Functional features of monovalent cation,MC, channels
Membrane currents attributable to non-selective cation channels are most likely buried in the leakage compensation of many conventional patch clamp measurements, and they annoy experimentalists who regard them as artifactual leaks. Recent discovery of a novel family of cation channels, which belong to the protein family of the ‘transient receptor potential' signalling molecules, accounts for an amazing revived interest of these unloved leakage conductances. Channels that reveal permeation for monovalent but not divalent cations (therefore referred to as MC channels), and which are unmasked by withdrawal of extracellular Ca2+, were discovered approximately 15 years ago in several types of epithelial cells, in Xenopus oocytes, and also in cardiac and smooth muscle cells (van driessche et al., 1988; Mubagwa et al., 1997).
Obviously, MC currents contribute to the total currents that determine the membrane potential of various cell types and, therefore, may regulate the electrical activity of excitable cells. In addition, they may be involved in cation transport, they are supposed to critically influence the electro-chemical gradients for diverse transport functions, and they may also be involved in a phenomenon occurring under pathophysiological conditions, the ‘Ca2+ paradox'. This accounts for influx of Na+ under conditions of a reduced extracellular Ca2+ concentration for membrane depolarization and secondary Ca2+ overload, and for cell injury when the tissues are re-exposed to extracellular Ca2+ (Zimmermann & Hülsmann, 1966).
In this issue, Zakharov et al. (2002) extend the functional characterization of MC channels in heart and smooth muscle. They have investigated the link to a still somewhat mysterious store-operated cation channel (SOC). MC channels are inhibited by intracellular Mg2+ and are blocked by extracellular polycations like spermine in μM ranges. More importantly, activation of MC does not require the depletion of intracellular Ca2+ stores. This paper shows convincingly that MC currents are not due to unmasking of SOC currents by withdrawal of extracellular Ca2+.
Block by intracellular Mg2+ is a well-known mechanism for modulating the activity of a variety of ion channels including inwardly rectifying K+ channels of the Kir family, L-type Ca2+ channels and TRP channels, as discussed below. Importantly, inhibition of MC by Mg2+ may link this channel to the metabolic state of a cell. MC are likely to be down-regulated by reduction of intracellular ATP, which may result in unbinding of Mg2+. Surprisingly, extracellular spermine blocks MC in a voltage-independent manner. In addition, the authors show that extracellular spermine also inhibits vascular contraction induced by the Ca2+ paradox. These findings are interesting because a rather powerful tool is now demonstrated to get rid of MC under conditions of Ca2+ withdrawal. It is very likely that this tool (other related polycationic compounds may follow) can be used successfully to estimate the contribution of MC on the net membrane current under diverse physiological and pathophysiological conditions, and it may open possible approaches to determine the still elusive functional role of MC channels. It might even be a useful tool to discriminate between different ionic currents in over-expression systems and in permeation studies, which often require measurements to be made under Ca2+ free conditions.
Why are these findings now worthy of comment? Perhaps because several interesting and fundamental questions remain. To ask them is indeed highly justified and timely. Some of these questions follow.
Is there any physiological relevance for MC?
It is a fair question to ask. When does the extracellular Ca2+ concentration ever fall below 60 μM, which is the halfmaximal concentration of MC block in cardiac tissue at −80 mV (Mubagwa et al., 1997)? Probably never!However, we do not yet have enough data to conclude that various modulatory events, e.g free radicals, lysophospholids, changes in intracellular nucleotide concentrations or especially changes in the redox state of the cell, might open these channels even under conditions of higher extracellular Ca2+ concentrations. An especially interesting question is whether Ca2+ alone might activate this channel. This type of experiment is of course difficult to perform in myocardial and smooth muscle cells. However, we cannot exclude per se the possibility that MS is a CAN (Ca2+ activated non-selective cation channel) (Petersen, 2002). At physiological extracellular Ca2+ concentrations we have no data yet as to how large the background Na+ currents via MC might be, given that the unblocked current is up to several nA. It is not unlikely that a very small percentage of the MC current, which appears in Ca2+ free solution, might already be present (and functional) at physiological Ca2+ concentrations. The role of MC might be significant in smooth muscle cells, e.g. in the vascular system, where small changes in the intracellular Na+ concentration may substantially change the contractile state of these cells and, in turn, influence the blood pressure. Such fine-tuning of the intracellular Na+ concentrations may indeed also depend on an influx of Na+ through MC. The situation is different under non-physiological conditions, in which the extracellular Ca2+ concentration is reduced for several reasons. Needless to say the list of players that may account for the Ca2+ paradox has been extended by a reliable candidate.
Is there any relation between MC and other cation channels which are modulated by [Mg2+]i?
In general, modulation by intracellular Mg2+ is a key feature of several cation channels, including the Kir family of inwardly rectifying K+ channels, voltage-operated L-type Ca2+ channels, and the novel family of TRP channels. Recently, much ado appeared concerning the molecular nature of store-operated Ca2+ channels (SOCs). One of the intriguing aspects of the present paper also concerns this topic. Many highly Ca2+-selective cation channels turn into non-selective cation channels by removing extracellular Ca2+. Until recently, the highly Ca2+-selective SOC (the Ca2+-release-activated Ca2+ ‘channel', CRAC), which has been analysed in detail in blood cells, was considered as such a channel with a fairly high conductance for monovalent cations in the absence of extracellular Ca2+. However, these data now seem to characterize another channel, a Mg2+ inhibitable channel, MIC, with a single channel conductance in the absence of extracellular divalent cations of 40–45 pS (Kozak et al., 2002). Block by intracellular Mg2+ is characterized by an IC50 of approximately 0.6 mM for MIC and 0.25 mM for MC. MIC is also blocked by μM concentrations of extracellular spermine, and this occurs with a very similar efficiency as for MC. It is now obvious that MIC is not CRAC (Kozak et al., 2002), and the same is true for MC. It is now of course intriguing to speculate whether MC is MIC. Permeability for monovalent cations is different for MC and MIC. For MC, an Eisenman IV like permeation sequence has been reported with PK>PCs>PNa=PLi (Mubagwa et al., 1997), which suggests a weak field strength binding site for the monovalent cations. MIC channels show a nearly identical permeation for Na+ and Cs+ (Kozak et al., 2002), which differ from MIC. Regretfully, some hallmarks of MIC, the dramatic outward rectification at positive potentials, have not (or cannot) been tested for MC.
Single channel data for MC are still missing. Importantly, the striking effect of intracellular Mg2+ on TRP channels point us – at least suggests – that we think in the direction of the recently discovered TRP channels.
What is the molecular nature of MC?
It is always challenging to identify a function for which a molecular candidate is still elusive. The readership of a journal like The British Journal of Pharmacology can hardly escape the wide fascination and attraction of TRP cation channels. Therefore, an intriguing question remains whether MC might have some relation to this novel family of cation channels? These channels, which were first characterized in the eye of Drosophila, are divided for mammals into three main subfamilies: the TRPC (‘Canonical') group, the TRPV (‘Vanilloid') group and the TRPM (‘Melastatin') group (Montell et al., 2002). All of these subfamilies likely host some members that can be activated by the depletion of intracellular Ca2+ stores. This – in addition – induces a further interest in these novel channels.
Is there any link between MC and candidate channels within the TRP family? TRPC: TRPC channels are mainly activated via the PLC signalling pathway. However, it is a well-accepted phenomenon that most if not all currents through TRPCs are increased in the absence of extracellular divalent cations, but they are all Ca2+ permeable. TRPV: All TRPV channels are permeable for Ca2+. At least two members, TRPV5 and TRV6 bind Mg2+ in their pore and are therefore sensitively regulated by both extra and intracellular Mg2+. They are also blocked by spermine (Nilius, Prenen unpublished and see Nilius et al., 2001). However, their permeation profile is completely different from MC, including a highly selective permeation for Ca2+. Only in the absence of extracellular divalent cations do TRPV channels provide large monovalent currents, which are blocked by intracellular Mg2+ (Voets et al., 2001). TRPM: The TRPM subfamily may provide some more reasonable candidates. Block by Mg2+ is a key feature of a non-selective cation channel, TRPM7 (also known as TRP-PLIK, LTRPC7 or MagNuM from ‘magnesium-nucleotide-regulated metal ion current'). The previously discussed MIC channels share several features with TRPM7. However, LTRPM7 is Ca2+ permeable (Runnels et al., 2001) and by definition not MC. Just recently, a Ca2+ impermeable non-selective cation channel has ben described as a member of the TRPM family, TRPM4. In fact, these data would fit with MC. However, this channel is very likely activated by an increase in the intracellular concentration of Ca2+, [Ca2+]i. TRPM4 has a conductance of 25 pS and is half maximally activated by 400 nM [Ca2+]i (Launay et al., 2002). However, some puzzles also remain for the mechanism of TRPM4 activation, e.g. the reason for the substantial delay between an increase in [Ca2+]i and the activation of the channel. In addition, no thorough permeation analysis has been provided so far showing the Eisenman type or giving any idea on critical amino acid residues in the pore, which prevent Ca2+ permeation. In addition, contradictory data on Ca2+ permeation have been reported for two TRPM isoforms, the short TRPM4a and the longer TRPM4b. TRPM4a is protein nearly identical to TRPM4b but lacking 174 N-terminal amino acids (Launay et al., 2002). In contrast to TRPM4b, TRPM4a is permeable for Ca2+ (for discussion see Launay et al., 2002). Unfortunately, no data has been published showing that TRPM4 is unblocked by removal of extracellular Ca2+ (preliminary data show that this is indeed the case, Nilius, Prenen, unpublished). Is the MC channel TRPM4? This question has not yet been answered. No data exists showing that MC might be activated or modulated by [Ca2+]i, no single channel data can be compared, and no data on block by spermine of TRPM4 are available. Another interesting candidate might be TRPM2. This channel is potently activated by oxidants, but again is Ca2+ permeable. For all other members of the TRPM family, functional data is too sparse to allow any comparison with MC. So far, none of the TRPs seem to be identical with MC (see Table 1), however, data are still too sparse and too incomplete.
Table 1.
Any ‘candidate' channels for MC?
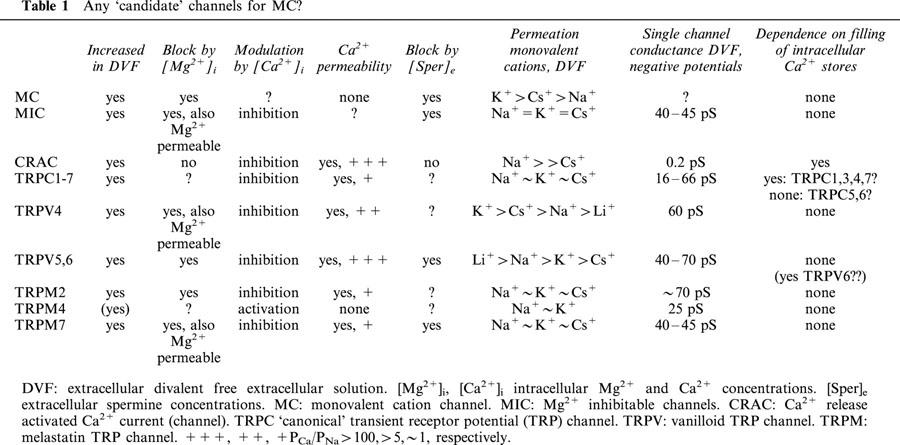
We are again confronted with the fact that unmasking of non-selective cation channels by reduction of the concentration of extracellular divalent cations, especially Ca2+, is a frequently occurring phenomenon in many cell types. Appearance of such current has nothing to do per se with activation of a CRAC or SOC like current. As the bottom line, we are left with an exciting set of data but still have much to learn about the cellular function of such channels. However, we are now tempted to pursue by novel approaches the molecular nature of such channels. Background currents remain a hot topic and the arrival of promising molecular candidates announces some light on the horizon.
Abbreviations
- CRAC
Ca2+ release activated Ca2+ (currents) channel
- MIC
Mg2+ inhibitable cation channel
- MC
monovalent cation (current) channel
- SOC
store operated Ca2+ entry
- TRP
transient receptor potential
- TRPC
TRPV, TRPM, TRP channels subfamilies ‘canonical', ‘vanilloid', ‘melastatin'
References
- KOZAK J.A., KERSCHBAUM H.H., CAHALAN M.D. Distinct properties of CRAC and MIC channels in RBL cells. J. Gen. Physiol. 2002;120:221–235. doi: 10.1085/jgp.20028601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAUNAY P., FLEIG A., PERRAUD A.L., SCHARENBERG A.M., PENNER R., KINET J.P. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- MONTELL C., BIRNBAUMER L., FLOCKERZI V., BINDELS R.J., BRUFORD E.A., CATERINA M.J., CLAPHAM D., HARTENECK C., HELLER S., JULIUS D., KOJIMA I., MORI Y., PENNER R., PRAWITT D., SCHARENBERG A.M., SCHULTZ G., SHIMIZU S., ZHU M.X. A unified nomenclature for the superfamily of TRP cation channels. Molecular Cell. 2002;9:229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- MUBAGWA K., STENGL M., FLAMENG W. Extracellular divalent cations block a cation non-selective conductance unrelated to calcium channels in rat cardiac muscle. J. Physiol. (Lond). 1997;502:235–247. doi: 10.1111/j.1469-7793.1997.235bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., PRENEN J., VENNEKENS R., HOENDEROP J.G.J., BINDELS R.J.M., DROOGMANS G. Pharmacological modulation of monovalent cation currents through the epithelial Ca2+ channel ECaC. Br. J. Pharmacol. 2001;134:453–462. doi: 10.1038/sj.bjp.0704272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSEN O.H. Cation channels: homing in on the elusive CAN channels. Curr. Biol. 2002;12:R520–R522. doi: 10.1016/s0960-9822(02)01027-8. [DOI] [PubMed] [Google Scholar]
- RUNNELS L.W., YUE L., CLAPHAM D.E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- VAN DRIESSCHE W., SIMAELS J., AELVOET I., ERLIJ D. Cation-selective channels in amphibian epithelia: electrophysiological properties and activation. Comp. Biochem. Physiol. A. 1988;90:693–699. doi: 10.1016/0300-9629(88)90686-x. [DOI] [PubMed] [Google Scholar]
- VOETS T., PRENEN J., FLEIG A., VENNEKENS R., WATANABE H., HOENDEROP J.G.J., BINDELS R.J.M., DROOGMANS G., PENNER R., NILIUS B. CaT1 and the calcium release-activated calcium channel manifest distinct pore properties. J. Biol. Chem. 2001;276:47767–47770. doi: 10.1074/jbc.C100607200. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN A.N.E., HÜLSMANN W.C. Paradoxical influence of calcium ions of the permeability of the cell membranes of isolated rat hearts. Nature. 1966;211:646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]


