Abstract
The mediators of nonadrenergic, noncholinergic (NANC) relaxation in longitudinal muscle of the jejunum and ileum of Wistar rats were examined in vitro.
Treatment of the jejunal and ileal segments with α-chymotrypsin resulted in decreases in the NANC relaxations induced by electrical field stimulation (EFS) by about one half.
The NANC relaxations were also decreased by about one half after the segments had been desensitized to neurotensin. A neurotensin receptor antagonist, SR48692 (10 μM) inhibited the NANC relaxation by 56 and 34% in the jejunal and ileal segments, respectively.
An inhibitor of small conductance Ca2+-activated K+ channel (SK channel), apamin (100 nM) also inhibited the NANC relaxation by 83 and 63%, respectively. Exogenous neurotensin-induced relaxations of the two segments were abolished by apamin.
In the ileal segments, NG-nitro-L-arginine (L-NOARG, 100 μM), inhibited the NANC relaxation by 43%. L-NOARG, but not apamin, further inhibited the relaxation which persisted after the desensitization to neurotensin. Apamin with SR48692 inhibited the relaxation only to the same extent as apamin alone.
EFS induced inhibitory junction potentials (i.j.ps) in the longitudinal muscle cells of the ileum. I.j.ps consisted of a rapid and a delayed phase. L-NOARG significantly inhibited only the delayed phase.
EFS induced only a rapid i.j.ps in the jejunum. SR48692 and apamin inhibited the i.j.ps.
These findings suggest that neurotensin and unknown substance(s) mediate NANC relaxation via SK channels in the jejunum of Wistar rats, and that neurotensin via SK channels and nitric oxide not via SK channels separately mediate the relaxation in the ileum.
Keywords: Neurotensin, NANC relaxation, rat jejunum, rat ileum, apamin, nitric oxide
Introduction
In the rat intestine, nitric oxide has been suggested to participate in nonadrenergic noncholinergic (NANC) relaxation of longitudinal muscle. However, an extent of its participation was different among intestinal regions. For example, in NANC relaxation of longitudinal muscle of 8-week-old Wistar rat intestine, nitric oxide has a very significant role in the proximal colon and a moderate role in the ileum and the distal colon, but no role in the jejunum and rectum (Takeuchi et al., 1998). Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating peptide (PACAP) were also suggested to partially participate the relaxation only in the distal colon among the regions studied (Okishio et al., 2000). These results suggest that nitric oxide solely mediates NANC relaxation in the proximal colon and nitric oxide, VIP and PACAP mediate that in the distal colon of Wistar rats. Thus, the mediators of NANC relaxation in the regions other than the proximal and distal colon of Wistar rat intestine have not been determined thoroughly.
A high concentration of neurotensin is present in the gastrointestinal tract of the rat (Carraway & Leeman, 1976) as well as the bovine (Kitabgi et al., 1976) and human intestine (Hammer et al., 1980). A relaxant effect of neurotensin was first reported in the rat duodenum (Carraway & Leeman, 1973). Subsequent studies also show that neurotensin induces relaxation of the rat ileum (Kitabgi & Freychet, 1978) and the proximal colon (Mulè & Serio, 1997), the dog gastric corpus (McLean & Fox, 1983), the guinea-pig ileum (Goedert et al., 1984) and colon (Kitabgi & Vincent, 1981). The role of neurotensin as a mediator of NANC relaxation was first suggested by Goedert et al. (1984) according to indirect evidence that apamin, an antagonist of small conductance Ca2+-activated K+ channel (SK channel, Castle et al., 1989), inhibits both the neurotensin- and the nerve stimulation-induced relaxations of the ileal smooth muscle of guinea-pigs. However, no persuasive evidence for the role of neurotensin in NANC relaxation has been presented so far. In the present study the mediator of NANC relaxation in longitudinal muscle of the jejunum and ileum of 8-week-old Wistar rats was searched. A role of neurotensin as a mediator of the relaxation in these intestinal regions will be shown.
Methods
Preparations of jejunal and ileal segments of rat intestine
Male Wistar rats (8-week-old) purchased from JCL Inc. (Osaka, Japan) were used in the present study. Although we had indicated the strain of rats used as just Wistar in our previous papers, Wistar-ST rats belonging to a subclass of Wistar strain were used in those studies. The rats were lightly anaesthetized with ether and then stunned by a blow on the head and bled via the carotid arteries. Segments of the jejunum and ileum were removed and placed in Tyrode solution consisting of (in mM): NaCl 137, KCl 2.7, CaCl2 1.8, MgCl2 1.1, NaH2PO4 0.42, NaHCO3 11.9 and glucose 5.6. The contents of the excised segments were gently flushed out with Tyrode solution. Jejunal and ileal segments, 2.5 cm in length, were excised from the proximal part of the jejunum and the central part of the ileum, respectively.
Recording of responses of longitudinal muscle of rat jejunum and ileum to electrical field stimulation (EFS)
Intact segments of the jejunum and ileum were suspended in an organ bath containing 5 ml of Tyrode solution maintained at 37°C and bubbled with 95% O2: 5% CO2. One end of each segment was attached to a transducer and the other end was mounted on an anodal electrode placed at the bottom of the bath. Atropine (1 μM) and guanethidine (5 μM) were present throughout the experiment to block cholinergic and adrenergic responses, respectively. After an equilibration period of 30 min, responses of the longitudinal muscle to EFS with trains of 100 pulses of 0.5-ms width, 30 V intensity, and 0.3–10 Hz frequency, were recorded isotonically with a 10-min interval between tests. Drugs were added to the bathing fluid, when responses to EFS became reproducible. The longitudinal muscle was subjected to a resting load of 0.75 g. Since apamin shortened only duration of the relaxation without affecting amplitude at lower concentrations and it at higher concentrations inhibited the amplitude, too (see the text and Figure 5), the extent of relaxation was expressed as the area under the line of resting tone that was drawn on the bottom of resting spontaneous contractile activity (broken lines in Figure 1) as described elsewhere (Kishi et al., 2000) throughout the present study.
Figure 5.
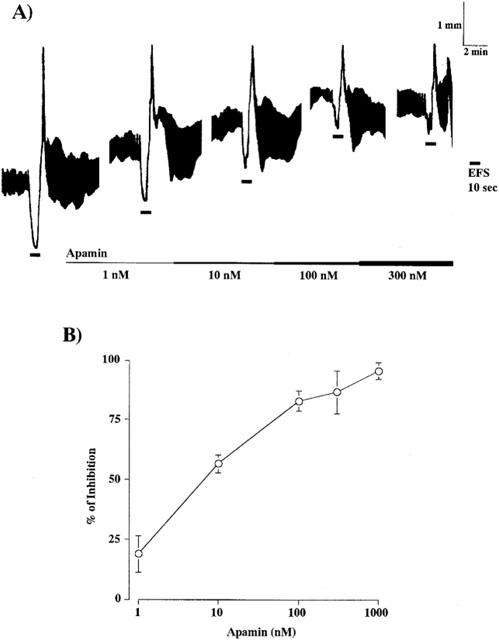
Effect of apamin on EFS-induced relaxation of longitudinal muscle in the jejunum. (A) Relaxations were induced by EFS at 10 Hz for 10 s before and after the treatment with apamin at the indicated concentrations. The lines indicate the presence of apamin in the bathing fluid. (B) Summary of effect of apamin on EFS-induced relaxation. Relaxations in the presence of indicated concentrations of apamin are expressed as a percentage of those obtained before the addition of apamin. Values are means±s.e.mean (vertical bars) for three to six experiments. For further details, see legend of Figure 1.
Figure 1.
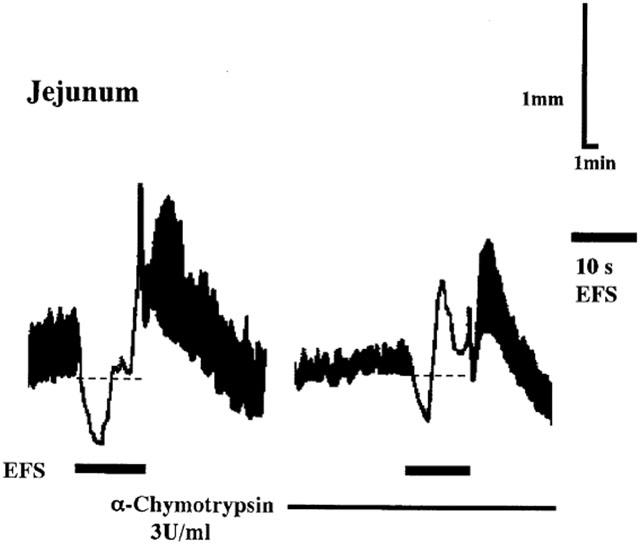
Effect of α-chymotrypsin on EFS-induced relaxation of longitudinal muscle in the jejunum of Wistar rats. When repetitive EFS at 10 Hz for 10 s led to reproducible relaxation, jejunal segments were treated with α-chymotrypsin (3 U ml−1) for 20 min. The relaxation after the treatment was compared to the control relaxation immediately before the treatment. The continuous line indicates the presence of α-chymotrypsin in the bathing fluid. After recording normal spontaneous movements, the chart was run at a fast speed immediately before the stimulation to make the relaxant response clear. Black bold lines indicate the duration of EFS for 10 s.
Treatment of the jejunal and ileal segments with α-chymotrypsin
Jejunal and ileal segments were treated with α-chymotrypsin by adding the agent to the organ bath at a final concentration of 3 units ml−1, which is known to reduce the response of the tissue to peptide agonists (De Beurme & Lefebvre, 1987). Also in our preliminary experiments, exogenously added neurotensin did not affect mechanical responses of the segments at all after a pretreatment of the segments with this concentration of α-chymotrypsin for 20 min at 37°C.
Recording of membrane potentials in longitudinal muscle of jejunum and ileum
The segments of the jejunum and ileum were mounted in a 1.5 ml organ bath maintained at 30°C and perfused continuously with Tyrode solution at a rate of 3 ml min−1. This temperature allowed stable recording of the membrane potentials, since the spontaneous and evoked mechanical responses were reduced. Atropine (1 μM) and guanethidine (5 μM) were added to the bathing solution throughout the experiment. Membrane potentials were recorded with a conventional glass microelectrode filled with 3 M KCl with a resistance of 50–80 MΩ. Inhibitory junction potentials were elicited by EFS to intramural nerves within the segment with square-wave pulses of 0.5 ms duration at an appropriate intensity (10–30 V). The electrode impalement was made into the longitudinal muscle cells of the superficial layer from the serosal side (Takewaki & Ohashi, 1977). The stimulus pulses were delivered with a pair of Ag-AgCl wire electrodes, one on the serosal surface 1–2 mm away from the impaled glass microelectrode and the other in the solution. The distance between the two electrodes was about 20 mm.
Drugs
The neurotensin antagonist SR48692 was a kind gift from Sanofi Recherche, Toulouse, France. NG-Nitro-L-arginine (L-NOARG), α-chymotrypsin and apamin were purchased from Sigma Chemical Co., St. Louis, U.S.A. Neurotensin was from the Peptide Institute, Osaka, Japan. Atropine sulphate and tetrodotoxin were from Wako Pure Chemical, Osaka, Japan. 1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one (ODQ) was from Dojindo Laboratories, Kumamoto, Japan. Drugs were added to the organ bath in a volume of less than 1.0% of the bathing solution. SR48692 was dissolved in dimethyl sulphoxide at stock solution. The final dimethyl sulphoxide concentration was 0.1%, which did not have any effect on preparations. These volumes of the vehicle of the drugs, redistilled water, did not affect the spontaneous contractile activity or muscle tone.
Results
Effects of α-chymotrypsin on EFS-induced NANC relaxation of longitudinal muscle of the jejunum and ileum of Wistar rats
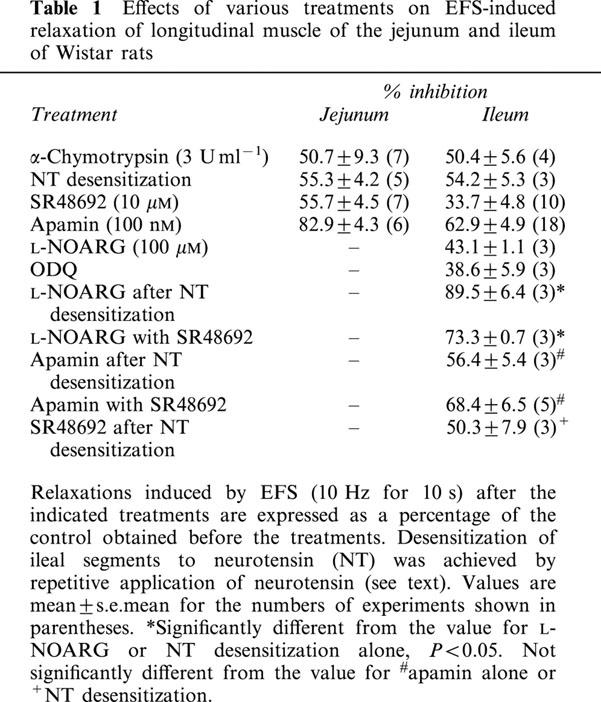
The effect of α-chymotrypsin treatment was examined to determine the possible participation of peptidergic neurons in NANC relaxation of longitudinal muscle of the jejunum and ileum. Treatment of the segments of the jejunum and ileum with α-chymotrypsin (3 units ml−1) for 30 min had no significant effect on the muscle tone, spontaneous contractile activity, nor EFS-induced off contraction. In some preparations, however, this treatment moderately inhibited the spontaneous contractile activity and slightly decreased the muscle tone. In the segments of the jejunum, treatment with α-chymotrypsin for 20 min resulted in a decrease in EFS-induced NANC relaxation by 51% (Figure 1; areas under the resting tone in this experiment were 640 and 270 in arbitrary unit for the relaxations before and after the treatment, respectively. Data are summarized in Table 1). The treatment of the segments of the ileum also resulted in a decrease in the relaxation by 50% (Table 1). These data suggest that some inhibitory peptidergic neurons mediate NANC relaxation. In our previous study, the relaxations in the jejunum and ileum were not affected by a VIP antagonist and a PACAP antagonist (Okishio et al., 2000). So we next studied a possible role of neurotensin as one of candidates for the peptide mediator of the relaxation.
Table 1.
Effects of various treatments on EFS-induced relaxation of longitudinal muscle of the jejunum and ileum of Wistar rats
Effects of neurotensin and a neurotensin antagonist on motility and EFS-induced NANC relaxation in jejunal and ileal segments
Neurotensin at 100 nM caused slow gradual relaxation of longitudinal muscle of the jejunum and inhibited the spontaneous contractile activity. Extent of the relaxation induced by 100 nM neurotensin was 44.7±4.2% (n=5) of the maximum relaxation induced by 30 μM papaverine. However, the spontaneous contractile activity and muscle tone were restored within 10 min, even in the presence of neurotensin. Repetitive application of 100 nM neurotensin, every 10 min without washing, markedly decreased the sensitivity of the segments to neurotensin, and complete desensitization was consistently achieved after 2 or 3 consecutive applications of neurotensin. EFS-induced NANC relaxation significantly decreased in the desensitized preparations by 55% (Table 1). Similar desensitization to neurotensin and a decrease in the NANC relaxation after desensitization were also observed in the ileal segment (Figure 2, Table 1).
Figure 2.
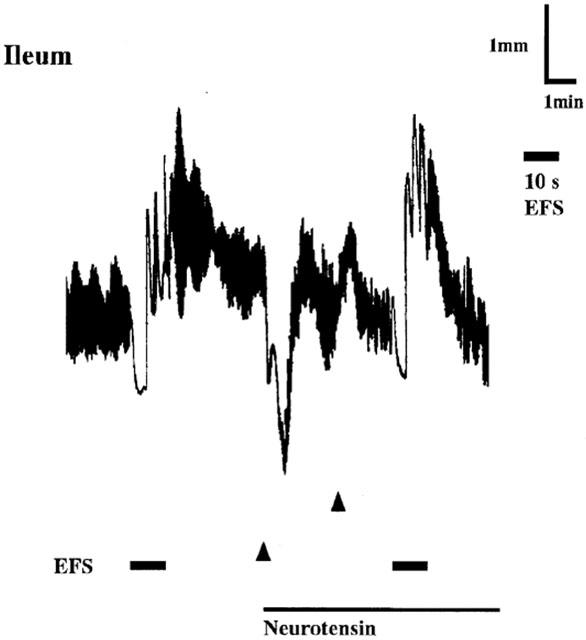
EFS-induced relaxation of longitudinal muscle in the ileal segments before and after desensitization of the preparation to neurotensin. Neurotensin (100 nM) was added at the times indicated by triangles. The line indicates the presence of neurotensin. For further details, see legend of Figure 1.
In another series of experiments, we examined the inhibitory effect of the desensitization on relaxation induced by EFS at different frequencies. The magnitude of the inhibition in the jejunum and ileum was roughly equal at different frequencies (Figure 3). In subsequent studies, the segments were stimulated at 10 Hz with good reproducibility of response.
Figure 3.
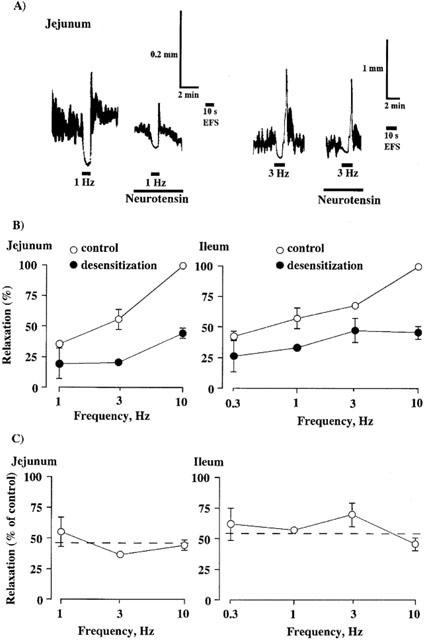
Inhibitory effects of desensitization of the preparations to neurotensin on relaxations induced by EFS at various frequencies in the jejunal and ileal segments. (A) Relaxations were induced by EFS at 1 Hz (left) or 3 Hz (right) for 10 s before (control) and after the desensitization to neurotensin (100 nM) in the jejunal segments. For further details, see legends of Figures 1 and 2. (B) Relaxations induced by EFS at the various frequencies for 10 s before and after the desensitization are summarized. Values are expressed as percentages of control relaxation induced at 10 Hz. (C) Inhibitory effects of the desensitization on relaxation at various frequencies are expressed as percentages of those obtained before the desensitization. Values are expressed as percentages of the corresponding control relaxation. Points are means±s.e.mean for three to seven experiments.
SR48692 at 10 μM, a neurotensin receptor antagonist (Labbé-Jullié et al., 1995), had a slight inhibitory effect on the basal tone but no effect on spontaneous contractile activity of the longitudinal muscle of the jejunum and ileum. SR48692 partially inhibited the NANC relaxation induced by EFS at 10 Hz for 10 s in a concentration dependent manner, causing maximum inhibition at 3 μM within 20–40 min in the jejunum (Figure 4 and Table 1). SR48692 also partially inhibited the relaxation in the ileum, although it was less effective, resulting in 34% inhibition at 10 μM (Figure 4, Table 1). We were not able to examine higher concentrations than 10 μM owing to its significant inhibitory effect on the muscle tone under the present experimental conditions. Neurotensin-induced relaxations were significantly inhibited by 10 μM SR48692 but not affected by tetrodotoxin (1 μM) (data not shown).
Figure 4.
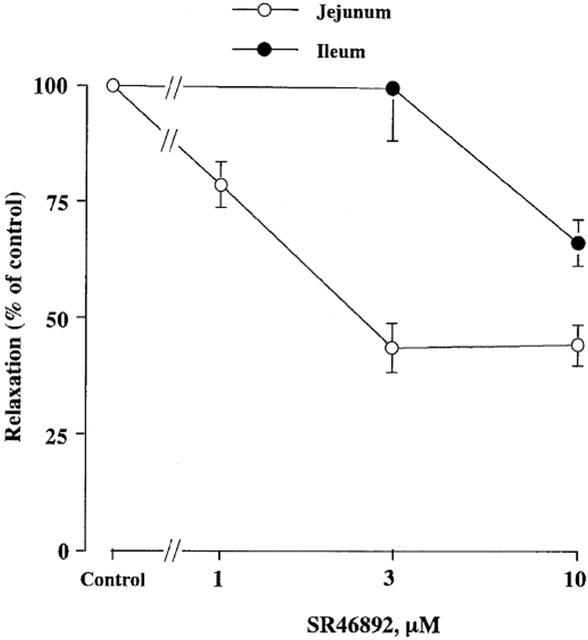
Effects of SR48692 on EFS-induced relaxation of longitudinal muscle in the jejunum and ileum. Relaxations were induced by EFS at 10 Hz for 10 s before (control) and after the treatment with various concentrations of SR48692 indicated. Values are expressed as percentages of the corresponding control relaxation. Points are means±s.e.mean for five to seven experiments.
Effects of apamin on EFS- and exogenous neurotensin-induced relaxations in jejunum
In our previous study using the Wistar-ST rat jejunum, it was suggested that major component of NANC relaxation in the preparation was an unknown substance-mediated apamin-sensitive one that was inhibited by α-chymotrypsin treatment (Niioka et al., 1997), so we next examined the effect of apamin on EFS- and exogenous neurotensin-induced relaxation in the jejunum. Apamin had slight stimulatory effects on the muscle tone and spontaneous contractile activity of the jejunal segments. Apamin at concentrations ranging from 1 nM to 1 μM concentration-dependently inhibited EFS-induced relaxation and at 100 nM, maximally inhibited by 83% within 10 min (Figure 5, Table 1). However, it did not show any effect on EFS-induced off contraction. Apamin at 100 nM abolished relaxation induced by 100 nM neurotensin added exogenously (n=3). These results suggest that neurotensin and unknown mediator(s) induce NANC relaxation of longitudinal muscle of the jejunum in the Wistar rat via opening of apamin-sensitive K+ channels.
Effects of apamin on EFS- and exogenous neurotensin-induced relaxations in ileum
Apamin also had slight stimulatory effects on the muscle tone and spontaneous contractile activity of the ileal segments. Apamin at 100 nM inhibited EFS-induced relaxation by 63% within 10 min (Table 1). Apamin at 100 nM abolished the neurotensin (100 nM)-induced relaxation (n=5). These results suggest that neurotensin induces NANC relaxation of longitudinal muscle of the ileum via opening of apamin-sensitive K+ channels.
Correlation between the neurotensin- and nitric oxide-mediated components in NANC relaxation of the ileum of Wistar rats
Since nitric oxide was shown to partially mediate NANC relaxation of longitudinal muscle of the ileum, but not jejunum, in 8-week-old Wistar rats (Takeuchi et al., 1998), we next examined correlation between the neurotensin- and nitric oxide-mediated NANC relaxation in the ileum.
An inhibitor of nitric oxide synthase, NG-nitro-L-arginine (L-NOARG) at 100 μM partially inhibited EFS-induced NANC relaxation by about 45% as shown previously (Takeuchi et al., 1998). ODQ (1 μM), an inhibitor of soluble guanylyl cyclase, also inhibited the relaxation by about 40%. L-NOARG significantly inhibited the relaxation within 20 min which persisted after desensitization of the ileal segments to neurotensin (Table 1). L-NOARG (100 μM) together with SR48692 (10 μM) inhibited the relaxation more significantly than inhibitions induced by each drug alone (Table 2). On the other hand, SR48692 (10 μM) and apamin (100 nM) did not have any significant effect on the relaxation which persisted after the desensitization. Apamin (100 nM) together with SR48692 (10 μM) did not result in a greater inhibition than apamin alone (Table 1). L-NOARG (100 μM) did not affect the neurotensin (100 nM)-induced relaxation (data not shown). Thus, neurotensin and nitric oxide are suggested to separately mediate NANC relaxation of longitudinal muscle of the Wistar rat ileum.
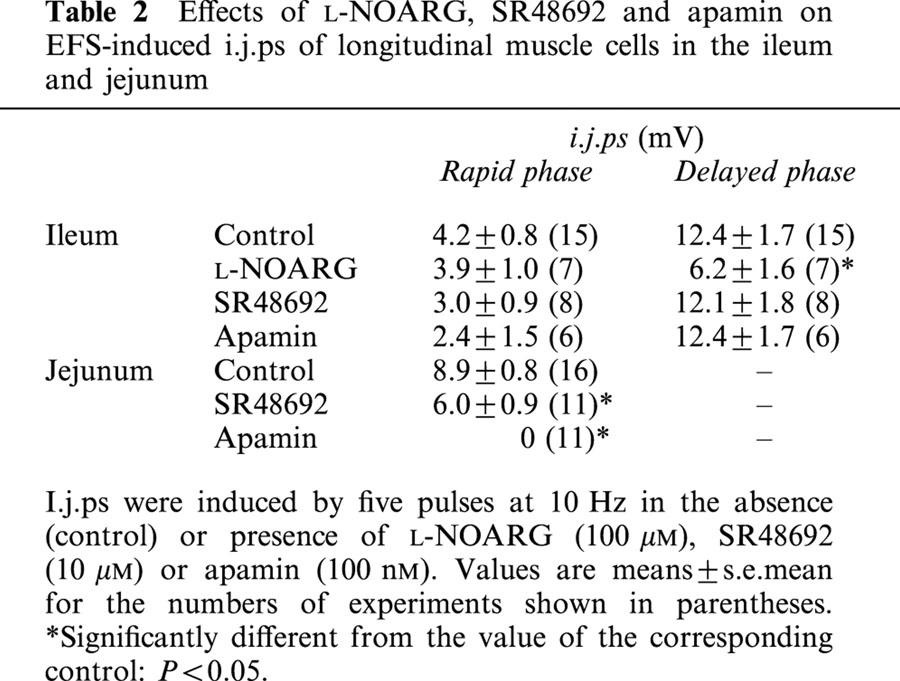
Table 2.
Effects of L-NOARG, SR48692 and apamin on EFS-induced i.j.ps of longitudinal muscle cells in the ileum and jejunum
Effects of L-NOARG and SR48692 on the changes in membrane potentials induced by EFS or neurotensin in longitudinal muscle cells of the ileum and jejunum of Wistar rats
The resting membrane potential of longitudinal muscle cells of the ileum was −55.6±2.8 mV (n=39). In the presence of atropine (1 μM) and guanethidine (5 μM), EFS with five pulses at 10 Hz induced inhibitory junction potentials (i.j.ps) which consisted of two phases, rapid and subsequent slow hyperpolarization. L-NOARG at 100 μM significantly inhibited the delayed phase, slow hyperpolarization, but it did not affect the rapid phase (Figure 6, Table 2). On the other hand, SR48692 at 10 μM and apamin at 100 nM did not show a significant effect on the two phases (Table 2).
Figure 6.
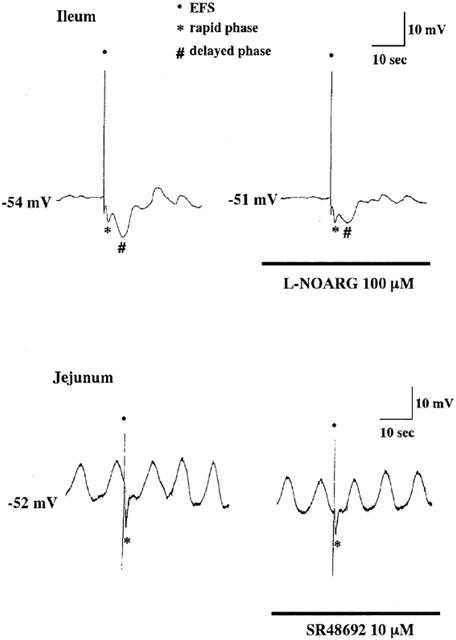
Effects of L-NOARG and SR48692 on EFS-induced i.j.ps in the longitudinal muscle cell of the ileal and jejunal segments, respectively. I.j.ps were induced by five pulses at 10 Hz in the absence or presence of 100 μM L-NOARG (ileum, upper traces) or 10 μM SR48692 (jejunum, lower traces). Atropine (1 μM) and guanethidine (5 μM) were added to the bathing fluid throughout the experiment. The smooth muscle cells were treated with the drugs for 10 min before EFS. EFS was applied at the points indicated by the dots. Following the artifact noise with large amplitude i.j.ps with two phases, rapid and delayed one or single rapid phase were induced in the ileum or jejunum, respectively. Note that in the L-NOARG (in the ileum) and SR48692 (in the jejunum) selectively inhibited the slow and rapid phase, respectively.
The resting membrane potential of longitudinal muscle cells of the jejunum was −53.6±1.1 mV (n=43). EFS induced mono-phasic rapid i.j.ps, amplitude of which was larger than that of the rapid phase in the ileum cells (Figure 6, Table 2). SR48692 and apamin inhibited the i.j.ps significantly and completely, respectively (Figure 6, Table 2).
Bath application of neurotensin at 100 nM did not have any significant effect on membrane potential of longitudinal muscle cells of the ileum, but it at 1 μM induced slow hyperpolarization in the cells (4.8±1.4 mV, n=6). Apamin at 100 nM completely inhibited the hyperpolarization (Figure 7).
Figure 7.
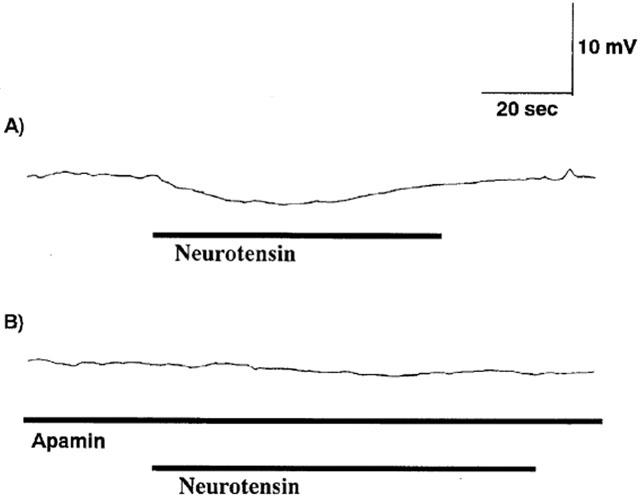
Effects of neurotensin in the absence or presence of 100 nM apamin on membrane potential of longitudinal smooth muscle cell of the ileum. (A) Exogenously added neurotensin at 1 μM induced slow hyperpolarization. (B) Inhibition of NT-induced hyperpolarization by apamin (100 nM). Lines indicate the presence of drugs. Apamin was added 10 min before NT-treatment. Records in (A) and (B) were from the same longitudinal muscle cell.
Discussion
Although a role of neurotensin as a mediator of NANC relaxation had been suggested in the ileum of the guinea-pig (Goedert et al., 1984), there has been no report of this in the rat intestine. This report suggests association of neurotensin with NANC relaxation in the jejunum and ileum of Wistar rat by using a selective neurotensin receptor antagonist, SR48692 (Gully et al., 1993, 1997) and a desensitization method. Since the association was not found in the proximal colon (Hata et al., 1990), distal colon and rectum (data not shown), the regional difference of neurotensin as a mediator of NANC relaxation was also shown to be similar to that of other NANC mediators (Hata et al., 2000).
The mediators of NANC relaxation in the ileum of 8-week-old Wistar rat
Previously nitric oxide was suggested to partially participate in the NANC relaxation in the Wistar rat ileum: 43% of the relaxation was nitric oxide-mediated component (Takeuchi et al., 1998).
Neurotensin was suggested to be associated with about one half of the relaxation based on the results obtained by SR48692 and a desensitization method. L-NOARG, but not apamin, further inhibited the relaxation which persisted after treatment with SR48692 or after desensitization to neurotensin of the segments. These results suggest that neurotensin, via opening of SK channels, and nitric oxide, via SK channel-independent mechanism, separately mediate the NANC relaxation in the ileum of Wistar rats. However, L-NOARG significantly inhibited the delayed i.j.ps, but inhibitory effects of SR48692 and apamin on the rapid phase were not statistically significant (Table 2). Because the rapid phase induced by EFS in the ileum was too small to confirm the statistical significance of the changes in the presence of the both inhibitors, the results do not necessarily deny the results of mechanical responses.
The mediator of NANC relaxation in the jejunum of 8-week-old Wistar rats
Neurotensin was suggested to mediate about one half of the NANC relaxation in the jejunum in the present study, but no participation of nitric oxide was shown in the previous study (Takeuchi et al., 1998). Apamin inhibited the relaxation by 80%. Apamin also very significantly inhibited EFS-induced i.j.ps. Therefore, it seems that neurotensin and unknown mediator(s) participate the relaxation via opening of SK channels in the jejunum of 8-week-old Wistar rats.
Association of SK channels with NANC relaxation
Involvement of SK channels in exogenous neurotensin-induced relaxation of intestinal smooth muscle was first suggested in the guinea-pig proximal colon and the rabbit ileum. It has been reported that apamin inhibited the exogenous neurotensin-induced relaxation in the mouse distal colon, the rat ileum and duodenum, the canine intestine and the guinea-pig jejunum and ileum (for review see Hata et al., 2000). Involvement of SK channels in neurotensin-mediated neurogenic relaxation was first suggested in the guinea-pig ileum (Goedert et al., 1984). Association of SK channels with neurotensin-mediated neurogenic relaxation in the jejunum and ileum was also suggested in the present study.
However, our previous studies indicate that NANC relaxation mediated by pituitary adenylate cyclase activating peptide (PACAP) in the distal colon of Wistar-ST rats is associated with SK channels (Kishi et al., 1996; Takeuchi et al., 1999). Nitric oxide-mediated NANC relaxation was also suggested to be associated with the SK channels in the various tissue preparations (He & Goyal, 1993; Matsuyama et al., 1999; Watson et al., 1996; Keef et al., 1993). These results indicate that the NANC relaxant mediator which is associated with SK channels differs among regions of the gastrointestinal tract and species of animals. Therefore, the mechanism of coupling of the NANC relaxant mediators and the SK channels seems one of the most interesting theme to be solved.
Association of nitric oxide-mediated NANC relaxation with i.j.ps in longitudinal muscle cells of the ileum
Inhibitors of nitric oxide synthesis moderately or significantly inhibited EFS-induced i.j.ps in circular smooth muscle cells of the various tissues such as the opossum esophagus and lower esophageal sphincter, the guinea-pig and hamster ileum, the canine proximal colon, the guinea-pig proximal and distal colon and the guinea-pig internal anal sphincter. Exogenously added nitric oxide or nitroso compounds induced hyperpolarization of the membrane of circular muscle cells of the opossum esophageal sphincter and esophagus, the canine jejunum, the hamster ileum, the rat caecum, the human colon, the canine proximal colon and the guinea-pig proximal and distal colon. Although these numerous reports strongly suggest that NANC inhibitory pathway involves nitric oxide-mediated i.j.ps in various regions of gastrointestinal tract (for review see Hata et al., 2000), few reports have suggested involvement of the pathway in the rat intestine. Moreover, it was shown that nitric oxide-mediated NANC relaxation was independent of change in the membrane potentials in longitudinal muscle of the rat proximal colon (Suthamnatpong et al., 1994) but dependent on activation of sarcoplasmic reticulum Ca2+-ATPase (Takeuchi et al., 2001). However, the present results suggest that nitric oxide-mediated component of the relaxation of Wistar rat ileum is associated with apamin-resistant slow i.j.ps. These results indicate that the mechanism of nitric oxide-mediated relaxation differs among regions of the gastrointestinal tract and species of animals.
In summary, neurotensin was suggested to partially mediate NANC relaxation of longitudinal muscle of the jejunum and ileum of Wistar rats. SK channels were also suggested to be associated with the relaxation mediated by neurotensin.
Acknowledgments
This work was supported in part by Grant-in-Aid for advanced scientific research from Osaka Prefecture University and from Ministry of Education, Science, Sports and Culture of Japan, and by scholarship from Ono Pharmaceutical Company.
Abbreviations
- EFS
electrical field stimulation
- i.j.ps
inhibitory junction potentials, L-NOARG, NG-nitro-L-arginine, NANC, nonadrenergic, noncholinergic
- ODQ
1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one, PACAP, pituitary adenylate cyclase activating peptide, SK channel, small conductance Ca2+-activated K+ channel, VIP, vasoactive intestinal peptide
References
- CARRAWAY R., LEEMAN S.E. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J. Biol. Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- CARRAWAY R., LEEMAN S.E. Characterization of radioimmunoassayable neurotensin in the rat. J. Biol. Chem. 1976;251:7045–7052. [PubMed] [Google Scholar]
- CASTLE N.A., HAYLETT D.G., JENKINSON D.H. Toxins in the characterization of potassium channels. Trends Neurosci. 1989;12:59–66. doi: 10.1016/0166-2236(89)90137-9. [DOI] [PubMed] [Google Scholar]
- DE BEURME F.A., LEFEBVRE R.A. Influences of α-chymotrypsin and trypsin on the non-adrenergic, non-cholinergic relaxation in the rat gastric fundus. Br. J. Pharmacol. 1987;91:171–177. doi: 10.1111/j.1476-5381.1987.tb08996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOEDERT M., HUNTER J.C., NINKOVIC M. Evidence for neurotensin as a non-adrenergic, non-cholinergic neurotransmitter in guinea pig ileum. Nature. 1984;311:59–62. doi: 10.1038/311059a0. [DOI] [PubMed] [Google Scholar]
- GULLY D., CANTON M., BOIGEGRAIN R., JEANJEAN F., MOLIMARD J.C., PONCELET M., GUEUDET C., HEAULME M., LEYRIS R., BROUARD A., PELAPRAT D., LABBÉ-JULLIÉ C., MAZELLA J., SOUBRIÉ P., MAFFRAND J.P., ROSTÉNE W., KITABGI P., LE FUR , G. Biochemical and pharmacological profile of a potent and selective nonpeptide antagonist of the neurotensin receptor. Proc. Natl. Acad. Sci. U.S.A. 1993;90:65–69. doi: 10.1073/pnas.90.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GULLY D., LABEEUW B., BOIGEGRAIN R., OURY-DONAT F., BACHY A., PONCELET M., STEINBERG R., SUAUD-CHAGNY M.F., SANTUCCI V., VITA N., PECCEU F., LABBÉ-JULLIÉ C., KITABGI P., SOUBRIÉ P., LEFUR M.G., MAFFRAND J.P. Biochemical and pharmacological activities of SR 142948A, a new potent neurotensin receptor antagonist. J. Pharmacol. Exp. Ther. 1997;280:802–812. [PubMed] [Google Scholar]
- HAMMER R.A., LEEMAN S., CARRAWAY R.E., WILLIAMS R.H. Isolation of human intestine neurotensin. J. Biol. Chem. 1980;255:2476–2480. [PubMed] [Google Scholar]
- HATA F., ISHII T., KANADA A., YAMANO N., KATAOKA T., TAKEUCHI T., YAGASAKI O. Essential role of nitric oxide in descending inhibition in the rat proximal colon. Biochem. Biophys. Res. Commun. 1990;172:1400–1406. doi: 10.1016/0006-291x(90)91605-r. [DOI] [PubMed] [Google Scholar]
- HATA F., TAKEUCHI T., NISHIO H., FUJITA A. Mediators and intracellular mechanisms of NANC relaxation of smooth muscle in the gastrointestinal tract. J. Smooth Mus. Res. 2000;36:181–204. doi: 10.1540/jsmr.36.181. [DOI] [PubMed] [Google Scholar]
- HE X.D., GOYAL R.K. Nitric oxide involvement in the peptide VIP-associated inhibitory junction potential in the guinea-pig ileum. J. Physiol. (Lond.) 1993;461:485–499. doi: 10.1113/jphysiol.1993.sp019524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEEF K.D., DU. C., WARD S.M., MCGREGOR B., SANDERS K.M. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology. 1993;105:1009–1016. doi: 10.1016/0016-5085(93)90943-7. [DOI] [PubMed] [Google Scholar]
- KISHI M., TAKEUCHI T., KATAYAMA H., YAMAZAKI Y., NISHIO H., HATA F., TAKEWAKI T. Involvement of cyclic AMP-PKA pathway in VIP-induced, charybdotoxin-sensitive relaxation of longitudinal muscle of the distal colon of Wistar-ST rats. Br. J. Pharmacol. 2000;129:140–146. doi: 10.1038/sj.bjp.0702998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISHI M., TAKEUCHI T., SUTHAMNATPONG N., ISHII T., NISHIO H., HATA F., TAKEWAKI T. VIP- and PACAP-mediated nonadrenergic, noncholinergic inhibition in longitudinal muscle of rat distal colon: involvement of activation of charybdotoxin- and apamin-sensitive K+ channels. Br. J. Pharmacol. 1996;119:623–630. doi: 10.1111/j.1476-5381.1996.tb15719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITABGI P., CARRAWAY R.E., LEEMAN S. Isolation of a tridecapeptide from bovine intestine tissue and its partial characterization as neurotensin. J. Biol. Chem. 1976;251:7053–7058. [PubMed] [Google Scholar]
- KITABGI P., FREYCHET P. Effects of neurotensin on isolated intestinal smooth muscles. Eur. J. Pharmacol. 1978;50:349–357. doi: 10.1016/0014-2999(78)90140-1. [DOI] [PubMed] [Google Scholar]
- KITABGI P., VINCENT J.P. Neurotensin is a potent inhibitor of guinea-pig colon contractile activity. Eur. J. Pharmacol. 1981;74:311–318. doi: 10.1016/0014-2999(81)90050-9. [DOI] [PubMed] [Google Scholar]
- LABBÈ-JULLIÈ C., BOTTO J.-M., MAS M.-V., CHABRY J., MAZELLA J., VINCENT J.-P., GULLY D., MAFFRAND J.-P., KITABGI P. [3H]SR 48692, the first nonpeptide neurotensin antagonist radioligand: characterization of binding properties and evidence for distinct agonist and antagonist binding domains on the rat neurotensin receptor. J. Pharmacol. Exp. Ther. 1995;47:1050–1056. [PubMed] [Google Scholar]
- MATSUYAMA H., THAPALIYA S., TAKEWAKI T. Cyclic GMP-associated apamin-sensitive nitrergic slow inhibitory junction potential in the hamster ileum. Br. J. Pharmacol. 1999;128:830–836. doi: 10.1038/sj.bjp.0702851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEAN J., FOX J.E.T. Mechanism of action of neurotensin on motility of canine gastric corpus in vitro. Can. J. Physiol. Pharmacol. 1983;61:29–34. doi: 10.1139/y83-003. [DOI] [PubMed] [Google Scholar]
- MULÈ F., SERIO R. Mode and mechanism of neurotensin action in rat proximal colon. Eur. J. Pharmacol. 1997;319:269–272. doi: 10.1016/s0014-2999(96)00943-0. [DOI] [PubMed] [Google Scholar]
- NIIOKA S., TAKEUCHI T., KISHI M., ISHII T., NISHIO H., TAKEWAKI T., HATA F. Nonadrenergic, noncholinergic relaxation in longitudinal muscle of rat jejunum. Jpn. J. Pharmacol. 1997;73:155–161. doi: 10.1254/jjp.73.155. [DOI] [PubMed] [Google Scholar]
- OKISHIO Y., NIIOKA S., YAMAJI M., YAMAZAKI Y., NISHIO H., TAKEUCHI T., HATA F. Mediators of nonadrenergic, noncholinergic relaxation in Sprague Dawley rat intestine: comparison with the mediators of other strains. J. Vet. Med. Sci. 2000;62:821–828. doi: 10.1292/jvms.62.821. [DOI] [PubMed] [Google Scholar]
- SUTHAMNATPONG N., HOSOKAWA M., TAKEUCHI T., HATA F., TAKEWAKI T. Nitric oxide-mediated inhibitory response of rat proximal colon: independence from changes in membrane potential. Br. J. Pharmacol. 1994;112:676–682. doi: 10.1111/j.1476-5381.1994.tb13129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI T., KISHI M., HIRAYAMA N., YAMAJI M., ISHII T., NISHIO H., HATA F., TAKEWAKI T. Tyrosine kinase involvement in apamin-sensitive inhibitory responses of rat distal colon. J. Physiol. 1999;514:177–188. doi: 10.1111/j.1469-7793.1999.177af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI T., NIIOKA S., YAMAJI M., OKISHIO Y., ISHII T., NISHIO H., TAKATSUJI K., HATA F. Decrease in participation of nitric oxide in nonadrenergic, noncholinergic relaxation of rat intestine with age. Jpn. J. Pharmacol. 1998;78:293–302. doi: 10.1254/jjp.78.293. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI T., SUGIMOTO K., MORIMOTO H., FUJITA A., HATA F. Mechanism of a nitric oxide donor, NOR 1-induced relaxation in longitudinal muscle of rat proximal colon. Jpn. J. Pharmacol. 2001;86:390–398. doi: 10.1254/jjp.86.390. [DOI] [PubMed] [Google Scholar]
- TAKEWAKI T., OHASHI H. Non-cholinergic, excitatory transmission to intestinal smooth muscle cells. Nature. 1977;268:749–750. doi: 10.1038/268749a0. [DOI] [PubMed] [Google Scholar]
- WATSON M.J., LANG R.J., BYWATER R.A.R., TAYLOR G.S. Characterization of the membrane conductance changes underlying the apamin-resistant NANC inhibitory junction potential in the guinea-pig proximal and distal colon. J. Autonom. Nerv. Syst. 1996;60:31–42. doi: 10.1016/0165-1838(96)00024-0. [DOI] [PubMed] [Google Scholar]


