Abstract
Ezetimibe potently inhibits the transport of cholesterol across the intestinal wall, thereby reducing plasma cholesterol in preclinical animal models of hypercholesterolemia. The effect of ezetimibe on known absorptive processes was determined in the present studies.
Experiments were conducted in the hamster and/or rat to determine whether ezetimibe would affect the absorption of molecules other than free cholesterol, namely cholesteryl ester, triglyceride, ethinylestradiol, progesterone, vitamins A and D, and taurocholic acid. In addition, to determine whether exocrine pancreatic function is involved in the mechanism of action of ezetimibe, a biliary anastomosis model, which eliminates exocrine pancreatic function from the intestine while maintaining bile flow, was established in the rat.
Ezetimibe reduced plasma cholesterol and hepatic cholesterol accumulation in cholesterol-fed hamsters with an ED50 of 0.04 mg kg−1. Utilizing cholesteryl esters labelled on either the cholesterol or the fatty acid moiety, we demonstrated that ezetimibe did not affect cholesteryl ester hydrolysis and the absorption of fatty acid thus generated in both hamsters and rats. The free cholesterol from this hydrolysis, however, was not absorbed (92 – 96% inhibition) in the presence of ezetimibe. Eliminating pancreatic function in rats abolished hydrolysis of cholesteryl esters, but did not affect the ability of ezetimibe to block absorption of free cholesterol (−94%). Ezetimibe did not affect the absorption of triglyceride, ethinylestradiol, progesterone, vitamins A and D, and taurocholic acid in rats.
Ezetimibe is a potent inhibitor of intestinal free cholesterol absorption that does not require exocrine pancreatic function for activity. Ezetimibe does not affect the absorption of triglyceride as a pancreatic lipase inhibitor (Orlistat) would, nor does it affect the absorption of vitamin A, D or taurocholate, as a bile acid sequestrant (cholestyramine) would.
Keywords: Cholesterol, triglyceride, fat soluble vitamins, absorption, pancreas, rat, hamster
Introduction
We have previously described the discovery of ezetimibe (SCH58235; Figure 1), a novel cholesterol absorption inhibitor that lowers plasma cholesterol in cholesterol-fed mice, hamsters, rats, rabbits, dogs and monkeys (Davis et al., 1995; van Heek et al., 1997; 2000; Rosenblum et al., 1998). Ezetimibe also lowers LDL cholesterol and raises HDL in humans with hypercholesterolemia (Lipka et al., 2000). Data from a number of clinical trials indicate that reducing plasma cholesterol by pharmacological means leads to reductions in the incidence of death from cardiovascular disease (Scandinavian Simavastatin Study Group (4S), 1994; Shepherd et al., 1995; Sacks et al., 1996; LIPID, 1998).
Figure 1.
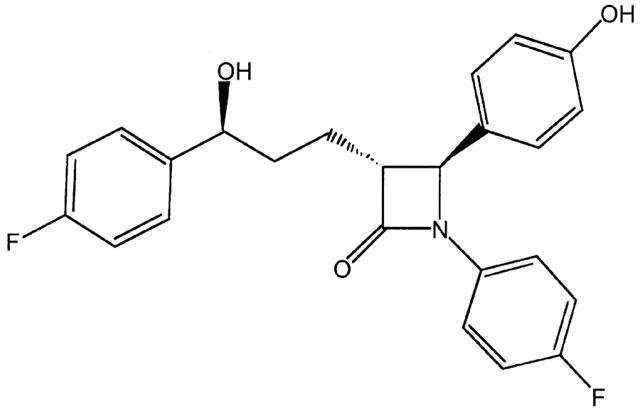
Structure of ezetimibe. (SCH58235; 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4-hydroxyphenyl)-2-azetidinone).
The mechanism by which free cholesterol is absorbed in the intestine is not clearly understood. Recently, interest in the field of cholesterol absorption has resurged. New findings regarding possible mechanisms of cholesterol absorption, and pharmacological agents which specifically inhibit cholesterol absorption, indicate that free cholesterol absorption is not a passive diffusion process as once thought (for review, please see Homan & Krause, 1997; Dawson & Rudel, 1999; Turley, 1999). Ezetimibe inhibits the transport of dietary and biliary cholesterol across the intestinal wall. It was important to demonstrate the effect of this compound on known processes of absorption in the intestine. Studies described herein were designed to determine whether ezetimibe inhibited the hydrolysis of cholesteryl esters in the intestine or interfered with the absorption of other lipids, steroids or fat-soluble vitamins. In addition, a biliary anastomosis rat model, which eliminates the flow of exocrine pancreatic secretions into the intestine, was established to answer two questions: Is a functional exocrine pancreas necessary for the absorption of free cholesterol? Are pancreatic secretions into the intestinal lumen necessary for the activity of ezetimibe?
Methods
Dose response of ezetimibe in hamsters
Male golden syrian hamsters (Charles River Labs, Wilmington, MA, U.S.A.) weighing between 100 – 125 g were fed rodent chow until study onset. At study onset, animals were separated into groups (n=4 – 6 per group) based on body weight, and were fed chow supplemented with 0.5% cholesterol (by weight; Research Diets Inc., New Brunswick, NJ, U.S.A.) for 7 days. Ezetimibe (0.03 – 1 mg kg−1) was administered once daily via oral gavage in 0.2 ml corn oil. After 7 days, animals were sacrificed by decapitation after anaesthesia, blood was collected, and plasma was separated by low speed centrifugation at 4°C for cholesterol (Wako; Osaka, Japan) and triglyceride (Sigma; St. Louis, MO, U.S.A.) analyses. Livers were harvested, weighed and 0.5 g samples were extracted (Folch et al., 1957) for neutral lipid analyses by HPLC as previously described in detail (Burrier et al., 1995).
Effect of ezetimibe on absorption of 3H-cholesteryl oleate, cholesteryl 14C-oleate and 3H-triolein in hamsters
Male golden syrian hamsters were fed a diet containing 0.5% cholesterol overnight and divided into four groups (n=5 per group). The following morning, two groups were dosed with vehicle (0.2 ml corn oil) and two with ezetimibe (10 mg kg−1). One hour later, one vehicle group and one ezetimibe-treated group were gavaged with 1 μCi each of 3H-cholesteryl-oleate and cholesteryl-14C-oleate, and 1 mg of unlabelled cholesteryl oleate in corn oil. The other vehicle-treated and ezetimibe-treated groups received an oral dose of corn oil containing 1 μCi 3H-triolein (NEN/Dupont). Unlabelled triglyceride was not included because the corn oil contained sufficient carrier. The high dose of ezetimibe was chosen to maximize the cholesterol absorption inhibition, as well as to detect any effect on cholesterol esterase and pancreatic lipase the compound might have, even at higher than pharmacologically active doses. Two hours later, animals were sacrificed, blood was collected and plasma was isolated. Aliquots of the plasma were analysed for 14C and 3H radioactivity.
Effect of ezetimibe on absorption of 14C-free cholesterol and 3H-cholesteryl oleate in rats
Fasted Sprague Dawley rats, which had been on normal chow diet, were anaesthetized with Inactin (100 mg kg−1 i.p.). All rats were fitted with tracheal tubes and were intraduodenally cannulated as previously described in detail (van Heek et al., 1997; 2000). For vehicle delivery, a control emulsion containing triolein (35.4 mg per dose), L-α-phosphatidylcholine (6.69 mg per dose) and sodium taurocholate (3 ml per dose; 19 mM in Dulbecco's phosphate buffered saline (DPBS), pH 6.4) was delivered directly into the intestine (n=5). For drug delivery, the same emulsion as described, with ezetimibe (1.0 mg kg−1; n=5), was delivered intraduodenally. This high dose (the effective dose at which 50% inhibition is observed (ED50) for rat=0.0016 mg kg−1) was chosen to capture any effect ezetimibe might have on the hydrolysis of cholesteryl ester. One hour after vehicle and drug delivery, 3 ml of another emulsion containing triolein, L-α-phosphatidylcholine, and sodium taurocholate as described above, as well as unlabelled (0.5 mg per dose) and 14C-free cholesterol (1 μCi per dose), and unlabelled (0.5 mg per dose) and 3H-cholesteryl oleate (2 μCi per dose: 3H on the cholesteryl moiety) was delivered intraduodenally to all rats. One and one half hours after the second emulsion, rats were sacrificed, blood was collected, and small intestines were rinsed with 50 ml of saline, which was retained (luminal contents). Triplicate aliquots of plasma and intestinal luminal contents were directly analysed for 14C and 3H. The entire small intestine was extracted in a 2 : 2 : 1 chloroform : methanol : water system (Folch et al., 1957); free cholesterol and cholesteryl ester were separated by thin layer chromatography in an 80 : 20 : 1 hexane : diethyl ether : glacial acetic acid solvent system. Free cholesterol and cholesteryl ester bands were scraped and analysed for 14C and 3H radioactivity.
Effect of ezetimibe on cholesterol absorption in rats with (sham-operated) and without (biliary anastomosis) exocrine pancreatic function
Surgical preparation of sham-operated and biliary anastomosed rats
The following surgical manipulation was a modification of the procedure described by Lin et al. (1957). Chow-fed male Sprague Dawley rats weighing between 300 and 350 grams were anaesthetized with an intramuscular injection of 30 mg kg−1 Ketamine and 3.3 mg kg−1 xylazine mixture. Animals were shaved of their ventral hair from xiphisternum to the inguinal region. The shaved area was scrubbed with a povidone-iodine solution (Betadine) and then wiped with 70% ethanol. Using a sterile technique, a midline incision was made along the linea alba and the abdominal wall retracted on both sides with hemostats. For sham-operated animals (n=10), the bile duct was located with sterile cotton applicators and manipulated similarly to that which was performed for the biliary anastomosis (see below). The animal's abdominal wall was then sutured with a continuous stitch and the skin was closed with wound clips. Triple antibiotic ointment (polymyxin B sulphate, bacitracin zinc, neomycin sulphate) was applied to the closed wound. For the biliary anastomosis rats (n=12), the bile duct was first ligated at its entry to the duodenum and retracted. The duct was cannulated with polyethylene tubing (PE 50; Becton Dickinson; Sparks, MD, U.S.A.) proximal to the liver and tied in place. A ligature was tied just below the cannulation site to prevent leakage of pancreatic secretions into the peritoneum. A second ligature was tied proximal to the ligature at the duct's entrance to the intestine and the duct was transected between the ligatures to ensure that no secretions could enter the gut. The cannula was manipulated to form a ‘dead loop' and redirected along the course of the intestine. A 5 mm circular purse stitch was sewn just below the site of the duct's original entry to the intestine. An 18G needle was used to puncture a hole in the middle of the purse. The cannula was placed inside the intestine approximately 1 cm and the purse stitch was drawn tight. The animal's abdominal wall and skin layers were then closed as was described above for sham-operated animals. After recovery from anaesthesia, the rats were housed individually with free access to chow and water for 30 h. Animals were fasted overnight prior to the cholesterol absorption portion of the study.
Acute cholesterol absorption experiment
The sham-operated and biliary anastomosed rats were anaesthetized with 100 mg kg−1 Inactin i.p. The intestine was intraduodenally cannulated as described previously (van Heek et al., 1997; 2000). Sham (n=5 per group) or biliary anastomosed (n=6 per group) rats were randomly assigned and given 1 ml of either 0.4% methyl cellulose vehicle or ezetimibe at 3 mg kg−1 in methyl cellulose. Vehicle or drug remaining in the cannula after delivery was rinsed into the intestine with 1 ml of saline. The 3 mg kg−1 dose was chosen because ezetimibe was not completely soluble in this vehicle and therefore absorption might not be as efficient as we had observed with corn oil or emulsion vehicle. These latter two vehicles could not be used because, without exocrine pancreatic function, the lipids in these vehicles would not be hydrolyzed and subsequently solubilized, and therefore their contents would not be absorbed. Within 5 min of vehicle or drug delivery, 3 ml of a solution containing monolein (3.2 mg per dose), oleic acid (2.7 mg per dose), equimolar free cholesterol (0.387 mg per dose) and cholesteryl oleate (0.651 mg per dose), 14C-cholesterol (1 μCi per dose), 3H-cholesteryl oleate (2 μCi per dose), and sodium taurocholate (32.2 mg per dose, in DPBS at pH 6.4) was delivered to the intestine via the cannula (Watt & Simmonds, 1981). After 1.5 h, the rats were sacrificed under anaesthesia by decapitation and trunk blood was collected. The liver was excised and frozen. The small intestine was removed and rinsed with 50 ml of ice-cold saline. Both intestinal luminal contents and small intestines were held on ice until frozen at −80°C. Triplicate aliquots of plasma were directly analysed for 14C and 3H.
Verification of biliary anastomosis surgery
Two functional assays were performed on the intestinal luminal contents to verify that the removal of exocrine pancreatic secretions by the biliary anastomosis procedure was complete. The Azocoll assay (Watt & Simmonds, 1981) for the determination of nonspecific luminal proteolytic activity was done as follows: (in duplicate) 10 mg of Azocoll substrate (Calbiochem) was weighed into glass tubes; 0.20 ml of intestinal luminal rinse was added. Two ml of DPBS adjusted to pH 7.0 was added and the tubes vortexed. The tubes were incubated for 5 min at 37°C. The tubes were mixed again and a 0.20 ml aliquot of the developed samples were taken into a 96-well filtration plate with a 0.45 micron mesh. The mixture was filtered under vacuum into a 96-well plate and read on a plate reader spectrophotometer at 540 nm. The data are reported as optical density units as they relate to the optical density for the blank. The second assay which determines the amylase activity of the intestinal luminal contents was performed as follows: a starch substrate solution was made (2.0 g starch, 0.04 gNaCl, DPBS (pH 6.9) to a final volume of 100 ml, maintained at >37°C). Standards (maltose) and intestinal luminal contents samples (0.01 – 0.09 ml) were added to 0.1 ml starch solution. These were then incubated for 5 min at 37°C and the reaction was stopped with 0.2 ml 3.5-dinitrosalicylate (stopping reagent). The tubes were boiled for 10 min and then cooled, 1 ml of water was added, and the optical density of the standards and samples were read at 530 nm. Data are expressed as a rate (mg of maltose formed in 5 min, standardized for the volume of intestinal luminal contents used). In addition, serum insulin (rat radioimmunoassay, LINCO Research, Inc., St. Charles, MO, U.S.A.) and glucose (Sigma Diagnostics, St. Louis, MO, U.S.A.) levels were determined on all samples to ensure that endocrine pancreatic function had not been compromised by the surgical manipulation.
Effect of ezetimibe on the acute absorption of ethinyl estradiol, progesterone, vitamins A and D and taurocholic acid in rats
Absorption of cholesterol, ethinyl estradiol, and progesterone
Male Sprague Dawley rats (n=5 per group) were gavaged with 0.3 mg kg−1 of ezetimibe or corn oil vehicle (0.25 ml) 1 h prior to 1 mg of unlabelled carrier and 1 μCi of either [14C]-cholesterol, [3H]-progesterone, or [3H]-ethinyl estradiol in 0.25 ml of corn oil. All radiolabelled compounds were purchased from NEN/Dupont (Boston, MA, U.S.A.). Four hours later, animals were sacrificed. Four hours was chosen because previous experiments showed that this timeframe was sufficient to allow significant entry of these radioactive compounds into plasma. Blood was collected, plasma was isolated, and aliquots of the plasma were analysed for radioactivity.
Absorption of vitamins A and D
Fasted rats, which had been on normal chow diet, were anaesthetized with Inactin (100 mg kg−1 i.p.). All rats were fitted with tracheal tubes and were intraduodenally cannulated as described before. At t=0 h, 3 ml of an emulsion containing triolein (35.4 mg per dose), L-α-phosphatidylcholine (6.69 mg per dose) in sodium taurocholate buffer (19 mM in DPBS, pH 6.4) without (n=5) or with ezetimibe (n=5; 10 mg kg−1) was delivered intraduodenally. After 1 h, 3 ml of an emulsion containing the same mass triolein and L-α-phosphatidylcholine as described above, and vitamin A (retinol; 1 μCi per dose; 1 mg unlabelled retinol per dose) was placed in the duodenum. At t=2.5 h, rats were sacrificed and 0.5 ml plasma in triplicate was analysed for 3H radioactivity. The timeframe chosen was based on the experimental design routinely used for an intraduodenal cholesterol absorption experiment. Data are expressed as total disintegrations per minute (DPM) in plasma by multiplying 0.04 (the volume of plasma as a percentage of body weight) by body weight. In a separate experiment, the same design as described above with one minor modification was utilized to study 3H-vitamin D absorption: the vitamin D emulsion contained 0.5 μCi per dose.
Absorption of 3H-taurocholic acid
Fasted rats (n=5 per group) which had been on normal chow diet were anaesthetized with Inactin (100 mg kg−1 i.p.). All rats were fitted with tracheal tubes, were intraduodenally cannulated, and were bile duct-cannulated for bile collection. At t=0 hours, either control bile, or control bile containing ezetimibe (2 mg kg−1) was placed into the duodenum via the cannula. At t=1 h, 3 ml of a radiolabelled sodium taurocholate buffer (19 mM in DPBS, pH 6.4; 1 μCi 3H-sodium taurocholate per 3 ml) was delivered to the duodenum. Bile was collected for 4 h to ensure that a substantial proportion of the labelled taurocholic dose had flowed into the bile. The data are expressed as total DPM in bile.
Statistical analyses
Power calculations determined that, with the group sizes used in these experiments, statistical significance could be detected with a power of 0.8. Statistical significance in the dose response was determined by analysis of variance, followed by Student's t-test. Statistical differences between control and ezetimibe-treated groups in all other experiments were tested using Student's t-test.
AAALAC statement
All studies were conducted in an AAALAC accredited facility following protocols approved by the Schering-Plough Research Institute's Animal Care and Use Committee. The procedures were performed in accordance with the principles and guidelines established by the NIH for the care and use of laboratory animals.
Results
Dose response of ezetimibe in hamsters
It has been repeatedly shown that feeding cholesterol-enriched diets to a number of different species leads to an increase in plasma cholesterol and accumulation of cholesterol in the liver. In cholesterol-fed hamsters, ezetimibe dose-dependently decreased both plasma and liver cholesterol with an ED50 of 0.04 mg kg−1 (calculated from liver cholesterol data; Figure 2).
Figure 2.
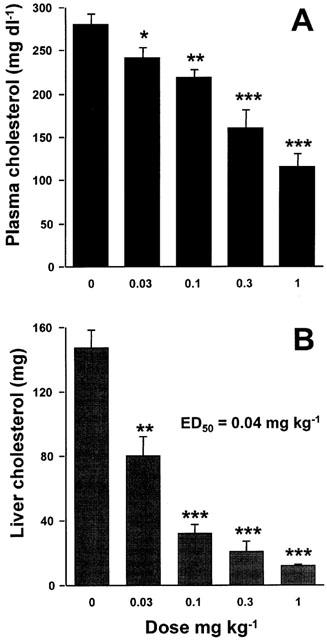
The effect of ezetimibe on plasma and liver cholesterol levels in the cholesterol-fed hamster. Hamsters were fed a diet containing 0.5% cholesterol for 7 days. The indicated doses of ezetimibe were orally gavaged once a day in 0.2 ml corn oil. Plasma cholesterol (A) and total liver cholesterol (B) were determined on day 7. Values are mean±s.e.mean, n=4 – 6 per group. *P<0.05; **P<0.01; ***P<0.001.
Effect of ezetimibe on absorption of 3H-cholesteryl oleate, cholesteryl 14C-oleate and 3H-triolein in hamsters
An initial mechanistic question was whether ezetimibe was inhibiting the hydrolysis of cholesteryl ester in the intestinal lumen, and thereby inhibiting the absorption of cholesterol. Hamsters that had been fed a single cholesterol-containing meal were orally gavaged with cholesteryl oleate labelled either on the cholesterol moiety (3H-cholesteryl oleate) or the fatty acid moiety (cholesteryl-14C-oleate) after being given vehicle or a single dose of ezetimibe (10 mg kg−1). Ezetimibe inhibited the appearance of 3H-cholesterol in plasma by 96%, but did not affect the appearance of 14C-oleate in plasma (Figure 3a, b). These data indicate that the hydrolysis of cholesteryl ester was unaffected by ezetimibe, that the absorption of free cholesterol thus generated was nearly completely inhibited, and that the absorption of fatty acids released by the hydrolysis of cholesteryl ester was also unaffected. It was also shown that this high dose of ezetimibe (10 mg kg−1) did not affect the absorption of radiolabelled triglyceride (Figure 3c).
Figure 3.
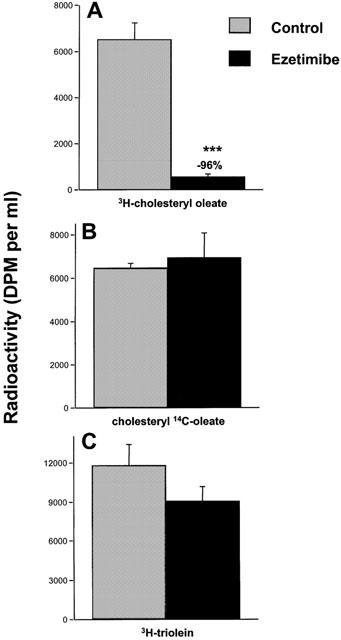
Effect of ezetimibe (10 mg kg−1) on absorption of 3H-cholesteryl oleate, cholesteryl 14C-oleate and 3H-triolein in hamsters. Hamsters were fed a diet containing 0.5% cholesterol overnight. Hamsters were orally gavaged vehicle (grey bars) or ezetimibe (black bars). One hour later hamsters representing (A) and (B) were orally gavaged with of 3H-cholesteryl oleate and cholesteryl 14C-oleate. The hamsters representing (C) were orally gavaged with 3H-triolein. All hamsters were euthanized 2 h later and plasma was analysed for radioactivity. Values are mean±s.e.mean, n=5 per group. ***P<0.001.
Effect of ezetimibe on absorption of 14C-free cholesterol and 3H-cholesteryl oleate in rats
A cholesterol absorption experiment in rats was conducted to determine the distribution of cholesterol in the intestinal lumen, intestinal wall and plasma in the absence or presence of ezetimibe (1 mg kg−1). In this experiment, 14C-free cholesterol and 3H-cholesteryl oleate (both labelled on the cholesterol moiety) were intraduodenally dosed after a single treatment of vehicle or ezetimibe (Figure 4). In the presence of ezetimibe, significantly more radiolabelled cholesterol remained in the intestinal lumen (Figure 4A), and 48% less was associated with the intestinal wall (Figure 4B), regardless of whether the cholesterol had originated in the free or esterified form. Ezetimibe reduced the appearance of radiolabelled cholesterol into the plasma by greater than 90% (Figure 4C). Analysis of free cholesterol versus cholesteryl ester in the intestinal wall indicated that ezetimibe markedly reduced pools of both the free and the esterified form, regardless of whether the cholesterol originated as 14C-free cholesterol or 3H-cholesteryl oleate (Figure 5).
Figure 4.
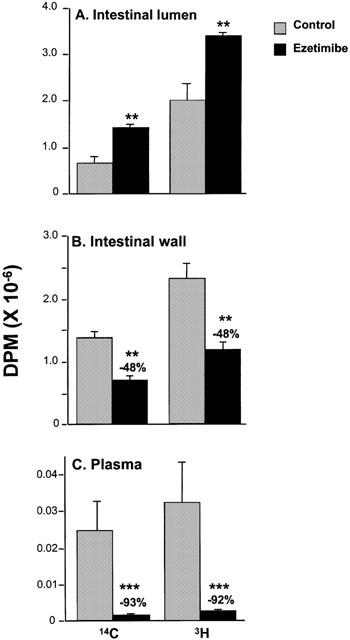
Effect of ezetimibe (1.0 mg kg−1) on appearance of 14C and 3H radioactivity in intestinal luminal contents (A), intestinal wall (B), and plasma (C) after intraduodenal delivery of 14C-free cholesterol and 3H-cholesteryl oleate in rats. 14C was originally delivered as 14C-free cholesterol and 3H as 3H-cholesteryl oleate with both radiolabels on the cholesterol moiety. Values are mean±s.e.mean, n=5 per group. **P<0.01; ***P<0.001.
Figure 5.
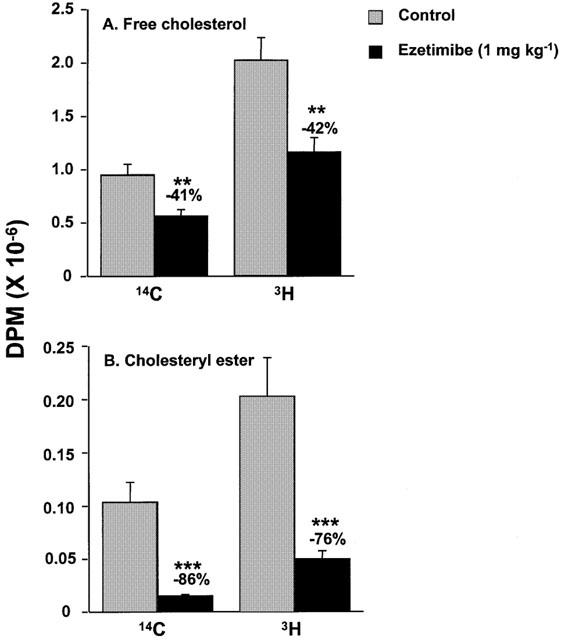
Distribution of 14C-and 3H- free cholesterol and cholesteryl ester in the intestinal wall in rats. These data are from the extracts of the intestines in Figure 4B. 14C was originally delivered as 14C-free cholesterol and 3H as 3H-cholesteryl oleate with both radiolabels on the cholesterol moiety. 14C- and 3H-free cholesterol are shown in (A). 14C- and 3H-cholesteryl ester are shown in (B). Values are mean±s.e.mean, n=5 per group. **P<0.01; ***P<0.001.
Effect of ezetimibe on cholesterol absorption in rats with (sham-operated) and without (biliary anastomosis) exocrine pancreatic function
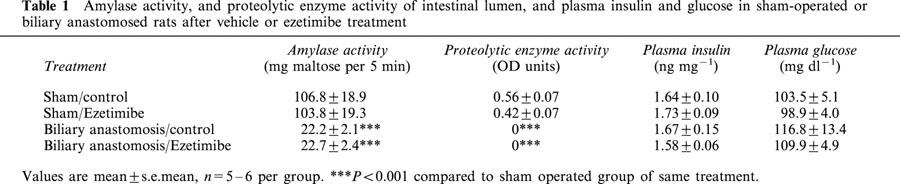
The results described above indicated that ezetimibe did not interfere with the intestinal hydrolysis of cholesteryl ester per se. Since the molecular mechanism of action of ezetimibe remains unknown, a more general question was whether exocrine pancreatic function was necessary for (1) cholesterol absorption; and (2) activity of ezetimibe. A biliary anastomosis model in which exocrine pancreatic function is eliminated, while endocrine pancreatic function and bile flow are maintained, was established in rats. Table 1 shows the amylase and proteolytic enzyme activities of the intestinal luminal contents, as well as the plasma insulin and glucose levels in all groups post mortem. Amylase activity was decreased > 80% in the biliary anastomosed rats and proteolytic activity was not detected in these animals, indicating that exocrine pancreatic secretions into the intestine were nearly absent. Plasma insulin and glucose levels were not different in the biliary anastomosed animals compared to sham-operated animals (Table 1), and were comparable to data for normal rats (data not shown), indicating that insulin secretion and glucose metabolism were normal. During the cholesterol absorption experiment, normal bile flow was observed in all rats.
Table 1.
Amylase activity, and proteolytic enzyme activity of intestinal lumen, and plasma insulin and glucose in sham-operated or biliary anastomosed rats after vehicle or ezetimibe treatment
In sham-operated rats, the appearance of 14C from free cholesterol and 3H from cholesteryl oleate in plasma after intraduodenal dosing of these lipids was decreased 87 and 81% respectively after treatment with ezetimibe (Figure 6). In vehicle-treated, biliary anastomosed rats, appearance of 14C which originated as 14C-free cholesterol was significantly greater than the sham-operated controls, indicating that exocrine pancreatic elimination did not lower the absorption of free cholesterol. The absorption of 14C-free cholesterol was inhibited 94% in anastomosed rats treated with ezetimibe. In vehicle-treated, biliary anastomosed rats, the appearance of 3H which had originally been on the cholesterol moiety of cholesteryl oleate was nearly absent, indicating that the capacity to cleave cholesteryl esters was nearly eliminated in this model. Ezetimibe decreased this remaining small window of cholesterol absorption by 34%, but this was not statistically significant. These data indicate that pancreatic secretions into the duodenum are needed for the hydrolysis and subsequent absorption of cholesteryl esters, but are not necessary for the absorption of free cholesterol per se. Furthermore, the inhibitory activity of ezetimibe is not affected after elimination of exocrine pancreatic activity.
Figure 6.
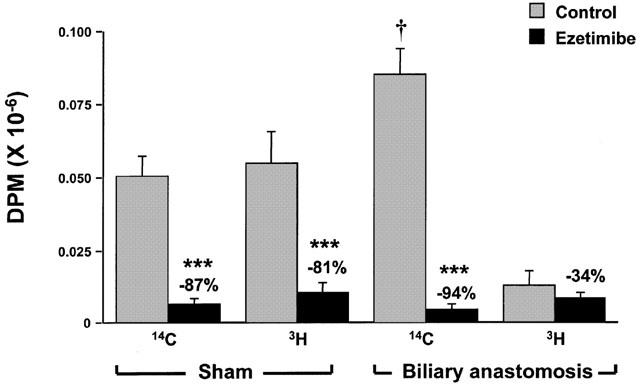
Effect of ezetimibe (3 mg kg−1) on appearance of 14C and 3H-free or esterified cholesterol in rat plasma after intraduodenal 14C- free cholesterol and 3H-cholesteryl oleate in sham-operated or biliary anastomosed rats. Radiolabels are on cholesterol moiety. Values are mean±s.e.mean, n=5 – 6 per group. ***P<0.001 compared to vehicle-treated control, †P<0.05 compared to the sham-operated rats given vehicle.
Effect of ezetimibe on the absorption of radiolabelled progesterone, ethinyl estradiol, vitamins A and D, and taurocholic acid
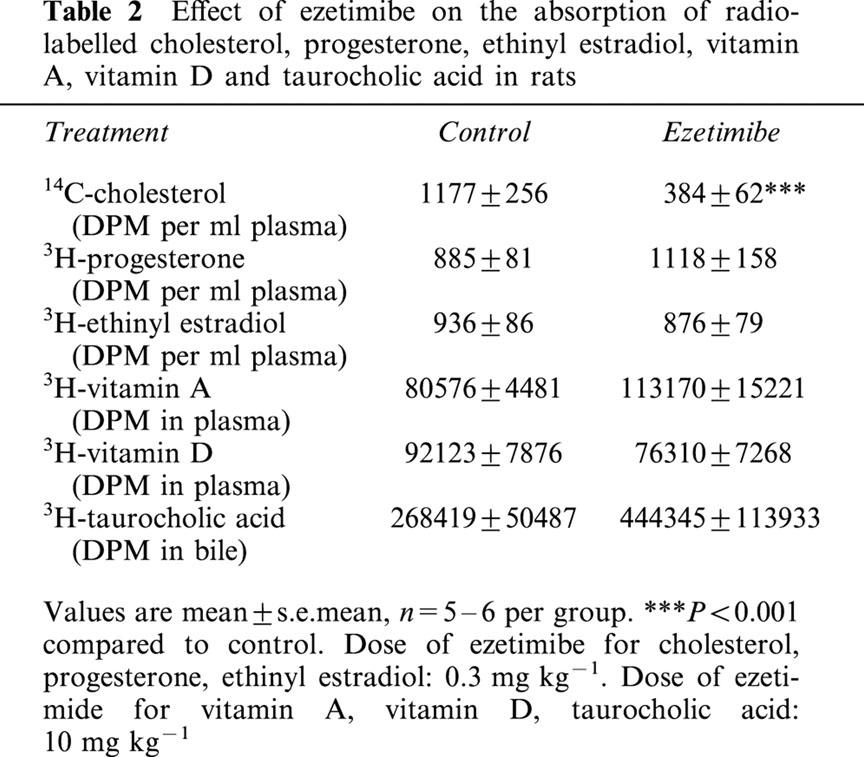
Ezetimibe did not have an effect on the acute absorption of progesterone, ethinyl estradiol, vitamins A and D, or taurocholic acid in rats. In this experiment, ezetimibe significantly decreased the absorption of radiolabelled cholesterol by 67% (Table 2).
Table 2.
Effect of ezetimibe on the absorption of radiolabelled cholesterol, progesterone, ethinyl estradiol, vitamin A, vitamin D and taurocholic acid in rats
Discussion
A number of studies indicate that ezetimibe is a potent and selective pharmacological agent that lowers plasma cholesterol, and thus may likely have an effect on the development of atherosclerosis. Ezetimibe is a cholesterol absorption inhibitor that lowers plasma cholesterol in a number of preclinical models of hypercholesterolemia (Davis et al., 1995; van Heek et al., 1997; 2000). Ezetimibe ablated combined hyperlipidemia in obese, hyperinsulinemic hamsters (van Heek et al., 2001a). A single dose of an ezetimibe analogue in cynomolgus monkeys reduced the cholesterol content of postprandial chylomicrons and chylomicron remnants, without affecting chylomicron triglyceride content (van Heek et al., 2001b). It has widely been suggested that a decrease in the cholesterol content of postprandial chylomicrons and their remnants may impact the potential atherogenicity of these particles (for reviews see Mamo, 1995; Zilversmit, 1995; Karpe, 1999; Ros, 2000). Chronic administration of ezetimibe to apo E knock-out mice inhibited the development of atherosclerosis (−97%) normally observed in these animals (Davis et al., 2000). Finally, ezetimibe significantly lowered LDL cholesterol and raised HDL cholesterol in hypercholesterolemic humans (Lipka et al., 2000), and is now in Phase III clinical trials.
Preclinical studies have demonstrated that ezetimibe inhibits the transport of radiolabelled cholesterol through the intestinal wall and ultimately into the plasma (van Heek et al., 1997; 2000). The precise molecular mechanism by which cholesterol is absorbed into and through the intestinal wall is not well understood (Homan & Krause, 1997; Dawson & Rudel, 1999; Turley, 1999). The studies presented here were conducted to further elucidate the effect that ezetimibe has on known intestinal absorptive processes, and to determine the selectivity of ezetimibe for cholesterol absorption. The data indicate that, in intact animals, the absorption of free cholesterol was inhibited by ezetimibe, even when it originated as cholesteryl ester. However, the absorption of the fatty acid generated from the hydrolysis of cholesteryl ester was unaffected by ezetimibe. This indicates that ezetimibe does not inhibit cholesterol ester hydrolase. In the absence of exocrine pancreatic function, cholesteryl ester could not be hydrolysed, and thus was not absorbed. The absorption of free cholesterol proceeded in the absence of exocrine pancreatic function, and ezetimibe inhibited its absorption to the same degree as animals that have intact exocrine pancreatic function (approximately 90% inhibition). These data indicate that a functional exocrine pancreas is not necessary for the absorption of free cholesterol or the activity of ezetimibe.
Ezetimibe inhibits the appearance of both free cholesterol and cholesteryl ester in the intestinal wall. The inhibition of free cholesterol in the intestinal wall differentiates ezetimibe from ACAT inhibitors, which prevent the formation of cholesteryl ester from free cholesterol, and therefore do not lower free cholesterol in the intestine (Burrier et al., 1995).
Ezetimibe does not affect the acute absorption of triglyceride, progesterone, ethinyl estradiol, vitamin A, vitamin D, or taurocholic acid. The lack of effect on the absorption of molecules other than free cholesterol may distinguish ezetimibe from other drugs that are active in the intestine, and that also lower plasma cholesterol. In addition, the lack of effect on triglyceride, vitamin A and vitamin D absorption may indicate that ezetimibe will have less side effect potential than other intestinally active drugs. Cholesteryramine sequesters bile acids in the intestine (Physician's Desk Reference, 2000a). This ultimately leads to a decrease in plasma cholesterol by upregulating the synthesis of bile acids from cholesterol in the liver. Cholestyramine must be taken in gram quantities daily for efficacy. Two potential side effects to the large quantities needed, as well as the mechanism by which cholestyramine acts, are gastrointestinal discomfort and the sequestration of fat-soluble vitamins. Ezetimibe is active in humans at 0.25 – 10 mg per day (Lipka et al., 2000), and does not appear to affect fat-soluble vitamin absorption in humans (Knopp et al., 2001), so the side effects observed with cholestyramine are not expected. In addition, ezetimibe does not inhibit the absorption of taurocholic acid, suggesting that the mechanism of action of ezetimibe is distinct from that of cholestyramine, or an inhibitor of the ileal Na+/bile acid cotransporter (Higaki et al., 1998). Orlistat is a pancreatic lipase inhibitor that is used as an anti-obesity agent that prevents the absorption of triglycerides (Physician's Desk Reference, 2000b). Due to its mechanism, orlistat may have an effect on cholesterol absorption. Orlistat prevents the hydrolysis of triglyceride in the intestinal lumen, which likely disrupts the solubilization of micelles. Micellar solubilization is critical for the absorption of cholesterol and fat-soluble vitamins, which may explain the cholesterol-lowering effect, as well as the side effect of decreased fat-soluble vitamin absorption, with orlistat treatment. Some patients treated with orlistat experience unpleasant gastrointestinal side effects, which is likely due to the unhydrolyzed triglyceride moving through the intestine. Since ezetimibe does not affect triglyceride absorption, the side effects observed with orlistat are not likely to occur. The lack of effect on triglyceride absorption also differentiates ezetimibe from the microsomal triglyceride transfer protein inhibitors which block both cholesterol and triglyceride absorption (Stein et al., 2000), and are associated with gastrointestinal side effects (Stein et al., 1999).
Ezetimibe is a very selective cholesterol absorption inhibitor. The results of the present experiments demonstrate that ezetimibe can cause a nearly complete inhibition of cholesterol absorption and block diet-induced hypercholesterolemia in rats and hamsters. These studies monitored the intestinal absorption of radiolabelled cholesterol and other molecules in animals, which has not been studied clinically yet in humans treated with ezetimibe. Ezetimibe is progressing through Phase III clinical trials, both as monotherapy and in combination with the statins, which inhibit cholesterol synthesis. In hypercholesterolemic humans, ezetimibe as monotherapy significantly decreased LDL cholesterol (−18.5%) and increased HDL cholesterol (+3.5%) with no notable side effects (Lipka et al., 2000). The combination of ezetimibe and the statins has shown significant synergy in lowering cholesterol in preclinical models (Davis et al., 1995; 2000). Studies in hypercholesterolemic humans demonstrated that combining simvastatin (10 – 20 mg day−1) and ezetimibe (10 mg day−1) led to a 50 – 60% decrease in LDL cholesterol in 2 weeks (Kosoglou et al., 2000a, 2000b). In conclusion, this novel class of cholesterol absorption inhibitors may provide a new approach for the treatment of hypercholesterolemia by selectively inhibiting the absorption of both dietary and biliary cholesterol in the intestine.
Abbreviations
- DPBS
Dulbecco's phosphate buffered saline
- DPM
disintegrations per minute
- ED50
effective dose at which 50% inhibition occurs
References
- BURRIER R.E., SMITH A.A., MCGREGOR D.G., HOOS L.M., ZILLI D.L., DAVIS H.R. The effect of acyl CoA: cholesterol acyltransferase inhibition on the uptake, esterification and secretion of cholesterol by the hamster small intestine. J. Pharm. Exp. Ther. 1995;272:156–163. [PubMed] [Google Scholar]
- DAVIS H.R., VAN HEEK M., WATKINS R.W., ROSENBLUM S.B., COMPTON D.S., HOOS L., MCGREGOR D.G., PULA K., SYBERTZ E.J. The hypocholesterolemic activity of the potent cholesterol absorption inhibitor SCH58235 alone and in combination with HMG CoA reductase inhibitors. (abstract) XII International Symposium on Drugs Affecting Lipid Metabolism (DALM) 1995.
- DAVIS H.R., WATKINS R.W., COMPTON D.S., COOK J.A., HOOS L., PULA K., VAN HEEK M. The cholesterol absorption inhibitor ezetimibe ( SCH58235) and lovastatin synergistically lower plasma cholesterol and inhibit the development of atherosclerosis (abstract) JACC. 2000;35:255A. [Google Scholar]
- DAWSON P.A., RUDEL L.L. Intestinal cholesterol absorption. Curr. Opin. Lipidol. 1999;10:315–320. doi: 10.1097/00041433-199908000-00005. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOAN-STANLEY G.H. A simple method for isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- HIGAKI J., HARA S., TAKASU N., TONDA K., SHIKE T., NAGATA K., MIZUI T. Inhibition of ileal Na+/bile acid cotransporter by S-8921 reduces serum cholesterol and prevents atherosclerosis in rabbits. Arterioscler. Thromb. Vasc. Biol. 1998;18:1304–1311. doi: 10.1161/01.atv.18.8.1304. [DOI] [PubMed] [Google Scholar]
- HOMAN R., KRAUSE B.R. Established and emerging strategies for inhibition of cholesterol absorption. Curr. Pharm. Des. 1997;3:29–44. [Google Scholar]
- KARPE F. Postprandial lipoprotein metabolism and atherosclerosis. J. Intern. Med. 1999;246:341–355. doi: 10.1046/j.1365-2796.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- KNOPP R.H., BAYS H., MANION C.V., LIPKA L.J., MELANI L., LE BEAUT A.P., SURESH R., VELTRI E.P. Effect of ezetimibe on serum concentrations of lipid-soluble vitamins. Atherosclerosis Suppl. 2001;2:90. [Google Scholar]
- KOSOGLOU T., MEYER I., MUSIOL B., CUTLER D.L., YANG B., VELTRI E.P., AFFRIME M.B. Coadministration of simvastatin and ezetimibe leads to significant reduction in LDL-cholesterol. (abstract) Third International Conference on Coronary Artery Disease–from Prevention to Intervention 2000aLyon, France; 71 [Google Scholar]
- KOSOGLOU T., MEYER I., MUSIOL B., MELLARS L., STATKEVICH P., MILLER M.F., SONI P.P., AFFRIME M.B. Pharmacodynamic interaction between the new selective cholesterol absorption inhibitor SCH 58235 and simvastatin (abstract) Atherosclerosis. 2000b;151:135. [Google Scholar]
- LIN T.M., KARVINEN E., IVY A.C. Role of pancreatic digestion in cholesterol absorption. Am. J. Physiol. 1957;190:214–220. doi: 10.1152/ajplegacy.1957.190.2.214. [DOI] [PubMed] [Google Scholar]
- LIPID: The Long-Term Intervention with Pravastatin in Ischaemic Disease Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Eng. J. Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- LIPKA L.J., LEBEAUT A.P., VELTRI E.P., MELLARS L.E., BAYS H.E., MOORE P.B. , and the EZETIMIBE (SCH58235) STUDY GROUP Reduction of LDL-cholesterol and elevation of HDL-cholesterol in subjects with primary hypercholesterolemia by ezetimibe ( SCH58235): pooled analysis of two Phase II studies (abstract) JACC. 2000;35:257A. [Google Scholar]
- MAMO J.C. Atherosclerosis as a post-prandial disease. Endocrin. and Metab. 1995;2:229–244. [Google Scholar]
- PHYSICIANS' DESK REFERENCE Colestid. 2000a. pp. 2427–2430.
- PHYSICIANS' DESK REFERENCE Xenical. 2000b. pp. 2693–2696.
- ROS E. Intestinal absorption of triglyceride and cholesterol. Dietary and pharmacological inhibition to reduce cardiovascular risk. Atherosclerosis. 2000;151:357–379. doi: 10.1016/s0021-9150(00)00456-1. [DOI] [PubMed] [Google Scholar]
- ROSENBLUM S.B., HUYNH T., AFONSO A., DAVIS H.R., YUMIBE N., CLADER J.W., BURNETT D.A. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4-hydroxyphenyl)-2-azetidinone ( SCH58235): a designed, potent, orally active inhibitor of cholesterol absorption. J. Med. Chem. 1998;41:973–980. doi: 10.1021/jm970701f. [DOI] [PubMed] [Google Scholar]
- SACKS F., PFEFFER M.A., MOYE L.A., ROULEAU J.L., RUTHERFORD J.D., COLE T.G., BROWN L., WARNICA J.W., ARNOLD J.M., WUN C.C., DAVIS B.R., BRAUNWALD E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- SCANDINAVIAN SIMVASTATIN SURVIVAL STUDY GROUP(4S) Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- SHEPHERD J., COBBE S.M., FORD I., ISLES C.G., LORIMER A.R., MACFARLANE P.W., MCKILLOP J.H., PACKARD C.J. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- STEIN E.A., AMES S.A., MOORE L.J., ISAACSOHN J.L., LASKERZEWSKI P.M. Inhibition of post prandial fat absorption with the MTP inhibitor Bay 13-9952 (abstract) Circulation. 2000;102 II:601. [Google Scholar]
- STEIN E.A., ISAACSOHN J.L., MAZZU A., ZEIGLER R. Effect of Bay 13-9952, a microsomal triglyceride transfer protein inhibitor on lipids and lipoproteins in dyslipoproteinemic patients (abstract) Circulation. 1999;100 I:258. [Google Scholar]
- TURLEY S.D. Dietary cholesterol and the mechanisms of cholesterol absorption. Eur. Heart J. Supplement. 1999. pp. S29–S35.
- VAN HEEK M., AUSTIN T.M., FARLEY C., COOK J.A., TETZLOFF G.G., DAVIS H.R. Ezetimibe, a potent cholesterol absorption inhibitor, normalizes combined dyslipidemia in obese, hyperinsulinemic hamsters. Diabetes. 2001a;50:1330–1335. doi: 10.2337/diabetes.50.6.1330. [DOI] [PubMed] [Google Scholar]
- VAN HEEK M., COMPTON D.S., DAVIS H.R. The cholesterol absorption inhibitor, ezetimibe, decreases diet-induced hypercholesterolemia in monkeys. Eur. J. Pharm. 2001b;415:79–84. doi: 10.1016/s0014-2999(01)00825-1. [DOI] [PubMed] [Google Scholar]
- VAN HEEK M., FARLEY C., COMPTON D.S., HOOS L., ALTON K.B., SYBERTZ E.J., DAVIS H.R. Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. Brit. J. Pharm. 2000;129:1748–1754. doi: 10.1038/sj.bjp.0703235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN HEEK M., FRANCE C.F., COMPTON D.S., MCLEOD R.L., YUMIBE N.P., ALTON K.B., SYBERTZ E.J., DAVIS H.R. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J. Pharm. Exp. Ther. 1997;283:157–163. [PubMed] [Google Scholar]
- WATT S.M., SIMMONDS W.J. The effect of pancreatic diversion on lymphatic absorption and esterification of cholesterol in the rat. J. of Lipid Res. 1981;22:157–165. [PubMed] [Google Scholar]
- ZILVERSMIT D.B. Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich remnant lipoproteins. Clin. Chem. 1995;41:153–158. [PubMed] [Google Scholar]


