizing the inhibitory effects of anandamide suggests that anandamide does not interact with the CB1 receptor on myenteric neurones. A concentration of 1 μM SR141716A shifted the concentration-response curves of anandamide slightly to the right, yet the approximate pKB values of 6.6 and 6.2 calculated from these shifts are not compatible with an action at CB1 receptors. Furthermore, the experiments with SR144528 show that CB2 receptors are likewise not involved in the effects of anandamide. Several studies have shown that the potency of cannabinoid receptor antagonists is diminished if anandamide is used as an agonist: Thus, SR141716A was much less effective in blocking behavioural effects of anandamide (antinociception, hypothermia, immobility) than in blocking these actions induced by other agonists such as CP 55940 or Δ9-THC (Adams et al., 1998, Welch et al., 1998). Similarly, in the isolated mouse vas deferens, the antagonist AM630 was more than 15 fold less effective in antagonizing the twitch inhibition induced by anandamide as compared to CP 55940 (Pertwee et al., 1995b). These findings have been explained with the possible existence of subtypes of the CB1 receptor to which SR141716A or AM630 bind with a lower affinity. Our data support this hypothesis and suggest that anandamide inhibits the electrically-evoked release of acetylcholine from cholinergic neurones innervating the longitudinal muscle via stimulation of a non-CB1 receptor that is susceptible to antagonism by SR141716A but with a lower KB value than that observed for this compound at the CB1 receptor. Alternatively, it may be that anandamide is acting on both CB1 and non-CB1 receptors to inhibit cholinergic neurotransmission which could explain the low potency of SR141716A.
It should be noted, though, that functional studies suggest that the cholinergic contraction of the circular muscle of the guinea-pig ileum is inhibited by anandamide acting at the CB1 receptor (Izzo et al., 1998). Whether this indicates that the cholinergic nerves innervating the circular muscle express the typical CB1 receptor is not clear and is beyond the scope of the present investigation.
The inhibition by anandamide of acetylcholine release might also result from the action of CGRP which is coreleased with tachykinins following stimulation of vanilloid receptor of primary afferent fibres (Holzer & Holzer-Petsche, 1997). CGRP is known to inhibit the electrically-evoked release of acetylcholine in the guinea-pig ileum (Schwörer et al., 1991). This possibility seems, however, unlikely, since the inhibitory effect of anandamide was not changed after blockade of the vanilloid receptor by capsazepine.
Conclusion
Anandamide increased basal acetylcholine release from the myenteric plexus-longitudinal muscle preparation via stimulation of vanilloid receptors. This effect involves the release of tachykinins from afferent nerves. In addition, anandamide inhibited the electrically-evoked release of acetylcholine and longitudinal muscle contraction. Likewise, the cannabinoid receptor agonist CP55940 decreased the evoked acetylcholine release and twitch contraction. The agonists differed, however, in that the CB1 receptor antagonist SR141716A was much less potent in blocking the inhibitory effect of anandamide than that of CP55940. We therefore conclude that anandamide inhibits the evoked acetylcholine release from guinea-pig myenteric neurones via a non-CB1 receptor.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (Ki 210/8). We wish to thank the pharmaceutical companies (Sanofi, Pfizer) for donation of drugs.
Abbreviations
- ANOVA
analysis of variance
- DMSO
dimethylsulphoxide
References
- ADAMS I.B., COMPTON D.R., MARTIN B.R. Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J. Pharmacol. Exp. Ther. 1998;284:1209–1217. [PubMed] [Google Scholar]
- BARTHO L., LENARD L., PATACCHINI R., HALMAI V., WILHELM M., HOLZER P., MAGGI C.A. Tachykinin receptors are involved in the ‘local efferent' motor response to capsaicin in the guinea-pig small intestine and oesophagus. Neuroscience. 1999;90:221–228. doi: 10.1016/s0306-4522(98)00459-x. [DOI] [PubMed] [Google Scholar]
- BEVAN S., HOTHI S., HUGHES G., JAMES I.F., RANG H.P., SHAH K., WALPOLE C.S., YEATS J.C. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUTTS A.A., PERTWEE R.G. Inhibition by cannabinoid receptor agonists of acetylcholine release from the guinea-pig myenteric plexus. Br. J. Pharmacol. 1997;121:1557–1566. doi: 10.1038/sj.bjp.0701301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI MARZO V., MELCK D., BISOGNO T., DEPETROCELLIS L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F.The classification of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory Catecholamines, Handb. Exp. Pharmakol. 1972Berlin: Springer-Verlag; 283–335.Vol. 33, ed. Blaschko, H., Muscholl, E. pp [Google Scholar]
- HOLZER P. Capsaicin:cellular targets, mechanisms of action and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- HOLZER P., HOLZER-PETSCHE U. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol. Ther. 1997;73:173–217. doi: 10.1016/s0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., BORRELLI F., CAPASSO F. Excitatory transmission to the circular muscle of the guinea-pig ileum: evidence for the involvement of cannabinoid CB1 receptors. Br. J. Pharmacol. 1998;124:1363–1368. doi: 10.1038/sj.bjp.0701964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERMAN J.C., BROUGH S.J., PRINJHA R., HARRIES M.H., DAVIS J.B., SMART D. Characterization using FLIPR of rat vanilloid receptor (rVR1) pharmacology. Br. J. Pharmacol. 2000;130:916–922. doi: 10.1038/sj.bjp.0703390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILBINGER H., WESSLER I. Inhibition by acetylcholine of stimulation-evoked release of [3H]acetylcholine from the guinea-pig myenteric plexus. Neuroscience. 1980;5:1331–1340. doi: 10.1016/0306-4522(80)90205-5. [DOI] [PubMed] [Google Scholar]
- MANG C.F., ERBELDING D., KILBINGER H. Differential effects of anandamide in basal and evoked release of acetylcholine (ACh) from guinea-pig ileum. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:R 24. [Google Scholar]
- MANG C.F., GEBER C., KILBINGER H. Effects of capsaicin on acetylcholine (ACh) release from guinea-pig myenteric plexus. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;361:R 22. [Google Scholar]
- PERTWEE R.G. Evidence for the presence of CB1 cannabinoid receptors on peripheral neurones and for the existence of neuronal non-CB1 cannabinoid receptors. Life Sci. 1999;65:597–605. doi: 10.1016/s0024-3205(99)00282-9. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G., FERNANDO S.R., GRIFFIN G., ABADJI V., MAKRIYANNIS A. Effect of phenylmethylsulphonyl fluoride on the potency of anandamide as an inhibitor of electrically evoked contractions in two isolated tissue preparations. Eur. J. Pharmacol. 1995a;272:73–78. doi: 10.1016/0014-2999(94)00618-h. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G., FERNANDO S.R., NASH J.E., COUTTS A.A. Further evidence for the presence of cannabinoid CB1 receptors in guinea-pig small intestine. Br. J. Pharmacol. 1996;118:2199–2205. doi: 10.1111/j.1476-5381.1996.tb15663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERTWEE R., GRIFFIN G., FERNANDO S., LI X., HILL A., MAKRIYANNIS A. AM630, a competitive cannabinoid receptor antagonist. Life Sci. 1995b;56:1949–1955. doi: 10.1016/0024-3205(95)00175-6. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NELIAT G., CAPUT D., FERRARA P., SOUBRIÉ P., BRELIÈRE J.C., LE FUR G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J.M., CASELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOUABOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIERE J.C., LE FUR G.L. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- SCHWÖRER H., SCHMIDT W.E., KATSOULIS S., CREUTZFELDT W. Calcitonin gene-related peptide (CGRP) modulates cholinergic neurotransmission in the small intestine of man, pig and guinea-pig via presynaptic CGRP receptors. Regul. Pept. 1991;36:345–358. doi: 10.1016/0167-0115(91)90068-r. [DOI] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- WELCH S.P., HUFFMAN J.W., LOWE J. Differential blockade of the antinociceptive effects of centrally administered cannabinoids by SR141716A. J. Pharmacol. Exp. Ther. 1998;286:1301–1308. [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H.H., SORGARD M., DIMARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


