Figure 1.
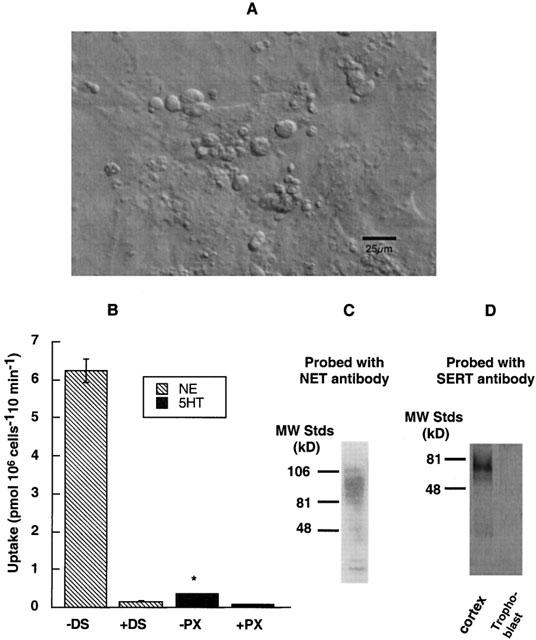
Trophoblast cultures and norepinephrine transport (A) Images of trophoblasts in tissue culture: The live cell images of 2-day cultures of rat placental trophoblasts are captured using Zeiss MC 100 35-mm camera system. The multinucleated giant trophoblasts along with small trophoblasts are seen along the varicosities. Bar, 2 μm. (B) Uptake of NE and 5HT by trophoblasts: Uptake of 50 nM [3H]-NE in the presence or absence of 1 μM DS, and uptake of 50 nM [3H]-5HT in the presence or absence of 10 nM paroxetine were measured during a 10 min incubation time in KRH buffer at 37°C. Specific NE was measured by subtracting NE uptake in the presence of 1 μM DS from total uptake in the absence of DS. Specific 5HT uptake was obtained in the same way using 10 nM PX. Results are given in pmoles of NE or 5HT pmol (106 cells)−1 10 min. Specific NE uptake by trophoblasts is ∼10 times higher than specific 5HT uptake. The data represent the mean±s.e.m. from two experiments carried out in triplicate using different trophoblast cultures. Asterisk denotes significant difference of PX-sensitive 5HT uptake as compared to DS-sensitive NE uptake, P<0.05 (Student's t-test and ANOVA). (C) Identification of NET protein: A heavily glycosylated protein band (∼85 kD) corresponding to NET is observed when trophoblast lysates (40 μg of protein) were run on SDS – PAGE and probed with NET-specific antibody N430. (D) Absence of SERT protein: There was no detectable level of SERT protein when same amount of protein (40 μg) from trophoblast lysate was analysed using SERT-specific antibody SR-12. SR12 antibody specifically recognizes a ∼76 kD protein band corresponding to SERT from 40 μg of protein extract of rat brain cortex.
