Abstract
The inhibitory effects of n-alcohols (methanol to dodecanol) on glycine-activated currents were studied in neurons freshly dissociated from the ventral tegmental area of neonatal rats using whole-cell patch-clamp recording technique.
Ethanol enhanced and depressed glycine-activated currents in 35% and 45%, respectively, of neurons of ventral tegmental area of neonatal rats. In this report, we extended our focus of ethanol-induced inhibition of glycine currents to other straight-chain alcohols.
Aliphatic n-alcohols, which have carbon numbers less than nine, suppressed glycine currents in 45% (71/158) of the neurons. All results from this study are obtained from the 45% of cells displaying inhibition; the other 55% of the neurons were not studied.
Alcohol potency increased as the number of carbon atoms increased from one to five, and was at a maximal plateau from five to nine; alcohols with 10 or more carbons did not inhibit glycine-activated currents. Thus, a ‘cutoff' point in their potency for inhibition of glycine receptor function occurred at about decanol.
A coapplication of dodecanol with ethanol eliminated the inhibition resulting from ethanol. Thus, dodecanol may bind to the receptor silently and compete with ethanol.
These observations indicate that straight-chain n-alcohols exhibit a ‘cutoff' point in their potency for inhibition of the glycine receptor function between nine and 10 carbon atoms. The inability of longer alcohols to change the activation properties of the receptors may contribute to the cutoff effect.
Keywords: Cutoff effect, foetal alcohol effects, freshly dissociated neuron, GABA, glycine receptor, neonatal neurons, patch clamp, aliphatic n-alcohol
Introduction
Despite numerous studies, the cellular and molecular mechanisms by which alcohols produce their actions are poorly understood. Recent studies indicate that alcohols may produce their effects by interacting directly with membrane proteins (Li et al., 1994; Peoples et al., 1996; Wick et al., 1998; Jenkins et al., 2001). This is supported by findings that several types of neuronal membrane receptors, such as nicotinic acetylcholine receptors (Murrell et al., 1991; McKenzie et al., 1995), N-methyl-D-aspartate receptors (Peoples & Weight, 1995), non-NMDA receptors (Akinshola, 2001), 5-HT3 receptors (Lovinger & White, 1991; Machu & Harris, 1994; Jenkins et al., 1996), γ-aminobutyric acid receptors (GABAA) (Mihic & Harris, 1995), and glycine receptors (GlyRs) (Mascia et al., 1996), are sensitive to alcohols.
Like GABA (Krnjevic', 1997), glycine is a major inhibitory neurotransmitter in the mature mammalian central nervous system (CNS) (Werman et al., 1968). Glycine is best known for its role in the spinal cord and brain stem, but GlyRs are found throughout the CNS, including the ventral tegmental area (VTA) (Betz, 1991; Ye et al., 1998). The VTA contains the cells of origin of the mesolimbic system, which plays a pivotal role in the medication of the rewarding effects of drugs of abuse, including alcohol (Gatto et al., 1994; Koob & Bloom, 1988; Langosch et al., 1990; Wise, 1996). Recent experiments in this laboratory have revealed that glycine-mediated responses can be recorded in the majority of VTA neurons, and that glycine-mediated responses of VTA neurons are sensitive to pharmacologically relevant concentrations of ethanol (Ye et al., 2001a, 2001b). Since glycine has inhibitory effects on neuronal activity, modulation of GlyR function would contribute to the effects of alcohols on the neuronal excitability. However, despite the importance of the VTA in the reinforcement of drug abuse, alcohol effects on the GlyRs of the VTA have not been well studied.
A potentiation effect of ethanol on GlyRs has been reported from several groups (Celentano et al., 1988; Engblom & Akerman, 1991; Aguayo & Pancetti, 1994; Mascia et al., 1996; Ye et al., 2001a). Mascia et al. (1996) demonstrated that n-alcohols (ethanol to dodecanol) potentiated glycine responses in Xenopus oocytes expressing homomeric α1 or α2 GlyRs. The potencies of alcohols increased with the number of n-alcohol carbon atoms (up to decanol), and a ‘cutoff' occurred at about dodecanol. Based on these observations, they proposed that the α subunits of GlyRs contain sites of action for n-alcohols. In addition to the potentiating effect, which occurred in 35% of the VTA neurons (Ye et al., 2001a), ethanol (0.1–10 mM) suppressed glycine-activated current in 45% of the VTA neurons (Ye et al., 2001b). The mechanisms underlying the difference between these two sets of seemingly conflicting results are currently under investigation. In the current study, we investigated whether or not the other alcohols also suppress the GlyRs. We show here that a series of n-alcohols inhibit the GlyRs in a fraction of VTA neurons. The inhibiting potency increased as the number of carbon atoms in an aliphatic n-alcohol increased from one to five, reached the maximum at six to nine, and then abruptly disappeared.
Methods
Isolation of neurons and electrophysiological recording
The care and use of animals and the experimental protocol of this study were approved by the Institutional Animal Care and Use Committee of University of Medicine and Dentistry of New Jersey. We performed our experiments on VTA neurons prepared as described earlier (Ye et al., 2001b). Briefly, 5–14-day-old Sprague-Dawley rats were decapitated. The brain was quickly excised, placed into ice-cold saline saturated with 95% O2 and 5% CO2, glued to the chilled stage of a vibratome (Campden Instruments, U.K.) and sliced to thickness of 300–400 μm. Slices were transferred to the standard external solution saturated with O2, containing 1 mg pronase per 6 ml and incubated (31°C) for 20 min. After an additional 20 min incubation in 1 mg thermolysin per 6 ml, the VTA was identified medial to the accessory optic tract and lateral to the fasciculus retroflexus under a dissecting microscope. Micro-punches of the VTA were isolated and transferred to a 35 mm culture dish. Mild trituration of these tissue punches through heat polished pipettes of progressively smaller tip diameters dissociated single neurons. Within 20 min of trituration, isolated neurons attached to the bottom of the culture dish and were ready to electrophysiological experiments. Based on morphology under the light microscope, the cells acutely isolated from VTA could be divided into two types: bipolar and multipolar. The majority are bipolar with 1–3 dendritic processes emerging from each end of the soma. The soma is fusiform in shape and from 20–40 μm in length and 15–25 μm in diameter. The multipolar neurons are larger with a diameter of 35–60 μm, and have 4–5 major dendrites. In agreement with a recent report (Brodie et al., 1999), most of the cells are tyrosine hydroxylase-positive. There were no appreciable differences between these two groups of neurons in response to alcohols, thus we did not distinguish between data from neurons of both shapes.
The saline in which the brain was dissected contained (in mM): NaCl 128, KCl 5, NaH2PO4 1.2, NaHCO3 26, MgCl2 9, CaCl2 0.3, glucose 2.5. The standard external solution contained (mM): NaCl 140, KCl 5, MgCl2 1, CaCl2 2, glucose 10, 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES) 10. The pH was adjusted to 7.4 with Tris base and the osmolarity to 310 mM kg−1 with sucrose. The patch electrode had a resistance between 3 and 5 MΩ when filled with the solution containing (mM): CsCl 120, TEA-Cl 21, MgCl2 4, 1, ethyleneglycol bis-(-aminoethylether)-N,N,N′N′-tetraacetic acid (EGTA) 11, CaCl2 1, HEPES 10 and Mg-ATP 2. The pH was adjusted to 7.2 with Tris base and the osmolarity to 280 mM kg−1 with sucrose.
Whole-cell glycine currents were recorded under voltage-clamp with an Axopatch 1D amplifier (Axon Instruments, Forster City, CA, U.S.A.) interfaced to a Digidata 1200 (Axon Instruments) and directly digitized with pCLAMP 6 software for further off-line analysis. The junction potential between the patch pipette and the bath solutions was nulled immediately before forming the giga-seal. The liquid junction potential between the bath and the electrode was 3.3 mV as calculated from the generalized Henderson equation using the Axoscope junction potential calculator (Barry, 1996). In most experiments, the series resistance before compensation was 15–25 MΩ. Routinely, 80% of the series resistance was compensated; hence, there was a 3 mV error for 1 nA of current. All glycine-induced responses were elicited in this solution at an ambient temperature of 20–30°C.
Chemical application
Solutions of methanol, propranol, butanol, heptanol, pentanol, hexanol, octanol, nonanol, decanol, undecanol, dodecanol, glycine (Sigma, St. Louis, MO, U.S.A.) and ethanol (95%, prepared from grain, stored in glass bottles, Pharmco, Brookfield, CT, U.S.A.) were prepared on the day of the experiment. Solutions were applied to a dissociated neuron with a superfusion system having a multi-barreled pipette as described previously (Ye et al., 2001b). The tip of the superfusion pipette was normally placed 50–100 μM away from the cell, a position which allowed rapid and uniform drug application without disturbing the mechanical stability of the neuron. This system allows complete exchange of solutions in the vicinity of the neuron within 20 to 35 ms. Throughout all experimental procedures the bath was continuously perfused with the standard external solution. In our perfusion system, we used glass made containers and Teflon made tubes, instead of the plastic ones. This is to avoid the production of bis (2,3,6,6-tetramethyl-4-piperidinyl) sebacate (Tinuvin 770), a sterically hindered amine light and radiation stabilizer, used in a wide range of plastics. Tinuyin 770 is known to inhibit some receptors such as nicotinic acetylcholine receptors (Roger et al., 1994). To ensure dissolution, alcohols with five or more carbon atoms were added to extracellular medium from tightly sealed glass containers with negligible headspace, vigorously shaken, and sonicated for ⩾30 min. Dodecanol was first dissolved in dimethylsulphoxide (DMSO), then diluted in standard external solution followed by sonication to facilitate the equilibration with standard external solution. The final concentration of DMSO was not exceeding 0.05%; it did not induce any ionic current and had no effect on the glycine response at the concentration used in these studies. Loss of long-chain alcohols from solutions due to evaporation or absorption to the experimental apparatus was minimized by using a closed solution application apparatus composed entirely of Teflon and fused silica.
Statistical analyses
Statistical analyses of concentration-response data were performed using a non-linear curve-fitting program (Sigma Plot, Jandel Scientific). Values reported for slope factors (n) and alcohol concentrations producing half-maximal inhibition (IC50) are those obtained by fitting the data to the logistic equation:
where x is alcohol concentration, y is response (i.e., percentage inhibition), Emax is maximal response, and Emin is minimal response, n is slope factors, IC50 is alcohol concentrations producing half-maximal inhibition. In these studies, Emax and Emin were constrained to 0% and 100% inhibition, respectively. Data were statistically compared using Student's t-test at a significance level of P<0.05, otherwise as indicated. Values reported for glycine-activated current represent peak current. Average values are expressed as mean±s.e.mean with the number of neurons indicated in parentheses.
Results
Inhibition of glycine-induced chloride current by aliphatic n-alcohols
Our previous findings on neonatal VTA neurons can be summarized as follows: Most VTA neurons (82%) were sensitive to glycine. All glycine-induced responses were antagonized by 100 nM strychnine (Ye et al., 1998; Ye, 2000). Acute application of 0.1–40 mM ethanol enhanced (35%) or depressed (45%) glycine responses of VTA neurons (Ye et al., 2001a, b).
To better define the inhibitory actions of alcohols on GlyRs, we tested a series of straight-chain n-alcohols on the currents induced by 30 μM glycine. This concentration of glycine was chosen because it was close to the EC50 value of the VTA neurons of this age group (Ye et al., 1998; Ye, 2000). As shown in Figure 1, 3 mM ethanol markedly decreased the inward current activated by 30 μM glycine, which is consistent with our recent report (Ye et al., 2001b). On average, 3 mM ethanol reduced the peak amplitude of current activated by 30 μM glycine by 36±8% (n=6). Note that neurons used in this study were those in which 3 mM ethanol inhibited the current activated by 30 μM glycine by at least 10%. In agreement with a previous report (Ye et al., 2001b), such inhibition of glycine-activated currents occurred in 45% (71/158) of the neurons. Aliphatic n-alcohols, which have carbon numbers less than nine, suppressed glycine currents in this subset of neurons (see below). As shown in Figure 1, from methanol to nonanol the indicated concentrations also markedly decreased the amplitude of inward current activated by 30 μM glycine. On average, the peak amplitude of current activated by 30 μM glycine was decreased by 32±6% (n=6) by 100 mM methanol, 32±5% (n=5) by 3 mM propanol, 30±6% (n=6) by 1 mM butanol, 28±4% (n=5) by 1 μM pentanol, 33±7% (n=5) by 5 μM hexanol, 28±4% (n=6) by 10 μM heptanol, 31±4% (n=6) by 5 μM octanol and 22±5% (n=4) by 10 μM nonanol. Decanol (10 μM) to dodecanol (10 μM) did not affect the current activated by 30 μM glycine.
Figure 1.
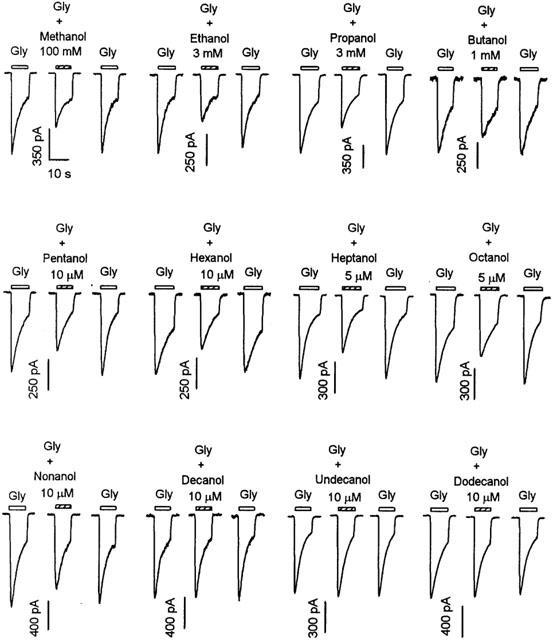
Inhibition of glycine-induced chloride current in VTA neurons by aliphatic n-alcohols from methanol to dodecanol. Currents activated by 30 μM glycine in the absence and presence of alcohols in the concentrations indicated. Each set of records is from a different neuron. Bars above each current trace indicate the duration of drug application. The time scale in the first set of records applies to all records. For all figures, all currents were recorded at a holding potential of −50 mV.
The graph in Figure 2 shows the concentration-response curves for inhibition of glycine-activated current by aliphatic n-alcohols from methanol to decanol. As the carbon chain length increased from methanol to pentanol, the curves successively shifted to the left in a parallel manner. However, the curves for pentanol to nonanol were crossed over. The curve for nonanol shifted to the left of pentanol between the concentration of 1 to 30 μM and to the right of pentanol between 30–10,000 μM. The concentration producing 50% inhibition (IC50) for each alcohol was in (μM) methanol 9.3, ethanol 180, propanol 8, butanol 2, pentanol 0.05, hexanol 0.03, heptanol 0.04, octanol 0.02 and nonanol 0.04. The IC50 value for decanol could not be determined, as maximal attainable concentration of this alcohol did not inhibit glycine-activated current.
Figure 2.
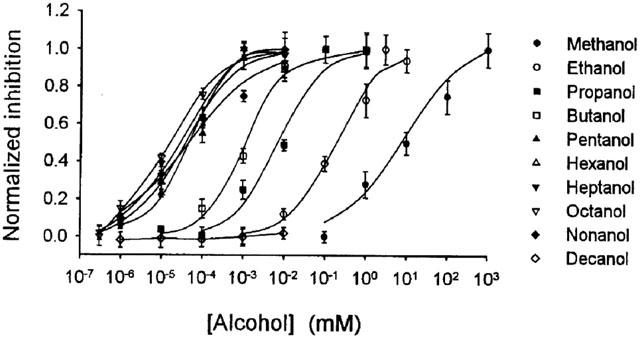
Concentration-response curves for inhibition of glycine-induced current by aliphatic n-alcohols. Data points are means±s.e.mean of five to nine neurons. Curves for alcohols from methanol to nonanol were obtained by fitting the data to the logistic equation given in Methods section. Curves for decanol are point-to-point fits. The IC50 values for decanol could not be determined, as maximal attainable concentration of this alcohol did not inhibit glycine-activated current.
A cutoff in the potency of alcohols for inhibiting the GlyRs
As the molecular size of a series of homologous alcohols is increased, a point is reached at which the biological potency attains a maximum and then declines with further increase in size. This effect of alcohols is named ‘cutoff effect' (Peoples et al., 1996).
We next tested whether a ‘cutoff effect' existed in alcohol's inhibition of the GlyRs. As illustrated in Figure 1, from methanol to nonanol, these alcohols significantly decrease current activated by 30 μM glycine. Alcohol inhibition of glycine-activated current disappears when carbon chain length increases to decanol. To better assess the relationship between the potency and carbon chain length, we plot the relative potency of aliphatic n-alcohols from methanol to decanol for inhibiting GlyRs (ethanol IC50/alcohol IC50) as a function of the carbon chain length. Figure 3 illustrates that alcohol potency for inhibiting GlyRs increased exponentially as the number of carbon atoms was increased from one to five, and reached a maximum from five to nine. Decanol to dodecanol did not inhibit glycine-activated current. Thus, a ‘cutoff' occurred at about decanol.
Figure 3.
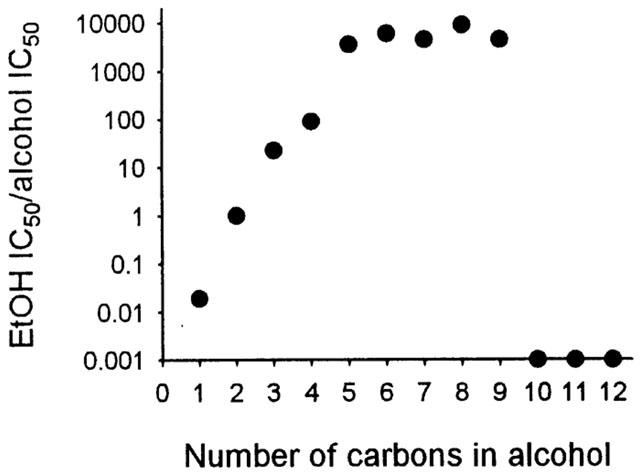
Cutoff in potency of aliphatic n-alcohols for inhibition of GlyRs. Relative potency of aliphatic alcohols for inhibiting GlyRs (ethanol IC50/alcohol IC50) plotted as a function of their carbon chain length. Data points for decanol to dodecanol are shown on the x-axis because IC50 values could not be obtained for these alcohols.
The observation of a cutoff could be explained by postulating that the size of this pocket is too small to contain longer alcohols (from decanol onwards in this case). To test further this possibility, we examined the effects of a coapplication of ethanol with dodecanol. As illustrated in Figure 4, when applied alone, ethanol inhibited, and dodecanol had no effect on the glycine current. However, simultaneous application of dodecanol together with ethanol eliminated the inhibition induced by ethanol. On average, 10 mM ethanol, 10 μM dodecanol, and 10 mM ethanol +10 μM dodecanol altered the peak current induced by 30 μM glycine to 81±3% (n=8), 102±2% (n=8), and 110±11% (n=8) of control, respectively. These data indicate that the size exclusion mechanism may not be adequate to explain the cutoff phenomenon.
Figure 4.
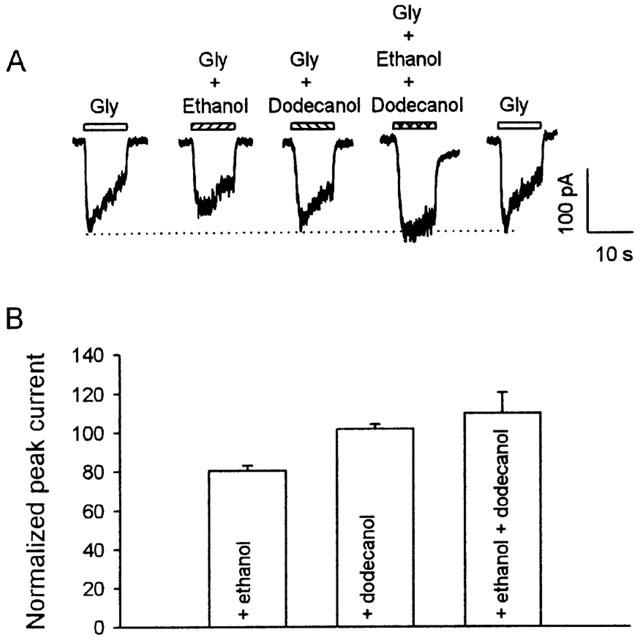
A coapplication of ethanol with dodecanol lessens the inhibition resulting from ethanol. (A) Current-activated by 30 μM glycine was inhibited by 10 mM ethanol, but not by 10 μM dodecanol. A coapplication of 10 mM ethanol with 10 μM dodecanol eliminated ethanol induced inhibition. All current traces were from the same VTA neuron. (B) The bar graph summarizes the normalized inhibition of peak current by 10 mM ethanol, 10 μM dodecanol, and the mixture of these two alcohols.
To further assess the cutoff effect of alcohol inhibition of glycine-activated current, we compared the effects of ethanol and decanol in the same neurons. Figure 5 illustrates that although ethanol significantly inhibited the glycine-activated current, decanol had virtually no effect on the glycine current. In the presence of 0.1 mM decanol, a concentration that would result in membrane alcohol concentration equivalent to that produced by over 1.9 M methanol (McCreery & Hunt, 1978), glycine current was 98.2±2.5% of control (n=6, P>0.05). To assess the possibility that the lack of effect of decanol on glycine-activated current was due to technical problem, such as incomplete dissolution or evaporative loss of the alcohol, we tested also the effect of decanol on GABA-activated current in the same neurons. By contrast, GABA-activated current was greatly increased in amplitude by decanol. On average, 0.1 mM decanol potentiated GABA-activated current to 265±12% of control (n=6, P<0.01).
Figure 5.
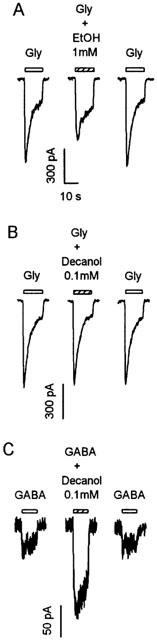
Effects of decanol on glycine (Gly) and GABA-activated currents. Current-activated by 30 μM glycine was inhibited by 1 mM ethanol (A) but not by 0.1 mM decanol (B). By contrast, 0.1 mM decanol potentiated current activated by 10 μM GABA (C). All current traces were from the same VTA neuron.
The above studies used a single concentration (30 μM) of glycine. To further support the cutoff data, we tested also the effect of alcohols on currents induced by a wide range of glycine concentration (3–1000 μM). In accordance with our recent report (Ye et al., 2001b), ethanol inhibition of glycine current depends on glycine concentration. While 1 mM ethanol had a significant inhibitory effect on the current induced by low concentrations of glycine, ethanol had virtually no effect on the response induced by saturating concentration of glycine. Specifically, while 1 mM ethanol inhibited current induced by 30 μM glycine to 73±4% of control (n=10, P<0.05), in the presence of 1 mM ethanol, currents induced by 1000 μM glycine were essentially the same, being 98±4% (n=8, P>0.5) of control. By contrast, in the presence of 10 μM dodecanol, the current induced by 10 and 30 μM glycine were the same, being 98±5% and 97±4% of control (n=5, P>0.5), respectively.
Discussion
In the present study, we investigated the inhibitory effect of n-alcohols on glycine-activated currents in neurons freshly isolated from VTA of 5–14-day-old rats. Our results clearly show that short and long chain alcohols (up to nonanol) inhibit the function of GlyRs in a subset of VTA neurons of neonatal rats, which extend our previous finding on ethanol inhibition of GlyRs function in VTA neurons (Ye et al., 2001b) to the other straight-chain n-alcohols. We demonstrated that alcohol potency for inhibiting GlyRs increased as the number of carbon atoms was increased from one to five, and was at a maximal plateau from five to nine; alcohols with 10 or more carbon atoms did not inhibit glycine-activated current, despite enhanced GABA current, increased lipid solubility and membrane disordering potency (Chiou et al., 1990; Raines et al., 1993; Mascia et al., 1996). Thus, a cutoff point in potency for alcohol inhibition of GlyRs occurred at between nine and 10 carbon atoms.
The potency of a series of aliphatic n-alcohols for producing intoxication was studied previously. Using either loss of righting reflex or ataxia as the experimental index of intoxication, it was found that as the number of carbon atoms was increased from six to eight, the intoxicating potency of the alcohols reached a maximum and then declined, despite increased membrane/buffer partition coefficient and membrane disordering potency (McCreery & Hunt, 1978). However, the underlying mechanisms are unclear. Lyon et al. (1981) noted that the cutoff observed after i.p. injection may be pharmacokinetic, that is the long chain alcohols do not reach the brain. This conclusion is supported by Dildy-Mayfield et al. (1996), who administered decanol intravenously and observed loss of righting reflex, showing that the cutoff is greater than 10. Thus, decanol is effective in vivo, and the apparent cutoff observed in both Lyon et al. (1981) and McCreery & Hunt (1978) are likely due to a lack of delivery of long chain alcohols to the brain.
Note that the cutoff for alcohol intoxication is similar to the cutoff for alcohol inhibition of GlyRs. In addition, previous studies revealed a cutoff point between eight and nine carbon atoms for alcohol inhibition of N-methyl-D-aspartate receptors (Peoples & Weight, 1995), and non-NMDA receptors (Akinshola, 2001). This is close to the cutoff point (between nine and 10 carbon atoms) for alcohol inhibition of GlyR of the native VTA neurons. By contrast, Mascia et al. (1996) reported a cutoff point at about 12 carbon atoms for alcohol potentiation of the homomeric GlyRs expressed in Xenopus oocyte. The mechanisms underlying the difference are unclear. However, in addition to the difference in preparations used, in contrast to the potentiating effect on alcohols on GlyRs expressed in Xenopus oocytes, the inhibitory effect of alcohols was examined in VTA neurons. We are currently studying the cutoff point for the potentiating effect of alcohol for GlyRs in the VTA neurons. As discussed by Peoples et al. (1996), even though an observation of cutoff with respect to the function of a membrane protein could result from the action of the alcohol on either the protein or the surrounding lipids, these possibilities could be identified by the difference between the cutoff point for modulation of the protein and for disordering of the membrane lipids. The present study indicates that n-alcohols exhibit a cutoff in potency for inhibition of native GlyRs between nine and 10 carbon atoms. This cutoff point is significantly lower than the cutoff point reported for alcohol effects on lipids, which is more than 12 carbon atoms (Chiou et al., 1990; Miller et al., 1989; Raines et al., 1993). Thus, it would not be appropriate to attribute the effect of alcohols on GlyRs to membrane disordering.
In the current study, we investigated the basis of the cutoff effect by looking at competition between ethanol and dodecanol. The hypothesis underlying the coapplication experiments of Figure 4 is that longer alcohols may also bind to the receptor, but silently. If the binding site for the alcohols is the same, it would be possible to observe an interaction by simultaneously applying a long chain alcohol together with ethanol. Our data support this hypothesis. The presence of dodecanol eliminated ethanol-induced inhibition of glycine current. This result suggests that the cutoff effect on GlyRs may result from the inability of longer alcohols to change the activation properties of the receptors. The fact that short chain alcohols can but the long chain ones cannot change the activation properties of GlyRs, however, may indicate a contribution of additional factors to the cutoff effect.
It has recently been demonstrated that alcohols interact with multiple intracellular second messenger systems (Slater et al., 1993; Snell et al., 1994; Tao & Ye, 2002), and these interactions may modulate the sensitivity of GlyRs to its agonists. However, the rapid nature of alcohol effect observed in current study weakens the case for an intracellular site of action. Most recently, Peoples & Ren (2002) reported that the cutoff effect observed in inhibition of NMDA receptor-channels by alcohols appears to be attributable primarily to an inability of higher alcohols to achieve adequate aqueous concentrations, rather than an ability to bind to the alcohol site due to their physical dimensions. However, data of Figure 5 are not in favour of this notion, as decanol (0.1 mM) markedly potentiated GABA current but did not inhibit glycine current when measured from the same VTA neurons. One may argue that GABAA receptor function may be potentiated by a lower aqueous concentration of decanol than is required to inhibit GlyR function. Nevertheless, the IC50s of alcohol inhibition of GlyRs of VTA neurons were much lower compared to the EC50s of alcohols to potentiate GABA current in hippocampal neurons (Peoples & Weight, 1999). Thus, the inability of decanol to achieve adequate aqueous concentration may not be the primary mechanism underlying the lack of effect of decanol on GlyRs.
The concentrations of ethanol requires to suppress glycine response are lower than that needed to produce behaviour effects. It is not immediately clear whether ethanol is these levels could produce Foetal Alcohol Effect/Syndrome or any other physiological effect. Recent studies have revealed that neonatal cells have relatively high intracellular Cl−. Therefore, in contrast to their inhibitory effects in adult neurons, both glycine and GABA induced an outward-flux of Cl−, resulting in membrane depolarization (Cherubini et al., 1991; Ye, 2000), and activation of voltage-gated Ca2+ channels (Reichling et al., 1994). This Ca2+ influx is crucial for the formation of gephyrin and GlyR clusters at developing postsynaptic sites (Kirsch & Betz, 1998) and plays an important role in synaptogenesis (Betz et al., 1999). By attenuating growth-promoting increases in cytoplasmic [Ca2+], ethanol-mediated inhibition of GlyR function may be responsible for abnormal CNS development.
Currently, the mechanisms underlying Foetal Alcohol Effects/Syndrome remain unclear. The brain is particularly sensitive to the neurotoxic effects of ethanol during the period of rapid brain growth and synaptogenesis (Ikonomidou et al., 2000). It is possible that the response to ethanol of developing GlyRs differs from that of mature GlyRs. This possibility is supported by our recent observation that 1 mM ethanol potentiated 72% (28/39), or had no effect on 23% (9/39) of the VTA neurons isolated from 27–34-day-old rats prepared in an identical manner (Ye et al., 2001b), which is in sharp contrast to that observed in neonatal rats, where ethanol inhibited the GlyRs in 45% of the neurons.
In summary, our experiments on VTA neurons of neonatal rat show that a series of aliphatic n-alcohols inhibit glycine-induced chloride currents. These alcohols exhibit a ‘cutoff' point in their potency for inhibition of the glycine receptor function between nine and 10 carbon atoms. The inability of longer alcohols to change the activation properties of the receptors may contribute to the cutoff effect.
Acknowledgments
This work was supported by NIAAA, National Institue of Health Grant AA-11989 (to J.H. Ye). The authors are happy to thank Drs Kresimir Krnjevic' and Chaoying Li for their helpful comments on this report, and Dr Zhenglin Jiang for his excellent assistance.
Abbreviations
- GABA
γ-aminobutyric acid receptors
- GlyR
glycine receptor
- NMDA
N-methyl-D-aspartate
- VTA
ventral tegmental area
References
- AGUAYO L.G., PANCETTI F.C. Ethanol modulation of the γ-aminobutyric acidA- and glycine-activated Cl− currents in cultured mouse neurons. J. Pharmacol. Exp. Ther. 1994;270:61–69. [PubMed] [Google Scholar]
- AKINSHOLA B.E. Straight-chain alcohols exhibit a cutoff in potency for the inhibition of recombinant glutamate receptor subunits. Br. J. Pharmacol. 2001;133:651–658. doi: 10.1038/sj.bjp.0704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY P. Axoscope junction potential calculator. Axobits. 1996;18:3–4. [Google Scholar]
- BETZ H. Glycine receptors: heterogeneous and widespread in the mammalian brain. TINS. 1991;14:458–461. doi: 10.1016/0166-2236(91)90045-v. [DOI] [PubMed] [Google Scholar]
- BETZ H., KUHSE J., SCHMIEDEN V., LAUBE B., KIRSCH J., HARVEY R.J. Structure and functions of inhibitory and excitatory glycine receptors. Ann. N.Y. Acad. Sci. 1999;868:667–676. doi: 10.1111/j.1749-6632.1999.tb11343.x. [DOI] [PubMed] [Google Scholar]
- BRODIE M.S., PESOLD C., APPEL S.B. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcoholism: Clin. Exp. Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- CELENTANO J.J., GIBBS T.T., FARB D.H. Ethanol potentiates GABA and glycine induced currents in chick spinal cord neurons. Brain Res. 1988;455:377–380. doi: 10.1016/0006-8993(88)90098-4. [DOI] [PubMed] [Google Scholar]
- CHERUBINI E., GAIARSA J.L., BEN-ARI Y. GABA: an excitatory transmitter in early postnatal life. TINS. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- CHIOU J.S., MA S.M., KAMAYA H., UEDA I. Anesthesia cutoff phenomenon: interfacial hydrogen binding. Science. 1990;248:583–585. doi: 10.1126/science.2159183. [DOI] [PubMed] [Google Scholar]
- DILDY-MAYFIELD J.E., MIHIC S.J., LIU Y., DEITRICH R.A., HARRIS R.A. Action of long chain alcohols on GABAA and glutamate receptors: relation to in vivo effects. Br. J. Pharmacol. 1996;118:378–384. doi: 10.1111/j.1476-5381.1996.tb15413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGBLOM A.C., AKERMAN K.E.O. Effects of ethanol on γ-aminobutyric acid and glycine receptor-coupled Cl− fluxes in rat brain synaptoneurosomes. J. Neurochem. 1991;57:384–390. doi: 10.1111/j.1471-4159.1991.tb03764.x. [DOI] [PubMed] [Google Scholar]
- GATTO G.J., MCBRIDE W.J., MURPHY J.M., LUMENT L., LI T.K. Ethanol self-infusion into the ventral area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- IKONOMIDOU C., BITTIGAU P., ISHIMARU M.J., WOZNIAK D.F., KOCH C., GENZ K., PRICE M.T., STEFOVSKA V., HORSTER F., TENKOVA T., DIKRANIAN K., OLNEY J.W. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- JENKINS A., FRANKS N.P., LIEB W.R. Actions of general anasthetics on 5-HT3 receptors in N1E-115 neuroblastoma cells. Br. J. Pharmacol. 1996;117:1507–1515. doi: 10.1111/j.1476-5381.1996.tb15314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINS A., GREENBLATT E.P., FAULKNER H.J., BERTACCINI E., LIGHT A., LIN A., ANDREASEN A., VINER A., TRUDELL J.R., HARRISON N.L. Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J. Neurosci. 2001;21:RC136. doi: 10.1523/JNEUROSCI.21-06-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSCH J., BETZ H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature. 1998;392:717–720. doi: 10.1038/33694. [DOI] [PubMed] [Google Scholar]
- KOOB G.F., BLOOM F.E. Cellular and molecular mechanisms of drug dependence. Science. 1988;24:2715–2723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC' K. Role of GABA in cerebral cortex. Can. J. Physiol. Pharmacol. 1997;75:439–451. [PubMed] [Google Scholar]
- LANGOSCH D., BECKER C.M., BETZ H. The inhibitory glycine receptor: a ligand-gated chloride channel of the central nervous system. Eur. J. Biochem. 1990;194:1–8. doi: 10.1111/j.1432-1033.1990.tb19419.x. [DOI] [PubMed] [Google Scholar]
- LI C., PEOPLES R.W., WEIGHT F.F. Alcohol action on a neuronal membrane receptor: Evidence for a direct interaction with the receptor protein. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8200–8204. doi: 10.1073/pnas.91.17.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVINGER D.M., WHITE G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol. Pharmacol. 1991;40:263–270. [PubMed] [Google Scholar]
- LYON R.C., MCCOMB J.A., SCHREURS J., GOLDSTEIN D.B. A relationship between alcohol intoxication and the disordering of brain membranes by a series of short-chain alcohols. J. Pharmacol. Exp. Ther. 1981;218:669–675. [PubMed] [Google Scholar]
- MACHU T.K., HARRIS R.A. Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. J. Pharmacol. Exp. Ther. 1994;271:898–905. [PubMed] [Google Scholar]
- MASCIA M.P., MACHU T.K., HARRIS R.A. Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br. J. Pharmacol. 1996;119:1331–1336. doi: 10.1111/j.1476-5381.1996.tb16042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCREERY M.J., HUNT W.A. Physico-chemical correlates of alcohol intoxication. Neuropharmacology. 1978;17:451–461. doi: 10.1016/0028-3908(78)90050-3. [DOI] [PubMed] [Google Scholar]
- MCKENZIE D., FRANKS N.P., LIEB W.R. Actions of general anaesthetics on a neuronal nicotinic acetylcholine receptor in isolated identified neurones of Lymnaea stagnalis. Br. J. Pharmacol. 1995;115:275–282. doi: 10.1111/j.1476-5381.1995.tb15874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIHIC S.J., HARRIS R.A. Potentiation of GABAergic currents by a series of alcohols: studies of receptor composition and the cut-off phenomenon. Alcohol. Clin. Exp. Res. 1995;19:7A. [Google Scholar]
- MILLER K.W., FIRESTONE L.L., ALIFIMOFF J.K., STREICHER P. Nonanesthetic alcohols dissolved in synaptic membranes without perturbing their lipids. Proc. Natl. Acad. Sci. U.S.A. 1989;86:1084–1087. doi: 10.1073/pnas.86.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRELL R.D., BRAUN M.S., HAYDON D.A. Action of n-alcohols on nicotinic acetylcholine receptor channels in cultured rat myotubes. J. Physiol. 1991;437:431–448. doi: 10.1113/jphysiol.1991.sp018604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEOPLES R.W., REN H. Inhibition of N-methyl-D-aspartate receptors by straight-chain diols: implications for the mechanism of the alcohol cutoff effect. Mol. Pharmacol. 2002;61:169–176. doi: 10.1124/mol.61.1.169. [DOI] [PubMed] [Google Scholar]
- PEOPLES R.W., WEIGHT F.F. Cutoff in potency implicates alcohol inhibition of N-methyl-D-aspartate receptors in alcohol intoxication. Proc. Natl. Acad. Sci. U.S.A. 1995;92:2825–2829. doi: 10.1073/pnas.92.7.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEOPLES R.W., WEIGHT F.F. Differential alcohol modulation of GABAA and NMDA receptors. Neuroreport. 1999;10:97–101. doi: 10.1097/00001756-199901180-00019. [DOI] [PubMed] [Google Scholar]
- PEOPLES R.W., CHAOYINGAND L.I., WEIGHT F.F. Lipid vs protein theories of alcohol action in the nervous system. Ann. Rev. Pharmacol. Toxicol. 1996;36:185–201. doi: 10.1146/annurev.pa.36.040196.001153. [DOI] [PubMed] [Google Scholar]
- RAINES D.E., KORTEN S.E., HILL W.A.G., MILLER K.W. Anesthetic cutoff in cycloalkanemethanols: a test of current theories. Anesthesiology. 1993;78:918–927. doi: 10.1097/00000542-199305000-00017. [DOI] [PubMed] [Google Scholar]
- REICHLING D.B., KYROZIS A., WANG J., MACDERMOTT A.B. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J. Physiol. 1994;476:411–421. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGER L., PAPKE A., GREY C., STEPHEN F.H. Inhibition of nicotinic receptors by Bis (2,2,6,6-tetramethyl-4-piperidinyl) sebacate (Tinuvin 770), an additive to medical plastics. J. Pharmacol. Exp. Ther. 1994;268:718–726. [PubMed] [Google Scholar]
- SLATER S.J., COX K.J.A., LOMBARDI J.V., HO C., KELLY M.B., RUBIN E., STUBBS C.D. Inhibition of protein kinase C by alcohols and anaesthetics. Nature. 1993;364:82–84. doi: 10.1038/364082a0. [DOI] [PubMed] [Google Scholar]
- SNELL L.D., TABAKOFF B., HOFFMAN P.L. Protein kinase C activation attenuates N-methyl-D-aspartate-induced increases in intracellular calcium in cerebellar granule cells. J. Neurochem. 1994;62:1783–1789. doi: 10.1046/j.1471-4159.1994.62051783.x. [DOI] [PubMed] [Google Scholar]
- TAO L., YE J.H. Protein kinase C modulation of ethanol inhibition of glycine-activated current in dissociated neurons of rat ventral tegmental area. J. Pharmacol. Exp. Ther. 2002;300:967–975. doi: 10.1124/jpet.300.3.967. [DOI] [PubMed] [Google Scholar]
- WERMAN R., DAVIDOFF R.A., APRISON M.H. Inhibitory action of glycine on spinal neurons in the cat. J. Neurophysiol. 1968;31:81–95. doi: 10.1152/jn.1968.31.1.81. [DOI] [PubMed] [Google Scholar]
- WICK M.J., MIHIC S.J., UENO S., MASCIA M.P., TRUDELL J.R., BROZOWSKI S.J., YE Q., HARRISON N.L., HARRIS R.A. Mutations of gamma-aminobutyric acid and glycine receptors change alcohol cutoff: evidence for an alcohol receptor. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6504–6509. doi: 10.1073/pnas.95.11.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISE R.A. Additive drugs and brain stimulation reward. Ann. Rev. Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- YE J.H. Physiology and pharmacology of native glycine receptors in developing rat ventral tegmental area neurons. Brain Res. 2000;862:74–82. doi: 10.1016/s0006-8993(00)02073-4. [DOI] [PubMed] [Google Scholar]
- YE J.H., REN J., LIU P.L., MCARDLE J.J. Glycine-activated chloride currents of neurons freshly isolated from the ventral tegmental area of rats. Brain Res. 1998;796:53–62. doi: 10.1016/s0006-8993(98)00317-5. [DOI] [PubMed] [Google Scholar]
- YE J.H., TAO L., REN J., SCHAFFER R., KRNJEVIC' K., LIU P.L., SCHILLER D.A., MCARDLE J.J. Ethanol potentiation of glycine-induced responses in dissociated neurons of rat ventral tegmental area. J. Pharmacol. Exp. Ther. 2001a;296:77–83. [PubMed] [Google Scholar]
- YE J.H., TAO L., ZHU L.I., KRNJEVIC' K., MCARDLE J.J. Ethanol inhibition of glycine-activated response in neurons of rat ventral tegmental area of neonatal rats. J. Neurophysiol. 2001b;86:2426–2434. doi: 10.1152/jn.2001.86.5.2426. [DOI] [PubMed] [Google Scholar]


