Abstract
Taxanes are antineoplastic drugs which have cardiovascular side effects of unknown mechanism. We investigated their influence on blood viscosity and erythrocyte morphology.
Whole blood was incubated in vitro with increasing concentrations of Taxol®, Taxotere®, paclitaxel (0 – 100 μM) and the vehicles Cremophor-EL and Tween 80 (0 – 5% vol) for 1 h at 37°C. Plasma and whole blood viscosity (Haematocrit 45%) were measured and erythrocyte morphology was assessed on glutaraldehyde-fixed cells. The same investigations were performed in seven patients before and after a Taxol®-infusion.
Taxol® and Taxotere® induced a dose- and time-dependent stomatocytic shape transformation of erythrocytes. Paclitaxel alone had no effect, but the vehicles cremophor-EL and Tween 80, used in Taxol® and Taxotere®, respectively, induced a comparable degree of stomatocytosis. This suggests a preferential intercalation of these substances into the inner hemileaflet of the membrane lipid bilayer. Associated with this shape change a dose-dependent increase in plasma and whole blood viscosity was observed. Neither shape nor viscosity changes were reversible upon removal of the agents. After the infusion of 130 – 300 mg Taxol® in patients a slight shift towards stomatocytosis and an increase in whole blood viscosity at high shear rate from 5.09±0.30 to 5.44±0.38 mPa.s (P<0.05) were confirmed.
Commercial taxane drug formulations induce stomatocytosis and increase blood viscosity, which is due to their formulation vehicles. These findings may contribute to the understanding of the cardiovascular side effects of these drugs.
Keywords: Blood rheology, blood viscosity, cardiovascular side effect, erythrocyte shape, taxanes
Introduction
Taxanes are antimicrotubule agents with activity against a broad range of cancers, including ovarian, breast, non-small-cell lung, and head and neck cancers (Crown & O'leary, 2000; Rowinsky & Donehower, 1995). A problem encountered with these drugs is their low aqueous solubility. For this reason, paclitaxel (Taxol®) is administered in a mixture of cremophor-EL (a derivative of castor oil and ethylene oxide):ethanol and docetaxel (Taxotere®) in tween 80 (polyoxyethylene sorbitanmonooleate):ethanol (Sparreboom et al., 1998).
Taxol® can cause cardiovascular side effects. Although they are usually transient and asymptomatic, fatal events have been reported (Rowinsky & Donehower, 1995; Arbuck et al., 1993; Rowinsky et al., 1991; Laher & Karp, 1997; Hekmat, 1996; Roth et al., 1993). The underlying mechanism remains elusive. A Taxol®-induced alteration of the plasma membrane composition has been suggested (Hamm-Alvarez et al., 1994). The formulation vehicle cremophor-EL itself has cardiovascular side effects, e.g. a decrease in cardiac output, mean arterial pressure and organ blood flow in a canine model (Bowers et al., 1991), decreased renal blood flow and glomerular filtration rate and increased renal vascular resistance in rats (Besarab et al., 1987). Major hypersensitivity reactions after Taxol®-based chemotherapies had been attributed to the propensity of cremophor-EL to release histamine (Rowinsky et al., 1991). There is only scant knowledge on the influence of cremophor-EL on blood rheology and blood cell morphology. Both decreased and increased plasma viscosity have been reported after the application of cremophor-EL containing anaesthetics (Gramstad & Stovner, 1979; Orr et al., 1982; Gibbs et al., 1984). Poikilocytosis and rouleaux formation of red cells has recently been observed after the application of Taxol® and has been attributed to cremophor-EL (Shimomura et al., 1998).
Based on these findings, we hypothesized that taxane-based chemotherapies may influence blood rheology, which would offer an explanation for cardiovascular side effects. The present study was designed to test this hypothesis.
Methods
Drugs
Commercial solutions of Taxol® and Taxotere® were obtained (Bristol-Myers Squibb AG, Baar, Switzerland; and Rhône-Poulenc Rorer AG, Thalwil, Switzerland (now distributed by Aventis), respectively). Cremophor-EL and paclitaxel (from Taxus brevifolia) were purchased from Sigma (Buchs, Switzerland). A stock solution of paclitaxel (5 mM) was dissolved in methanol containing 0.1% acetic acid (both from Fluka, Buchs, Switzerland), light-protected, and stored at −20°C prior to use. Tween® 80 (polyoxyethylene sorbitanmonooleate) was from Fluka.
In vitro study
Blood from healthy volunteers was drawn into K3-EDTA (ethylene-diaminetetraacetic acid) containing tubes (Becton Dickinson Vacutainer® System, Plymouth, U.K.). The haematocrit was determined with an electronic particle counter (Sysmex K-1000, Kobe, Japan). Samples were centrifuged at 1000 × g for 10 min. Plasma samples were separated, incubated with different concentrations of one drug for 60 min in a water bath at 37°C and plasma viscosity was measured as described below. For these experiments, autologous plasma was used for dilution of drugs as required. Experiments measuring blood viscosity were prepared as follows: the haematocrit of whole blood was first adjusted to 50% by removing a calculated volume of plasma. Several aliquots were prepared. One part of drug diluted in autologous plasma was added to nine parts of whole blood to yield a final haematocrit of 45% and final drug concentrations of 0, 5, 10, 25, 50, and 100 μM. Blood from one donor was used for two control experiments (addition of autologous plasma only) and experiments with the addition of one drug. When not stated otherwise, the specimens were incubated for 60 min in a water bath at 37°C.
Viscosity measurements
Whole blood and plasma viscosity were measured in a Couette viscometer (Contraves LS 30, Mettler-Toledo, Greifensee, Switzerland) at 37°C. For whole blood, shear rates of 94.5 s−1 and 0.945 s−1 were applied. For plasma, the mean of the viscosity's measured at shear rates of 94.5 s−1 and 37.6 s−1 was calculated (note: in contrast to whole blood, plasma is a Newtonian fluid, i.e. its viscosity is shear rate-independent (Chien, 1975)).
Red blood cell (RBC) morphology
RBCs were fixed in 1% glutaraldehyde (Fluka) in phosphate-buffered saline (mM: PBS: NaCl 131, Na2KPO4 30, D-glucose 11, pH 7.4, mOsm kg−1 300) RBC morphology was quantified on fixed cells by light microscopy according to the slightly modified nomenclature of Bessis (Bessis, 1972) with the help of a scoring system (Ferrell & Huestis, 1984): a discocyte obtained a score of zero; echinocytic transformation was classified as: echinocyte I (score: +1; irregularly contoured discocyte with up to five protrusions); echinocyte II (score: +2; flat cell with multiple spicules); echinocyte III (score: +3; ovoid or spherical erythrocyte with multiple spicules); spheroechinocyte (score:+4; a sphere with multiple short and thin spicules). Stomatocytic transformation was classified as: stomatocyte I (score: −1; cup-shaped erythrocyte (convex-concave erythrocyte)); stomatocyte II (score: −2; RBC with a pronounced, often longitudinal concavity); stomatocyte III (score: −3; RBC with more than one concavity); and spherostomatocyte (score: −4; spherical RBC with an irregular area of pits). For each specimen, 200 RBCs were classified and the morphological index calculated as Σ scores/200 (Ferrell & Huestis, 1984). Selected samples of fixed RBCs were also prepared for scanning electron microscopy with methods described before (Reinhart et al., 1999).
Ex vivo study
For ex vivo experiments, seven consecutive patients undergoing chemotherapies which included Taxol® were recruited. Their characteristics are shown in Table 1. They gave their informed consent for the necessary additional blood withdrawal. Ten ml of blood anticoagulated with EDTA was drawn directly before and after the exclusive intravenous administration of Taxol®. Samples of plasma and whole blood with an adjusted haematocrit of 45% were prepared. Viscosity and RBC morphology were determined after incubation for 30 min in a water bath at 37°C as described above.
Table 1.
Characteristics of patients undergoing chemotherapy with Taxol®
Statistical analysis
Results are presented as means±s.d. Paired t-tests (ex vivo study) or one-way analysis of variance (ANOVA) for repeated measures with Tukey-Kramer's Multiple Comparisons Test were performed as appropriate (GraphPad InStat 3.00, San Diego, CA, U.S.A.). P<0.05 was considered significant.
Results
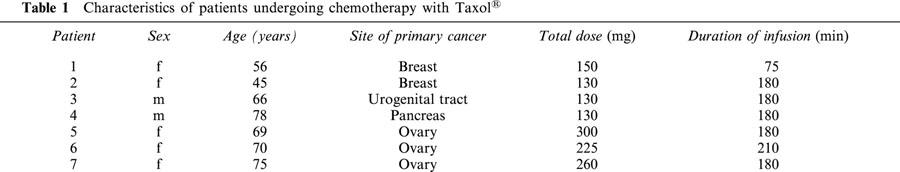
In vitro incubation of whole blood with a haematocrit adjusted to 45% and plasma of healthy volunteers with Taxol® for 1 h resulted in a dose-dependent increase of both whole blood viscosity at high (94.5 s−1) and low (0.945 s−1) shear rate as well as plasma viscosity (Figure 1). At the highest dose examined, yielding a concentration of 100 μM paclitaxel, the increase of whole blood viscosity was 20% at high shear rate and 61% at low shear rate, whereas plasma viscosity increased by 6% as compared to corresponding controls.
Figure 1.
Effect of Taxol® on viscosity of whole blood and plasma. Increasing concentrations of Taxol® were incubated with whole blood (haematocrit 45%) or plasma for 60 min at 37°C prior to determination of viscosity. (A) High shear (94.5 s−1) viscosity of whole blood; (B) Low shear (0.945 s−1) viscosity of whole blood; (C) Plasma viscosity. n=6. *P<0.01 and **P<0.001 compared to corresponding control.
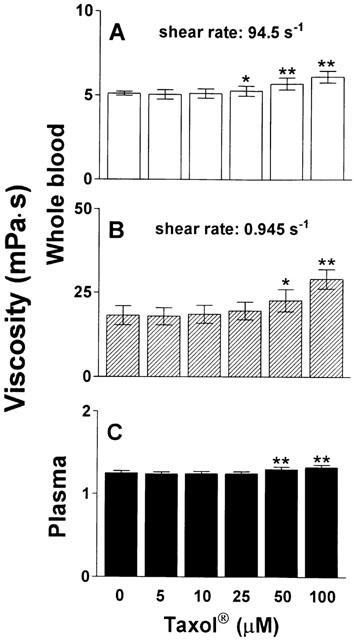
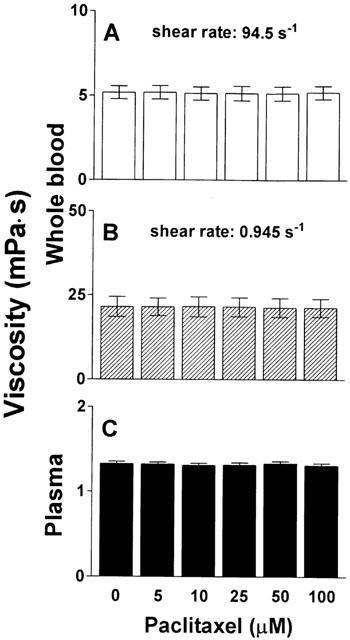
In contrast, neither whole blood viscosity nor plasma viscosity changed significantly when blood was incubated with pure paclitaxel at similar concentrations without the formulation vehicle cremophor-EL (Figure 2). In vitro incubation of whole blood and plasma of healthy volunteers with cremophor-EL for 1 h, however, dose-dependently increased both whole blood viscosity at high and low shear rate as well as plasma viscosity, as shown in Figure 3. The highest concentration examined (5%) resulted in an increase of whole blood viscosity by 34% at high shear rate and 82% at low shear rate, and plasma viscosity increased by 17% as compared to corresponding controls.
Figure 2.
Effect of paclitaxel on viscosity of whole blood and plasma. Paclitaxel was incubated at increasing concentrations with whole blood (haematocrit 45%) or plasma for 60 min at 37°C prior to determination of viscosity. (A) High shear viscosity of whole blood; (B) Low shear viscosity of whole blood; (C) Plasma viscosity. n=6.
Figure 3.
Effect of cremophor-EL on viscosity of whole blood and plasma. Increasing concentrations of cremophor-EL were incubated with whole blood (haematocrit 45%) or plasma for 60 min at 37°C prior to determination of viscosity. (A) High shear viscosity of whole blood; (B) Low shear viscosity of whole blood; (C) Plasma viscosity. n=5. *P<0.05 and **P<0.001 compared to corresponding control.
As shown in Figure 4, in vitro incubation of whole blood and plasma of healthy volunteers with Taxotere® for 1 h dose-dependently increased whole blood viscosity both at high and low shear rate, but did not change plasma viscosity. At the highest dose examined (resulting in a concentration of 100 μM docetaxel) the increase of whole blood viscosity was 19% at high and 50% at low shear rate, respectively. The formulation vehicle of Taxotere®, namely tween 80, caused a dose-dependent increase of whole blood viscosity at both shear rates as well as of plasma viscosity (Figure 5). The highest concentration assessed (5%) increased blood viscosity at high shear rate by 59%, at low shear rate by 71%, and plasma viscosity by 18%.
Figure 4.
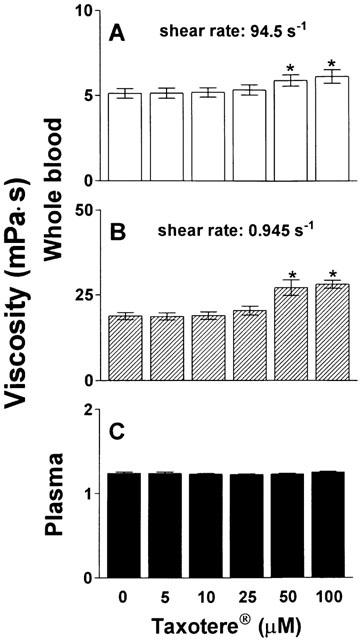
Effect of Taxotere® on viscosity of whole blood and plasma. Increasing concentrations of taxotere were incubated with whole blood (haematocrit 45%) or plasma for 60 min at 37°C prior to determination of viscosity. (A) High shear viscosity of whole blood; (B) Low shear viscosity of whole blood; (C) Plasma viscosity. n=6. *P<0.001 compared to control.
Figure 5.
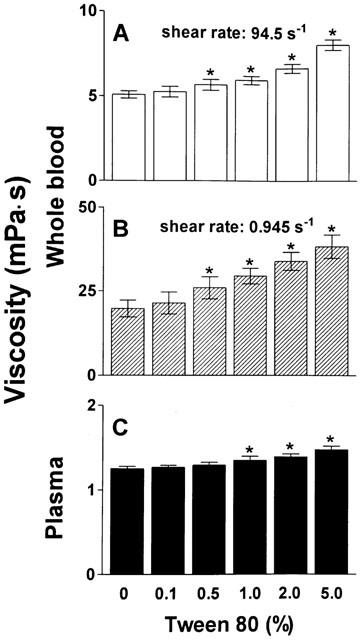
Effect of tween 80 on viscosity of whole blood and plasma. The formulation vehicle of Taxotere®, tween 80, was incubated at increasing concentrations with whole blood (haematocrit 45%) or plasma for 60 min at 37°C prior to determination of viscosity. (A) High shear viscosity of whole blood; (B) Low shear viscosity of whole blood; (C) Plasma viscosity. n=6. *P<0.001 compared to corresponding control.
A dose-dependent stomatocytic shape transformation was observed, which is illustrated in Figure 6. Profound stomatocytosis was found with either 100 μM Taxol® or Taxotere® (Figure 6A,C), which was also seen with the formulation vehicle of Taxotere®, tween 80 (Figure 6D), but not with pure paclitaxel (Figure 6B). The quantification of these shape changes with the help of the morphological index is shown in Figures 7 and 8. Taxol® dose-dependently decreased the morphological index (indicating dose-dependent stomatocytic shape transformation; Figure 7A), which was not seen with pure paclitaxel (Figure 7B). The formulation vehicle cremophor-EL had a stomatogenic potential (Figure 7C) although to a lesser degree than expected from Taxol® (Figure 7A). A dose-dependent decrease in the morphological index was also seen with Taxotere® (Figure 8A) and by a similar extent with the vehicle of Taxotere®, tween 80 (Figure 8B).
Figure 6.
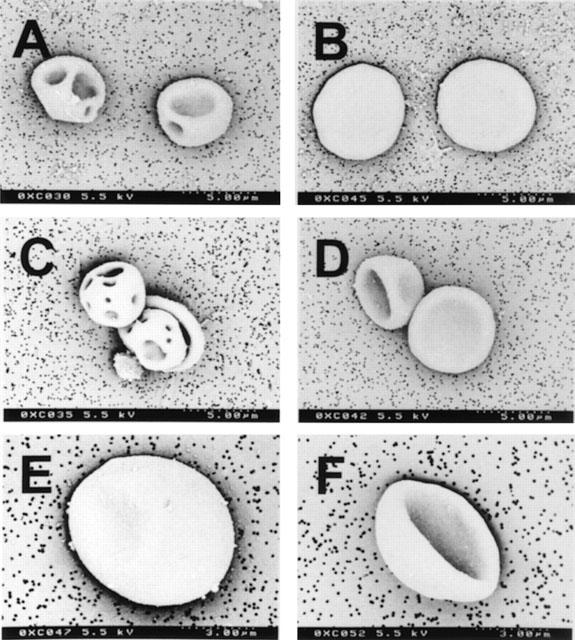
Scanning electron micrographs of RBCs. (A) incubation with 100 μM Taxol® in vitro generating stomatocytes III; (B) Incubation with 100 μM paclitaxel in vitro leaving the normal discocytic RBC shape unaffected; (C) Incubation with 100 μM Taxotere® in vitro resulting in stomatocytosis II and III; (D) Incubation with the carrier of Taxotere®, tween 80 (5%), in vitro with stomatocytes I and II. (E) Discocytic RBC of patient 4 before therapy; (F) A stomatocyte II after the infusion of 130 mg Taxol® (ex vivo).
Figure 7.
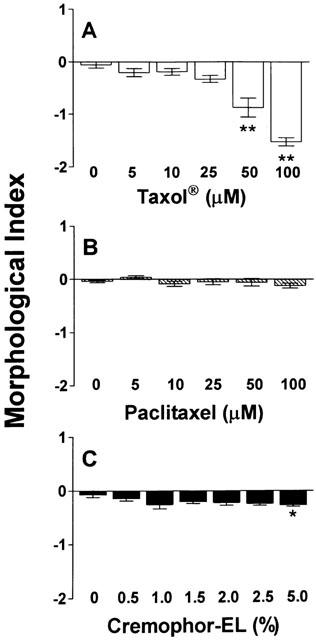
Influence of Taxol® and its constituents paclitaxel and cremophor-EL on the RBC morphology. Increasing concentrations of Taxol® (A), paclitaxel (B), and cremophor-EL (C) were incubated with whole blood (haematocrit 45%) for 60 min at 37°C, and RBC morphology, expressed as morphological index, was assessed by light microscopy. n=6. *P<0.05 and **P<0.001 compared to control.
Figure 8.
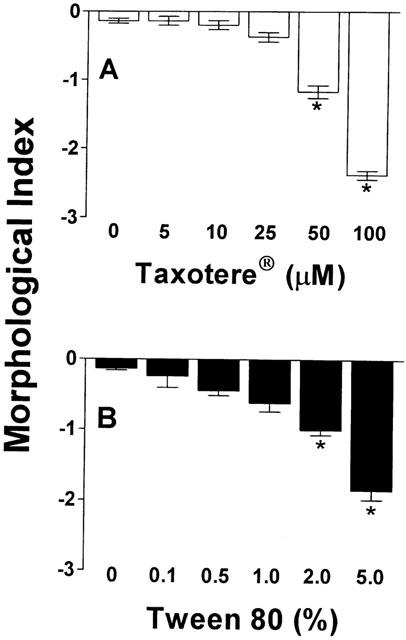
Influence of Taxotere® and tween 80 on RBC morphology. Increasing concentrations of Taxotere® (A) and tween 80 (B) were incubated with whole blood (haematocrit 45%) for 60 min at 37°C, and RBC morphology, expressed as morphological index, was assessed by light microscopy. n=6. *P<0.001 compared to corresponding control.
As shown in Figure 9, both the Taxol®-induced changes in whole blood viscosity and stomatocytic shape transformation were time-dependent. It was almost not seen after 2 min, but obvious after 60 min. These changes were not, or at the most only partially reversible, which is also shown in Figure 9. When RBCs were washed three times in phosphate-buffered saline and then resuspended in autologous plasma, blood viscosity at high shear rate and the degree of stomatocytosis remained unaffected, whereas blood viscosity at low shear rate significantly decreased towards the control level.
Figure 9.
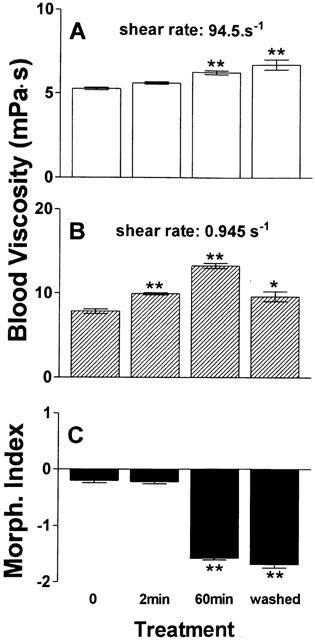
Time-dependency of Taxol®-induced effects on viscosity and RBC morphology and missing reversibility after wash-out. Taxol® (corresponding to 100 μM paclitaxel) was incubated with whole blood (haematocrit 45%) at 37°C, and whole blood viscosity at high (A) and low (B) shear rate as well as RBC morphology (C), were determined at two consecutive time points (2 min and 60 min). Afterwards, RBCs were washed with PBS, resuspended in autologous plasma, and measurements were repeated at 37°C. n=6. *P=0.01 and **P=0.001 compared to control.
Because of these in vitro observations we searched for similar findings in seven patients undergoing chemotherapies containing Taxol®. Characteristics of these patients are shown in Table 1. As shown in Figure 10, whole blood viscosity (adjusted haematocrit 45% by removal of autologous plasma) at 94.5 s−1 was significantly increased from 5.09±0.30 to 5.44±0.38 mPa.s (P=0.031) after Taxol®-infusion; individual data are shown in Figure 10A. Low shear viscosity remained unaffected (9.73±0.99 and 10.29±0.99 mPa.s; P=0.249; Figure 10B). These values are lower than those observed in the in vitro experiments, which may be due to plasma dilution by the hydration performed before Taxol®-infusion. As shown in Figure 10C, there was a tendency of plasma viscosity to increase (from 1.24±0.05 to 1.34±0.08 mPa.s; P=0.059). RBC morphology showed a significant stomatocytic shape transformation (decrease of the morphological index from 0.02±0.26 to −0.42±0.35; P=0.004; Figure 10D). Scanning electron micrographs of a RBC of patient 4 before and after administration of Taxol® are shown in Figure 6E,F, respectively.
Figure 10.
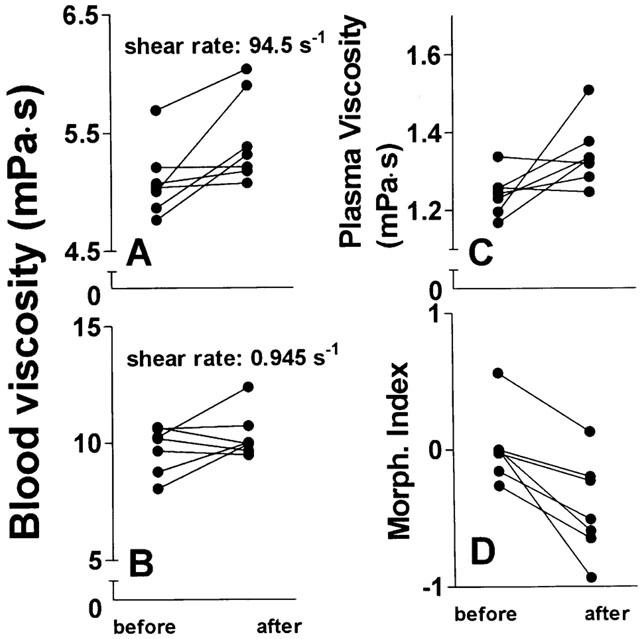
Changes of viscosity and the RBC morphology in patients undergoing Taxol®-based chemotherapies. Blood was drawn from patients directly prior to and after administration of Taxol®, for details see Table 1. Whole blood viscosity (haematocrit 45%) at high (A) and low (B) shear rate, plasma viscosity (C), and the RBC morphological index (D) were measured. n=7. P=0.031 for high shear viscosity; P=0.249 for low shear viscosity; P=0.059 for plasma viscosity; P=0.004 for morphological index (paired t-test).
Discussion
The main findings of the present study are that commercial taxane drug formulations induce a stomatocytic shape transformation of RBCs and increase blood viscosity in vitro and also in vivo. Since blood samples were directly drawn prior to (i.e. after the administration of premedications) and following the application of Taxol® alone, possible effects of other drugs were eliminated as confounders. Haematocrits were all adjusted to 45% to exclude influences of changing haematocrits on the results. Our in vitro experiments further identify the formulation vehicles cremophor-EL and tween 80, respectively, as most important inducers of these effects.
According to the bilayer couple theory (Sheetz & Singer, 1976) a stomatocytic shape transformation occurs when a compound is preferentially intercalated into the inner hemileaflet of the membrane lipid bilayer, leading to a relative expansion of the inner hemileaflet and hence of the membrane towards the cell interior. Taxol® induced stomatocytosis dose-dependently but paclitaxel alone without the formulation vehicle cremophor-EL had no influence on RBC shape. Since cremophor-EL used alone induced less stomatocytosis than expected from the Taxol® data (Figure 7), one must assume a synergy of the taxane and the formulation vehicle in intercalating into the inner hemileaflet of the RBC membrane and inducing stomatocytosis. This seems conceivable since cremophor-EL acts as a solubilizer and may increase the tendency of paclitaxel to interact with the lipid bilayer. Alternatively, the other drug contained in Taxol®, ethanol, may also play a role. Some alcohols may accumulate in the inner hemileaflet of the RBC membrane and induce stomatocytosis (Chabanel et al., 1985). It is noteworthy that cremophor-EL is also used as a formulation vehicle for other drugs, e.g. cyclosporine, which has been found to induce stomatocytosis (Reinhart, 1993). Taxotere® induced a stomatocytic shape transformation to an even stronger degree than Taxol®. This was paralleled by the ability of the vehicle tween 80 to induce significant stomatocytosis at concentrations at which cremophor-EL failed to influence RBC shape.
It is interesting that the RBC shape change and the impairment of blood viscosity was not reversible upon removal of Taxol® in vitro. This is in contrast to the reversibility of the echinocytosis induced by the cytostatic drug 5-fluorouracil (Baerlocher et al., 1997) or the stomatocytosis induced by ifosfamide (Reinhart et al., 1999). This indicates that commercial taxane drug formulations have a higher affinity for the RBC membrane.
In agreement with earlier observations stomatocytosis had a profound effect on blood rheology (Reinhart et al., 1992; 1999; Meiselman, 1978). The increase in whole blood viscosity at high shear rate, which is representative of flow conditions in arteries, arterioles, and non-venous portions of the microcirculation (Chien, 1975), is due to decreased RBC deformability induced by the stomatocytic shape transformation. Whole blood viscosity at low shear rate is primarily determined by RBC aggregation and is relevant for blood flow in veins and under stasis (Chien, 1975). Stomatocytes, also called cup-shaped RBCs, have a greater propensity to stack one in another and hereby form RBC aggregates (Reinhart et al., 1989), which explains the earlier observation of increased rouleaux formation under Taxol® (Shimomura et al., 1998). We did not observe any significant effect of Taxol® on low shear blood viscosity when administered in patients.
It is conceivable that the stomatocytic shape transformation and increase in blood viscosity induced by commercial taxane drug formulations may be a contributing pathophysiologic mechanism behind the various cardiovascular side effects of taxanes (Rowinsky & Donehower, 1995; Arbuck et al., 1993; Rowinsky et al., 1991; Hekmat, 1996; Roth et al., 1993) and cremophor-EL (Besarab et al., 1987; Bowers et al., 1991). The clinical significance, however, remains speculative. As most cancer patients have anaemia, their low haematocrits could compensate for the decreased RBC deformability and prevent an increase in whole blood viscosity. As the formulation vehicles seem to account for most of the influence on blood rheology, other galenic forms might be desirable. In this context it is noteworthy that a new taxane analogue, which needs three times less polyoxyethylated castor oil as a vehicle, is currently in clinical trials (Hidalgo et al., 2001). Various cytostatic drugs have a high affinity for cell membranes and hence affect cell shape. Vinblastine (Jacobs et al., 1972), ifosfamide (Reinhart et al., 1999), and taxanes induce stomatocytosis, 5-fluorouracil (Baerlocher et al., 1997) and mesna (Reinhart et al., 1999) echinocytosis. Stomatocytic agents counterbalance echinocytic agents and vice versa (Alhanaty & Sheetz, 1981; Reinhart & Chien, 1987). Certain drug combinations may, therefore, be equilibrative with regard to the interaction with cell membranes, not only of RBCs but of cells in general (e.g. endothelial cells), while other combinations may have additional or even potentiating effects.
Acknowledgments
We are indebted to all voluntary blood donors and patients participating in the study. We thank Dr V. Wüscher, Dr F. Egli, and Dr R. A. Steiner for allowing us to study their patients. We further acknowledge Dr R. G. Richards (AO/ASIF Research Institute, Davos, Switzerland) for consultations on the s.e.m.
Abbreviations
- EDTA
ethylenediaminetetraacetic acid
- PBS
phosphate buffered saline
- RBC
red blood cell
References
- ALHANATY E., SHEETZ M.P. Control of the erythrocyte membrane shape: Recovery from the effect of crenating agents. J. Cell. Biol. 1981;91:884–888. doi: 10.1083/jcb.91.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARBUCK S.G., STRAUSS H., ROWINSKY E., CHRISTIAN M., SUFFNESS M., ADAMS J., OAKES M., MCGUIRE W., REED E., GIBBS H., GREENFIELD R.A., MONTELLO M. A reassessment of cardiac toxicity associated with Taxol. J. Natl. Cancer Inst. Monogr. 1993;15:117–130. [PubMed] [Google Scholar]
- BAERLOCHER G.M., BEER J.H., OWEN G. RH., MEISELMAN H.J., REINHART W.H. The anti-neoplastic drug 5-fluorouracil produces echinocytosis and affects blood rheology. Br. J. Haematol. 1997;99:426–432. doi: 10.1046/j.1365-2141.1997.4003212.x. [DOI] [PubMed] [Google Scholar]
- BESARAB A., JARRELL B.E., HIRSCH S., CARABASI R.A., CRESSMAN M.D., GREEN P. Use of the isolated perfused kidney model to assess the acute pharmacologic effects of cyclosporine and its vehicle, cremophor EL. Transplantation. 1987;44:195–201. doi: 10.1097/00007890-198708000-00005. [DOI] [PubMed] [Google Scholar]
- BESSIS M. Red cell shapes: an illustrated classification and its rationales. Nouv. Rev. Fr. Hematol. 1972;12:721–746. [PubMed] [Google Scholar]
- BOWERS V.D., LOCKER S., AMES S., JENNINGS W., CORRY R.J. The hemodynamic effects of Cremophor-EL. Transplantation. 1991;51:847–850. doi: 10.1097/00007890-199104000-00021. [DOI] [PubMed] [Google Scholar]
- CHABANEL A., ABBOTT R.E., CHIEN S., SCHACHTER D. Effects of benzyl alcohol on erythrocyte shape, membrane hemileaflet fluidity and membrane viscoelasticity. Biochim. Biophys. Acta. 1985;816:142–152. doi: 10.1016/0005-2736(85)90402-x. [DOI] [PubMed] [Google Scholar]
- CHIEN S.Biophysical behaviour of red cells in suspension The red blood cell 1975New York: Academic Press; 1031–1133.D. Mac, N. Surgenor, (ed.) [Google Scholar]
- CROWN J., O'LEARY M. The taxanes: an update. Lancet. 2000;355:1176–1178. doi: 10.1016/S0140-6736(00)02074-2. [DOI] [PubMed] [Google Scholar]
- FERRELL J.E., HUESTIS W.H. Phosphoinositide metabolism and the morphology of human erythrocyte. J. Cell. Biol. 1984;98:1992–1998. doi: 10.1083/jcb.98.6.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS N.M., OH T.E., CHESTER A. Blood viscosity and blood film morphology during prolonged sedation with Althesin. Br. J. Anaesth. 1984;56:1179–1180. doi: 10.1093/bja/56.10.1179-b. [DOI] [PubMed] [Google Scholar]
- GRAMSTAD L., STOVNER J. Plasma viscosity and cremophor-containing anaesthetics. Br. J. Anaesth. 1979;51:1175–1179. doi: 10.1093/bja/51.12.1175. [DOI] [PubMed] [Google Scholar]
- HAMM-ALVAREZ S.F., ALAYOF B.E., HIMMEL H.M., KIM P.Y., CREWS A.L., STRAUSS H.C., SHEETZ M.P. Coordinate depression of bradykinin receptor recycling and microtubule-dependent transport by taxol. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7812–7816. doi: 10.1073/pnas.91.16.7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEKMAT E. Fatal myocardial infarction potentially induced by paclitaxel. Ann. Pharmacother. 1996;30:1110–1112. doi: 10.1177/106002809603001008. [DOI] [PubMed] [Google Scholar]
- HIDALGO M., AYLESWORTH C., HAMMOND L.A., BRITTEN C.D., WEISS G., STEPHENSON J., SCHWARTZ G., PATNAIK A., SMITH L., MOLPUS K., FELTON S., GUPTA E., FERRANTE K.J., TORTORA A., SONNICHSEN D.S., SKILLINGS J., ROWINSKY E.K. Phase I and pharmacokinetic study of BMS-184476, a taxane with greater potency and solubility than paclitaxel. J. Clin. Oncol. 2001;19:2493–2503. doi: 10.1200/JCO.2001.19.9.2493. [DOI] [PubMed] [Google Scholar]
- JACOBS H., AMSDEN T., WHITE T. Membrane microfilaments of erythrocytes: Alterations in intact cells reproduces the hereditary spherocytosis syndrome. Proc. Natl. Acad. Sci. U.S.A. 1972;69:471–474. doi: 10.1073/pnas.69.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAHER S., KARP S.J. Acute myocardial infarction following paclitaxel administration for ovarian carcinoma. Clin. Oncol. 1997;9:124–126. doi: 10.1016/s0936-6555(05)80452-2. [DOI] [PubMed] [Google Scholar]
- MEISELMAN H.J. Rheology of shape-transformed human red cells. Biorheol. 1978;15:225–237. doi: 10.3233/bir-1978-153-410. [DOI] [PubMed] [Google Scholar]
- ORR J.E., DAVIDSON R.J., RUSSELL I.F., ROBERTSON G.S. A haemorheological study of althesin. Br. J. Anaesth. 1982;54:1003–1006. doi: 10.1093/bja/54.9.1003. [DOI] [PubMed] [Google Scholar]
- REINHART W.H. Binding of cyclosporine by erythrocytes: influence on cell shape and deformability. Eur. J. Clin. Invest. 1993;23:177–181. doi: 10.1111/j.1365-2362.1993.tb00758.x. [DOI] [PubMed] [Google Scholar]
- REINHART W.H., BAERLOCHER G.M., CERNY T., OWEN G. RH., MEISELMAN H.J., BEER J.H. Ifosfamide-induced stomatocytosis and mesna-induced echinocytosis: influence on biorheological properties of blood. Eur. J. Haematol. 1999;62:223–230. doi: 10.1111/j.1600-0609.1999.tb01751.x. [DOI] [PubMed] [Google Scholar]
- REINHART W.H., CHIEN S. Echinocyte-stomatocyte-transformation and shape control of human red blood cells: Morphological aspects. Am. J. Hematol. 1987;24:1–4. doi: 10.1002/ajh.2830240102. [DOI] [PubMed] [Google Scholar]
- REINHART W.H., SINGH A., STRAUB P.W. Red blood cell aggregation and sedimentation: the role of the cell shape. Br. J. Haematol. 1989;73:551–556. doi: 10.1111/j.1365-2141.1989.tb00296.x. [DOI] [PubMed] [Google Scholar]
- REINHART W.H., SINGH-MARCHETTI M., STRAUB P.W. Influence of erythrocyte shape on suspension viscosities. Eur. J. Clin. Invest. 1992;22:38–44. doi: 10.1111/j.1365-2362.1992.tb01933.x. [DOI] [PubMed] [Google Scholar]
- ROTH B.J., YEAP B.Y., WILDING G., KASIMIS B., MCLEOD D., LOEHRER P.J. Taxol in advanced, hormone-refractory carcinoma of the prostate. A phase II trial of the Eastern Cooperative Oncology Group. Cancer. 1993;72:2457–2460. doi: 10.1002/1097-0142(19931015)72:8<2457::aid-cncr2820720825>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- ROWINSKY E.K., DONEHOWER R.C. Paclitaxel (Taxol) N. Engl. J. Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- ROWINSKY E.K., MCGUIRE W.P., GUARNIERI T., FISHERMAN J.S., CHRISTIAN M.C., DONEHOWER R.C. Cardiac disturbances during the administration of taxol. J. Clin. Oncol. 1991;9:1704–1712. doi: 10.1200/JCO.1991.9.9.1704. [DOI] [PubMed] [Google Scholar]
- SHEETZ M.P., SINGER S.J. Equilibrium and kinetic effects of drugs on the shape of human erythrocytes. J. Cell. Biol. 1976;70:247–251. doi: 10.1083/jcb.70.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMOMURA T., FUJIWARA H., IKAWA S., KIGAWA J., TERAKAWA N. Effects of taxol on blood cells. Lancet. 1998;352:541–542. doi: 10.1016/S0140-6736(05)79249-7. [DOI] [PubMed] [Google Scholar]
- SPARREBOOM A., VAN TELLINGEN O., NOOIJEN W.J., BEIJNEN J.H. Preclinical pharmacokinetics of paclitaxel and docetaxel. Anticancer Drugs. 1998a;9:1–17. doi: 10.1097/00001813-199801000-00001. [DOI] [PubMed] [Google Scholar]


