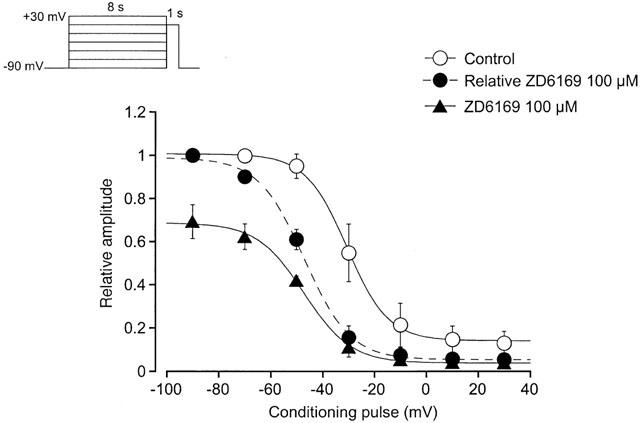
Effects of ZD6169 (100 μ
M) on the voltage-dependent inactivation of the Ba
2+ inward currents in pig urethra. Whole-cell recording, pipette solution Cs
+ – TEA
+ solution containing 5 m
M EGTA and the bath solution 10 m
M Ba
2+ containing 135 m
M TEA
+. The holding potential was −90 mV. Conditioning pulses of various amplitudes were applied (up to +30 mV, 8 s duration) before application of the test pulse (to +10 mV, 1 s duration). An interval of 20 ms was allowed between these two pulses to estimate possible contamination of the capacitive current. The peak amplitude of Ba
2+ current evoked by each test pulse was measured before and after application of 100 μ
M ZD6169. The curves with the solid line; the peak amplitude of Ba
2+ inward current in the absence and presence of ZD6169 without application of any conditioning pulse was normalized as one. The curve with the broken line was normalized to the current at +10 mV upon stepping from −90 mV in 100 μ
M ZD6169. The lines were drawn by fitting the data to the following equation in the least-squares method,
where
I,
Imax,
V,
Vhalf,
k and
C are the relative amplitude of Ba
2+ inward currents observed at various amplitudes of the conditioning pulse (
I) and observed with application of the conditioning pulse of −90 mV (
Imax), amplitude of the conditioning pulse (
V), and that where the amplitude of Ba
2+ inward current was reduced to half (
Vhalf), slope factor (
k) and fraction of the non-inactivating component of Ba
2+ inward current (
C). The curves in the absence or presence of ZD6169 were drawn using the following values: (control),
Imax=1.0,
Vhalf=−30.7,
k=8.5 and
C=0.14; (ZD6169, 100 μ
M),
Imax=0.69,
Vhalf=−47.2,
k=8.5 and
C=0.04. Each symbol indicates the mean of four observations with±s.d. shown by vertical lines. Some of the s.d. bars are less than the size of the symbol.