Abstract
Despite the potential importance of the mouse in studying the pharmacology of aqueous dynamics, measurement of intraocular pressure (IOP) in its very small eye has been problematic. Utilizing a novel servo-null electrophysiologic approach recently applied to the mouse, we have identified a diversity of adenosine-receptor mechanisms in modulating IOP in this species. We report the first evidence that A3 receptors increase IOP in any species, and verify in the mouse reports with larger mammals that A1 receptors lower and A2A receptors increase IOP.
Keywords: Aqueous humour, glaucoma, MRS 1191, MRS 1097, MRS1523, IB-MECA, Cl-IB-MECA, CPA, DCPCX, ZM241385
Introduction
The mouse provides a potentially powerful vehicle for studying the pharmacology of aqueous humour dynamics, particularly in view of the increasing availability of knockout animals. Further clarification of this pharmacologic area would be helpful in generating novel drugs in treating glaucoma, a major world-wide cause of blindness usually associated with elevated intraocular pressure (IOP). The mouse is especially suitable for this initiative since it and the human display similar functional responses to drugs reducing inflow and enhancing outflow of aqueous humour (Avila et al., 2001). However, progress has been limited by the technical challenge of measuring IOP in the small eye of the mouse, whose anterior chamber contains only 2 – 4 μl of aqueous humour (Lea et al., 2000; Avila et al., 2001). The novel application of an electrophysiologic, non-manometric approach has addressed this challenge, permitting reliable monitoring of mouse IOP (Avila et al., 2001).
As the initial extension of this technique to ocular pharmacology in the mouse, we sought a potential role for adenosine receptors in modulating IOP. A1 and A2 receptors have been associated with reduction and increase of IOP, respectively, in rabbits (Crosson, 1995; Crosson & Gray, 1996) and monkeys (Tian et al., 1997), an effect ascribed in monkeys entirely to actions on aqueous humour outflow (Tian et al., 1997). In contrast, A3 agonists activate Cl− channels of the nonpigmented ciliary epithelial cells which face the eye's aqueous humour in vivo (Carré et al., 1997; 2000; Mitchell et al., 1999). This effect is expected to increase inflow and consequently IOP, but until now A3 receptor mechanisms potentially related to IOP control have been studied only in vitro, not in living mammals.
Methods
Animals and anaesthesia
Black Swiss outbred mice of mixed sex, 7 – 9 weeks old and ∼30 gm in weight, were obtained from Taconic, Inc. (Germantown, NY, U.S.A.), maintained under 12-h light – dark illumination cycle and allowed unrestricted access to food and water. All procedures conformed to the ARVO Statement for Use of Animals in Ophthalmic and Vision Research. Mice were anaesthetized with intraperitoneal ketamine (250 mg kg−1) supplemented by topical proparacaine HCl 0.5% (Allergan, Hormigueros, Puerto Rico) for the IOP measurements, which were conducted at 6 – 11 h into the light phase.
Measurement of IOP
IOP was measured with the Servo-Null Micropipette System (SNMS) previously detailed in detail (Avila et al., 2001). The SNMS has been validated by comparing imposed and measured pressures in the mouse eye, by comparing repeated measurements in the same eye and between right and left eyes, and by monitoring the responses to anisosmotic solutions and drugs known to modulate inflow and outflow of aqueous humour in other species.
Drugs
Drugs were applied topically in 10 μl droplets with an Eppendorf pipette at the stated concentrations. Agents were initially dissolved in DMSO. The final droplet solution was isosmotic saline solution (310 mOsm) containing 1 – 2% DMSO and 0.003% benzalkonium chloride (Sigma, commonly used to enhance ocular penetration of topical drugs). We have previously found that the DMSO-benzalkonium solution itself has no effect on mouse IOP (Avila et al., 2001). We have also verified in three sets of experiments that topical administration alters IOP through local ocular, and not systemic, actions: (1) In our initial study, 1% pilocarpine HCl (Alcon, Fort Worth, TX, U.S.A.) triggered oculotensive responses (Avila et al., 2001) and miosis in the treated eye but no miosis contralaterally. (2) We now report that 10 μl droplets of 1% mydriacyl (Alcon Humacao, Puerto Rico) produced mydriasis 45 min later in the treated (Δ=+1.18±0.07 mm, n=8, P<0.001), but not in the contralateral, eye (Δ=−0.12±0.05 mm). (3) Topical application of 100 μM adenosine to one eye also had no effect on IOP in the contralateral eye (n=3), but topical adenosine application to the monitored eye produced the largest IOP change of all agents tested (Results, Table 1, Figures 1 and 2).
Table 1.
IOP effects of adenosine agonists and antagonists
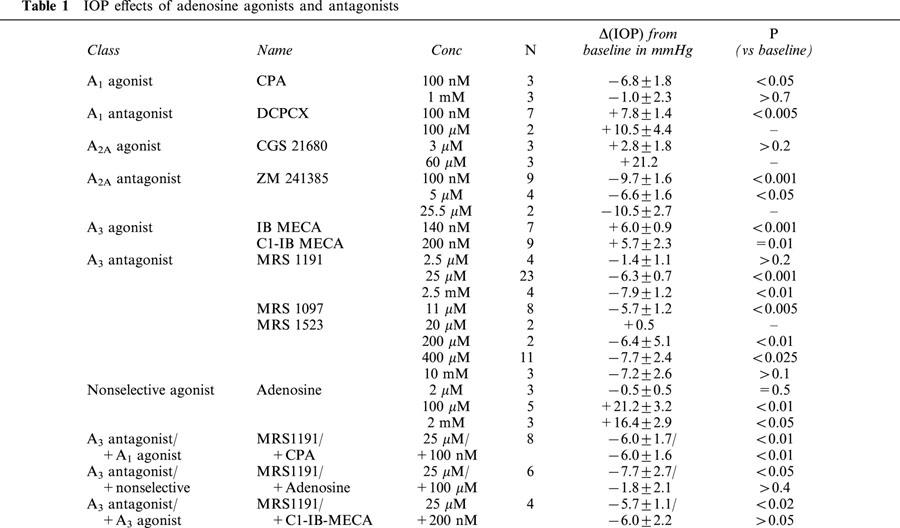
Figure 1.
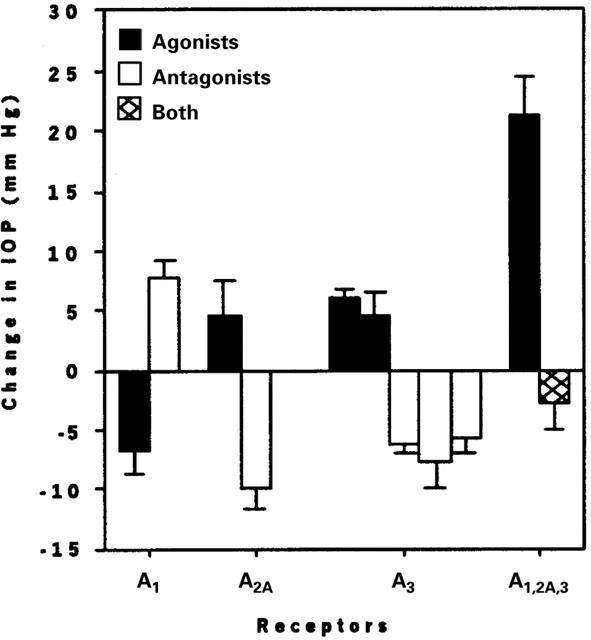
Means±s.e.mean of the responses to minimally effective concentrations of AR agonists and antagonists: 100 nM CPA, 100 nM DCPCX, 60 μM CGS 21680, 100 nM ZM241385, 200 nM Cl-IB-MECA, 140 nM IB-MECA, 25 μM MRS 1191, 11 μM MRS 1097, and 400 μM MRS 1523. At the extreme right of the bar graph, data are presented for 100 μM adenosine with and without pretreatment with the A3 AR antagonist MRS 1191.
Figure 2.
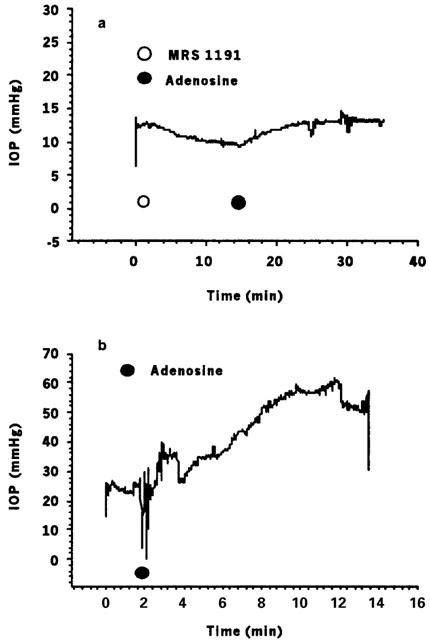
Representative IOP tracings assessing effects of MRS 1191 and adenosine on mouse IOP. (a) MRS 1191 (25 μM) lowered baseline IOP by 2.62±0.04 mmHg, following which the response to 100 μM adenosine (+2.52±0.03 mmHg) was substantially attenuated; see, e.g., Figure 2b). (b) In the absence of the A3 antagonist, 100 μM adenosine consistently produced several-fold larger increases in IOP, in this eye raising IOP by 31.9±0.1 mmHg.
9-chloro-2-(2-furyl)-5-([phenylacetyl]amino)[1,2,4]-triazolo-[1,5-c]quinazo-line (ZM241385) was purchased from Tocris (Ballwin, MO, U.S.A.) and 3,5-diethyl 2-methyl-6-phenyl-4-(trans-2-phenylvinyl)-1,4(R,S)-dihydro-pyridine-3,5-dicarboxylate (MRS 1097) was a gift from Drs Kenneth A. Jacobson and Bruce L Liang. The following agents were obtained from RBI Sigma (St. Louis, MO, U.S.A.): adenosine, N6-cyclopentyladenosine (CPA), 8-cyclopentyl-1,3-dipropyl-xanthine (DCPCX), 2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamido-adenosine (CGS-21680), N6-(3-iodo-benzyl)-adenosine-5′-N-methyl-uronamide (IB-MECA), 2-chloro-IB-MECA (Cl-IB-MECA), 3-ethyl 5-benzyl 2-methyl-6-phenyl-4-phenyl-ethynyl-1, 4-(±)-dihydropyridine-3,5-dicarboxylate (MRS 1191), and 2,3-diethyl-4,5-dipropyl-6-phenylpyridine-3-thiocarboxylate-5-carboxylate (MRS 1523).
Data analysis
Means±s.e.mean were calculated from recording periods of usually 3 – 5 min. Numbers of experiments or of eyes are indicated by ‘N'. For those few drug concentrations tested in duplicate, the uncertainty was estimated as half the difference between the two values. The probability P of the null hypothesis was estimated with the paired, 2-tailed Student's t-test.
Results
A3 receptors
As indicated in the Introduction, the primary aim was to test whether AR agonists and antagonists modulate IOP in the mouse and to focus on the putative role of A3 ARs. For the latter purpose, MRS 1191 is particularly favourable as a highly selective A3 antagonist of both human and murine ARs (Jacobson et al., 1997).
Consistent with our earlier data (Avila et al., 2001), baseline IOP was 17.2±0.8 mmHg (n=134). MRS 1191 (25 μM in the topical droplet) lowered IOP by 6.3±0.7 mmHg (n=23, P<0.001, Figure 1). A representative trace appears in Figure 2a. From measurements (Table 1) obtained at 2.5 μM, 25 μM and 2.5 mM, we calculated a half-maximal inhibitory concentration (ID50) for MRS 1191 in the droplet of 16±4 μM. Another dihydropyridine A3 antagonist MRS 1097 (Jacobson et al., 1997) similarly lowered IOP by 5.7±1.2 mmHg at 11 μM (n=8, P<0.005, Figure 1, Table 1). A structurally dissimilar third A3 AR antagonist MRS 1523 (Li et al., 1999) also reduced baseline IOP at higher concentrations (Figure 1, Table 1). These data indicated that antagonism of A3 ARs lowers baseline mouse IOP.
We tested whether the preferred murine A3 antagonist MRS 1191 also attenuated the responses to non-selective and A3-selective AR antagonists. Adenosine had no effect at 2 μM concentration, but increased IOP over baseline by 21.2±3.2 mmHg (n=5, P<0.01) at 100 μM (Figures 1 and 2b, Table 1). Increasing the adenosine concentration 20 fold to 2 mM had no further effect (Table 1). The concentration in the aqueous humour following topical application of 100 μM adenosine was likely high enough (Discussion) to stimulate A1, A2A and A3, but not A2B receptors (Fredholm et al., 1994).
Following pretreatment with 25 μM MRS 1191, 100 μM adenosine increased IOP by only 5.9±2.2 mmHg (n=6, P<0.05, Figures 1 and 2a, Table 1); thus, the adenosine-triggered elevation in IOP was ∼70%-attenuated by pretreatment with the A3 AR antagonist (compare Figures 2a and 2b). The combined effect of MRS 1191 and adenosine was to lower IOP slightly (with respect to the initial baseline level) by 1.8±2.1 mmHg (Figures 1 and 2a, Table 1).
Opposite to the reduction in IOP produced by A3 AR antagonists, A3 agonists increased pressure. IB-MECA (Jacobson et al., 1993) increased IOP by 6.0±0.9 mmHg (n=7, P<0.001, Figure 1, Table 1) at 140 nM. The more polar A3 AR agonist Cl-IB-MECA (Jacobson et al., 1995) raised IOP above baseline by 5.7±2.3 mmHg at 200 nM (n=9, Figure 1, P=0.01) and by 4.6±1.8 mmHg at 2 mM (n=3), and this response was abolished by pretreatment with the A3 AR antagonist MRS 1191 (25 μM, n=5, Table 1).
The foregoing results obtained with selective A3 antagonists and agonists and with adenosine constitute the first evidence that A3 ARs modulate mammalian OP in vivo. We also tested whether mouse IOP is modulated by A1 and A2 ARs, as reported for rabbit (Crosson, 1995; Crosson & Gray, 1996) and monkey (Tian et al., 1997).
A1 and A2 receptors
The A1-agonist CPA (Fredholm et al., 1994; Klotz et al., 1998) reduced IOP by 6.8±1.8 mmHg (100 nM, n=4, P<0.05, Figure 1, Table 1) and the A1-antagonist DCPCX (Haleen et al., 1987) increased IOP by 7.8±1.4 mmHg (100 nM, n=6, P<0.005, Figure 1, Table 1). At a substantially higher concentration (1 mM), CPA exerted no significant effect (Table 1). The A2A-agonist CGS-21680 (Jacobson et al., 1995) increased IOP at 60 μM (n=3), Table 1) but also produced corneal oedema and was associated with unstable records, complicating analysis of the IOP measurements. However, a role of A2A ARs was clearly detected by applying the A2A-antagonist ZM 241385 (Ongini et al., 1999) (100 nM, n=11), which triggered a transient increase in IOP (8.4±2.5 mmHg), followed by a sustained reduction of 9.7±1.6 mmHg (P<0.001, Figure 1, Table 1). Similar sustained reductions were noted at higher concentrations of this drug (Table 1).
Discussion
This manuscript provides the first information concerning modulation of mouse IOP by the three adenosine receptors (A1, A2A and A3) postulated to regulate human IOP. In principle, caution is necessary in analyzing the data in the mouse eye since we do not know the concentrations of agonists and antagonists in the small volume of aqueous humour. MRS 1191 is known to be a highly selective A3 antagonist for human and rat ARs (Jacobson et al., 1997). We can calculate an absolute, unrealistic upper bound to the true aqueous humour concentration of MRS 1191 resulting from topical administration by assuming that the cornea presents no permeability barrier whatsoever. The topically applied drug would have been thereby distributed uniformly and thus diluted throughout the applied droplet, tear and anterior chamber. Based on this assumption, the concentration in the aqueous humour would not have exceeded 40% of that in the applied droplet. Thus, topical application of 25 μM MRS 1191 could not have led to concentrations higher than 10 μM in the aqueous humour. Even at this unrealistically high level, only 25% of murine A1 and <10% of murine A2A receptors would have been occupied (Jacobson et al., 1997). We conclude that the direct effect of MRS 1191 to lower baseline IOP and its marked action to attenuate the subsequent response to adenosine are mediated by A3 receptors, consistent with results of in vitro studies (Carré et al., 1997; 2000; Mitchell et al., 1999).
In extending analysis of the data presented in Table 1, we have compared the minimally effective concentrations in the topical droplets with published functional estimates of binding, largely to human receptors. From this, drug penetration ratios (PR) of aqueous-to-drop concentration can be estimated. For example, the A3-selective agonist IB-MECA significantly raised IOP at a drop concentration of 140 nM (Figure 1, Table 1) and the Kd in mouse cerebellar membranes is 1.39±0.04 nM (Jacobson et al., 1993), so that the penetration ratio was ∼1 : 100. By this approximate measure, almost all adenosine agonists and antagonists displayed penetration ratios of 1 : 200 – 250. The exceptions were Cl-IB-MECA (PR ∼600) and MRS 1523 (PR ∼770), presumably reflecting slower penetrance of these agents. Thus, a conservatively high estimate of the drug concentration in the aqueous humour can be calculated as ∼1% of the applied droplet concentration. Interestingly, this observation conforms to the commonly-applied assumptions of ∼1% penetration of topically applied drug to rabbits (Honjo et al., 2001) and primates (Asseff et al., 1973). This rough rule of thumb apparently holds for the mouse eye, as well as for eyes with thicker corneas from other species.
Taking the likely aqueous humour concentrations to be no higher than 1% the droplet concentrations, the antagonists MRS 1097 and 1191, and the agonists IB-MECA and Cl-IB-MECA must have exerted their IOP effects by binding specifically to A3 ARs, whereas MRS 1523 (Li et al., 1999) may possibly have antagonized A2A as well as A3 receptors. Correspondingly, the A1 agonist CPA lowered IOP at a calculated equivalent aqueous concentration of ∼1 nM, at which <1% of the A2A and A3 receptors were likely occupied (Fredholm et al., 1994; Klotz et al., 1998). Conversely, the A1 antagonist DCPCX likely raised IOP by selective action at A1 ARs. When the droplet concentration was markedly raised to 1 mM, ensuring occupancy of A1, A2A and A2B adenosine receptors, CPA had no significant effect on IOP, suggesting that the negative oculotensive effect of the A1 receptors was offset by the opposing effects of the A3 and possibly A2A receptors. The role of A2A receptors in elevating mouse IOP was separately substantiated by the observation that the selective A2A antagonist ZM 241385 triggered a sustained reduction in IOP at 100 nM droplet concentration. At an equivalent aqueous concentration of 1 nM, ZM 241385 was occupying 2% of the A2B and <1% of the other receptors.
It is intriguing that A1 and A3 ARs exert opposing effects on IOP although both receptors trigger reductions in intracellular cyclic-AMP (Fredholm et al., 1994). This suggests that A1 and A3 agonists reduce IOP through other signalling pathways and/or that they act on different cells. Indeed, the major oculotensive effects of A1 and A3 agonists may be at the trabecular meshwork and/or inner wall of Schlemm's canal (Tian et al., 1997) and nonpigmented ciliary epithelial cells (Carré et al., 1997; 2000; Mitchell et al., 1999), respectively.
In summary, the current data provide the first evidence that A3-selective adenosine agents and antagonists modulate IOP in the mammalian eye, extending in vitro observations implicating A3 receptors in tissues controlling aqueous humour physiology. The pharmacologic responses in vivo conform to expectations based on A3 AR responses by ciliary epithelial cells in vitro suggesting that A3 antagonists would lower IOP. The results also agree with findings in other mammalian eyes implicating A1 and A2A receptors in control of IOP. The work further supports the potential utility of the mouse in studying the physiology and pharmacology of aqueous dynamics.
Acknowledgments
Supported in part by grants from The Research Foundation of the University of Pennsylvania (MMC), and EY05454 (RAS), EY08343 (MMC), EY12213 (MMC), EY13624 (MMC) and core grant EY01583 from the National Institutes of Health, Research to Prevent Blindness Physician-Scientist Merit Award (RAS) and The Paul and Evanina Mackall Foundation Trust (RAS). The authors thank Dr Evan Dreyer for Dr Avila's partial support salary. We are grateful to Drs Kenneth A. Jacobson and Bruce L. Liang for very helpful discussions and for gracious gifts of MRS 1097, and to Dr Claire H. Mitchell for critical reading of the manuscript.
References
- ASSEFF C.F., WEISMAN R.L., PODOS S.M., BECKER B. Ocular penetration of pilocarpine in primates. Am. J. Ophthalmol. 1973;75:212–215. doi: 10.1016/0002-9394(73)91015-5. [DOI] [PubMed] [Google Scholar]
- AVILA M.Y., CARRÉ D.A., STONE R.A., CIVAN M.M. Reliable measurement of mouse intraocular pressure by a servo-null micropipette system. Invest. Ophthalmol. Vis. Sci. 2001;42:1841–1846. [PubMed] [Google Scholar]
- CARRÉ D.A., MITCHELL C.H., PETERSON-YANTORNO K., COCA-PRADOS M., CIVAN M.M. Adenosine activates Cl− channels of nonpigmented ciliary epithelial cells. Am. J. Physiol. 1997;273:C1354–1361. doi: 10.1152/ajpcell.1997.273.4.C1354. [DOI] [PubMed] [Google Scholar]
- CARRÉ D.A., MITCHELL C.H., PETERSON-YANTORNO K., COCA-PRADOS M., CIVAN M.M. Functional similarity of A3-adenosine and swelling-activated Cl− channels in nonpigmented ciliary epithelial cells. Am. J. Physiol. 2000;279:C440–C451. doi: 10.1152/ajpcell.2000.279.2.C440. [DOI] [PubMed] [Google Scholar]
- CROSSON C.E. Adenosine receptor activation modulates introcular pressure in rabbits. J. Pharmacol. Exp. Ther. 1995;273:320–326. [PubMed] [Google Scholar]
- CROSSON C.E., GRAY T. Characterization of ocular hypertension induced by adenosine agonists. Invest. Ophthalmol. Vis. Sci. 1996;37:1833–1839. [PubMed] [Google Scholar]
- ONGINI E., DIONISOTTI S., GESSI S., IRENIUS E., FREDHOLM B.B. Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonist at human adenosine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1999;359:7–10. doi: 10.1007/pl00005326. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DALY J.W., HARDEN T.K., JACOBSON K.A., LEFF P., WILLIAMS M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- HALEEN S.J., STEFFEN R.P., HAMILTON H.W. PD 116,948, a highly selective A1 adenosine receptor antagonist. Life Sci. 1987;40:555–561. doi: 10.1016/0024-3205(87)90369-9. [DOI] [PubMed] [Google Scholar]
- HONJO M., TANIHARA H., INATANI M., KIDO N., SAWAMURA T., YUE B.Y.J.T., NARUMIYA S., HONDA Y. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest. Ophthalmol. Vis. Sci. 2001;42:137–144. [PubMed] [Google Scholar]
- JACOBSON K.A., KIM H.O., SIDDIQUI S.M., OLAH M.E., STILES G., VON LUBITZ D.K.J.E. A3-adenosine receptors: Design of selective ligands and therapeutic prospects. Drugs Future. 1995;20:689–699. doi: 10.1358/dof.1995.020.07.531583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON K.A., NIKODIJEVIAE O., SHI D., GALLO-RODRIGUEZ C., OLAH M.E., STILES G.L., DALY J.W. A role for central A3-adenosine receptors: Mediation of behavioral depressant effects. FEBS. 1993;336:57–60. doi: 10.1016/0014-5793(93)81608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON K.A., PARK K.S., JIANG J.-L., KIM Y.-C., OLAH M.E., STILES G.L., JI X.D. Pharmacological characterization of novel A3 adenosine receptor-selective antagonists. Neuropharmacology. 1997;36:1157–1165. doi: 10.1016/s0028-3908(97)00104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLOTZ K.-N., HESSLING J., HEGLER J., OWMAN C., KULL B., FREDHOLM B.B., LOHSE M.J. Comparative pharmacology of human adenosine receptor subtypes: characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch. Pharmacol. 1998;357:1–9. doi: 10.1007/pl00005131. [DOI] [PubMed] [Google Scholar]
- LEA G.S., AMANN J., CHAKRAVARTI S., LASS J., EDELHAUSER H.F. Intraocular volumes and surface area measurements of the mouse eye. Invest. Ophthalmol. Vis. Sci. 2000;41:S739. [Google Scholar]
- LI A.H., MORO S., FORSYTH N., MELMANN N., JI X.D., JACOBSON K.A. Synthesis, CoMFA analysis, and receptor docking of 3,5-diacyl-2, 4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists. J. of Med. Chem. 1999;42:706–721. doi: 10.1021/jm980550w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL C.H., PETERSON-YANTORNO K., MCGLINN A.M., COCA-PRADOS M., STONE R.A., CIVAN M.M. A3 adenosine receptors regulate Cl− channels of nonpigmented ciliary epithelial cells. Am. J. Physiol. 1999;276:C659–666. doi: 10.1152/ajpcell.1999.276.3.C659. [DOI] [PubMed] [Google Scholar]
- TIAN B., GABELT B., CROSSON C.E., KAUFMAN P.L. Effects of adenosine agonists on intraocular pressure and aqueous humour dynamics in cynomolgus monkeys. Exp. Eye Res. 1997;64:979–989. doi: 10.1006/exer.1997.0296. [DOI] [PubMed] [Google Scholar]


