Abstract
Theophylline possesses anti-inflammatory activities in asthma. We examined whether theophylline and agents that modulate cyclic AMP can determine the survival and proliferation of progenitor cells.
Progenitor cells from the blood of normal and asthmatic subjects were cultured for 14 days in methylcellulose with GM-CSF, stem cell factor, IL-3 and IL-5. Apoptosis was measured by flow cytometry of propidium-iodide-stained cells.
A greater number of colonies with a higher proportion of cells of eosinophil lineage from asthmatics compared to normal subjects were grown. Theophylline (at 5 and 20 μg ml−1) significantly inhibited colony formation and increased apoptotic cells in asthmatics compared to control. Salbutamol (0.1, 1, 10 μM), dibutyryl-cAMP (0.1, 1 mM) and rolipram (0.1, 1 mM), a phosphodiesterase IV inhibitor, also dose-dependently decreased colony numbers and increased apoptosis of progenitor cells from asthmatics.
There was no significant effect of theophylline, db-cAMP, salbutamol or rolipram on colony formation or the survival of progenitor cells from normal subjects. AMP did not affect the colony formation and apoptosis. Expression of Bcl-2 protein on progenitor cells of asthma was downregulated by theophylline, salbutamol, db-cAMP and rolipram.
Theophylline and rolipram decreased colony formation committed to the eosinophil lineage, together with an increase in apoptosis through an inhibition of Bcl-2 expression effects that may occur through cAMP. The anti-inflammatory properties of theophylline include an inhibition of circulating progenitor cells.
Keywords: Phosphodiesterase inhibition, theophylline, asthma, CD34+ progenitor cells, Bcl-2 protein, apoptosis
Introduction
Asthma is characterized by chronic airway inflammation, cellular infiltration, and cytokine production (Laitinen et al., 1985; Jeffery et al., 1989; Laitinen et al., 1993). Among the inflammatory cells, eosinophils may play an important role in bronchial asthma (Gleich, 1990). Increased numbers of progenitor cells expressing CD34+ committed to an eosinophilic lineage is found in the blood of patients with asthma (Chou et al., 1999; Wang et al., 1999). Furthermore, progenitor cells are increased during asthma exacerbation induced by controlled withdrawal of inhaled steroid therapy (Gibson et al., 1990), and by experimental exposure to allergen (Gibson et al., 1991). In addition to an increase found in peripheral blood, CD34+ cells expressing IL-5Rα receptor have also been found in the airways of patients with asthma and have been correlated with asthma severity (Robinson et al., 1999). Therefore, circulating CD34+ progenitor cells derived from bone marrow may migrate to airways and develop into mature cells under the effects of cytokines and haematopoietic factors, such as interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF) and interleukin-5 (IL-5).
Theophylline has been used in the treatment of asthma for several decades as a bronchodilator, but several lines of evidence indicate that it also has anti-inflammatory effects (Chung, 1996). Thus, theophylline reduces the numbers of eosinophils in the airway submucosa of patients with asthma (Sullivan et al., 1994; Lim et al., 2000). The mechanisms underlying this effect could be related to apoptosis or programmed cell death of eosinophils since theophylline increased apoptosis of IL-5-exposed eosinophils in vitro (Adachi et al., 1996; Ohta & Yamashita, 1999). Since progenitor cells, particularly those committed to an eosinophil lineage, are increased in the circulation and in the mucosal tissues of patients with asthma, we determined whether theophylline could also influence the survival and proliferation of progenitor CD34+ cells, similar to its effects on mature eosinophils. Apoptosis of haemopoietic and epithelial progenitor cells may be mediated by p53 and c-myc (Hockenbery et al., 1991; Ryan et al., 1994), and Bcl-2 acts as an inhibitor of apoptosis (Chao & Korsmeyer, 1998). We determined whether any potential effect of theophylline on apoptosis could be linked to modulation of Bcl-2 expression. Since the intracellular effects of theophylline could be secondary to phosphodiesterase (PDE) inhibition, we also examined the effects of a β-adrenergic agonist, salbutamol, of rolipram, a specific PDE type 4 inhibitor, and of a cell-permeant analog of cyclic adenosine monophosphate (cAMP).
Methods
Subjects and study design
A total of forty nonsmoking adults with mild intermittent asthma (17 males and 23 females) were recruited from outpatient clinics of Chang Gung Memorial Hospital (Table 1). All patients with asthma had a >15% improvement in forced expiratory volume in 1 s (FEV1) with inhaled albuterol (400 μg). These patients had mild asthma symptoms with intermittent daytime asthma symptoms less than once per week, night time symptoms less than twice a month, and normal lung function between exacerbations were defined as mild intermittent asthma. Of these, thirty of the asthmatic volunteers were atopic as determined by history and by increased serum specific imunoglobulin E (IgE) to common aeroallergens (Phadiatop, Pharmacia, Sweden). All patients had been stable for at least 6 weeks before enrolment and were only taking inhaled short-acting β2-agonists as a rescue medication. Inhaled β2-agonists were withheld for 12 h before venepuncture and methacholine testing.
Table 1.
Demographic features of patients
| Normal subjects | Asthmatic patients | |
|---|---|---|
| Age (years) | 29.8±1.7 | 30.5±1.5 |
| Sex (male/female) | 16/19 | 17/23 |
| FEV1 (% predicted) | 85.9±3.6 | 80.3±2.2 |
| FEV1/FVC (%) | 84.3±2.7 | 80.7±1.4 |
| PC20 (mg ml−1) | >25 | 3.6±0.7 |
PC20: concentration of methacholine to induce 20% decrease in FEV1. Data are mean±s.e.m.
A total of thirty-five normal nonsmoking subjects (16 males and 19 females) without evidence of allergic rhinitis and asthma with normal lung function and no evidence of bronchial hyper-responsiveness to methacholine (as described and defined below), were recruited from our hospital staff or medical students. They had negative serum specific IgE (Phadiatop) and normal total IgE level as well as eosinophil counts. This study was approved by our Ethics Committee and all subjects gave informed consent.
Methacholine challenge testing
FEV1 was measured by a Spiroanalyzer ST-350R (Fukuda Sangyo Co. Ltd., Tokyo, Japan). The best of three reproducible values (with a difference within or less than 5%) was used for the calculation. Methacholine dissolved in phosphate-buffered saline (PBS) or PBS alone (10 mM, pH=7.40) was delivered by a Rosenthal dosimeter (Model PF2A, Laboratory for Applied Immunology Inc., VA, U.S.A.) using 646 DeVilbiss nebulizer. PBS was inhaled first, followed by methacholine solution at increasing concentrations of 0.075, 0.15, 0.3, 0.6, 1.25, 2.50, 5.0, 10 and 25 mg ml−1 at 5-min intervals. Patients received five inhalations for each concentration. FEV1 was measured 5 min after the inhalation, followed by inhalation of the next higher concentration until FEV1 has fallen by more than 20% of the starting FEV. The log dose – response curves for methacholine was constructed as the percentage changes in FEV1 from the baseline (postbuffer) value. The dose of methacholine causing a 20% fall in FEV1 (PC20) was measured by linear interpolation. Bronchial hyper-responsiveness was defined as a PC20 of less than 8 mg ml−1.
Preparation of progenitor cells
Heparinized venous blood (100 ml) was obtained from each subject. Our method for preparing progenitor cells has been described previously (Wang et al., 1999). In brief, peripheral blood mononuclear cells were separated from the whole blood using Ficoll–Hypaque density gradient centrifugation at 400 × g for 30 min. The mononuclear cells at the interface were harvested, washed twice, and resuspended in Iscove modified Dulbecco medium (IMDM). The nonadherent mononuclear cell fraction was separated by adherence technique (Shih & Lee, 1994). Resuspended cell pellets were incubated in 25 cm2 flask at 37°C and 5% CO2 for 2 h. The nonadherent mononuclear cells were separated by centrifugation at 4°C and 1500 r.p.m. for 5 min and resuspended in IMDM. These nonadherent mononuclear cells were mixed well with 100 μl sheep red blood cells, then incubated at 4°C for 1 h, and depleted of E-rosette-forming cells by a second Ficoll–Hypaque centrifugation. Cells depleted of T-lymphocytes were obtained at the interface and these cells constitute the progenitor cell fraction we studied in this paper.
Granulocyte-macrophage progenitor (CFU-GM) assay
The progenitor cells (see above) were then cultured at a concentration of 2 × 105 cells in 1 ml of IMDM containing 0.9% (v v−1) of methylcellulose, 2-mercaptoethanol (50 μM), 1% bovine serum albumin (BSA), and 30% fetal calf serum, with growth factors, GM-CSF (500 U ml−1), stem cell factor (50 ng ml−1), IL-3 (100 U ml−1), and IL-5 (10 ng ml−1) in 35-mm dishes (Shih & Lee, 1994; Wang et al., 1999). Progenitor cells were treated with a range of concentrations of theophylline (0, 1, 5, 20, and 100 μg ml−1; Sigma, St Louis, MO, U.S.A.), salbutamol (0, 0.1, 1, 10 μM; Sigma, St Louis, MO, U.S.A.), dibutyryl adenosine 3′,5′-cyclic monophosphate (db-cAMP, 0, 0.01, 0.1, and 1 mM; Sigma, St Louis, MO, U.S.A.) or adenosine monophosphate (AMP, 0, 0.01, 0.1, and 1 mM; Sigma, St Louis, MO, U.S.A.). In order to study the effect of PDE on colony formation of progenitor cells, rolipram (Sigma, St Louis, MO, U.S.A.) was added at the concentrations of 0, 0.01, 0.1 or 1 mM. Duplicate cultures were prepared for each sample and incubated for 14 days in a humidified atmosphere of 5% CO2 in air. Colonies were scored 14 days after plating using an inverted light microscope. A colony was defined as a group of 40 or more cells. Cytocentrifuge preparation smears of the cultured colonies were made for morphological identification and differential cell count determination.
All experimental procedures were performed in endotoxin-free plastic wares; according to the manufacturer's information, the levels of endotoxin contamination in the cytokine preparations were three endotoxin units mg−1 by the limulus assay, and the antibodies used in all experiments contained <1 ng ml−1 of endotoxin.
Assessment of apoptosis
Progenitor cells (2 × 105 cells ml−1) were treated with or without theophylline, salbutamol, cAMP, AMP and rolipram at various concentrations in the IMDM containing 1% BSA, 30% fetal calf serum, with growth factors, GM-CSF (500 U ml−1), stem cell factor (50 ng ml−1), IL-3 (100 U ml−1), and IL-5 (10 ng ml−1), and incubated in 5% CO2 in air at 37°C for 3 days. To measure apoptosis of progenitor cells, the cells were first fixed and permeabilized by adding 500 μl iced 70% alcohol to the cell pellet and incubating at room temperature for 10 min. The cells were washed twice and resuspended in 0.5 ml PBS. The cells were treated with 10 μl RNase A (100 μg ml−1) and 10 μl propidium iodide (PI, 40 μg ml−1) at the same time, then incubated in a water bath at 37°C for 30 min. The proportion of cells within the hypodiploid DNA region was assessed by flow cytometry (Nicoletti et al., 1991).
Measurement of Bcl-2 expression in CD34+ cells
To measure the expression of intracellular Bcl-2 in CD34+ progenitor cells, cells were initially stained with anti-CD34-conjugated phycoerythrin (PE) monoclonal antibody (Becton Dickinson, Mountain View, CA, U.S.A.) for 30 min at 4°C. Then, the cells were washed with PBS and subsequently permeabilized with FACS™ permeabilizing solution (Becton Dickinson, Mountain View, CA, U.S.A.) for 20 min at room temperature. The cells were washed with PBS twice, then followed by staining with anti-Bcl-2 monoclonal antibody-conjugated FITC (Ancell Co., Bayport, MN, U.S.A.) for 30 min at 4°C. Subsequently, the cells were washed with ice-cold PBS twice and analyzed using a FACScan (Becton Dickinson & Co., Mountain View, CA, U.S.A.). Unrelated FITC-labelled anti-IgG1 (Ancell Co., Bayport, MN, U.S.A.) and PE-conjugated IgG (Becton Dickinson, Mountain View, CA, U.S.A.) monoclonal antibodies were used as negative control.
Materials
Theophylline, salbutamol, db-cAMP, AMP, rolipram, 2-mercaptoethanol, methylcellulose, BSA, RNase A, and PI were purchased from Sigma (St Louis, MO, U.S.A.). Growth factors of GM-CSF, IL-3, IL-5, and SCF were obtained from R&D systems Inc. (Minneapolis, MN, U.S.A.). IMDM, PBS, and fetal calf serum were purchased from Life Technologies (Grand Island, NY, U.S.A.). FACS™ permeabilizing solution, anti-CD34-conjugated PE and antimouse PE-conjugated IgG monoclonal antibodies were purchased from Becton Dickinson (Mountain View, CA, U.S.A.). Anti-Bcl-2 monoclonal antibody-conjugated-FITC and FITC-labelled anti-IgG1 were obtained from Ancell Co. (Bayport, MN, U.S.A.).
Statistical analysis
Data were analyzed for normal distribution and all data except for the data regarding the effect of rolipram on apoptosis were normally distributed. Data are presented as mean±s.e.m. Parametric statistical analyses were therefore employed and analysis of variance was used to analyze the overall effects of various in vitro treatments. Subsequent paired t-tests were used to assess the significance of differences between groups, and Bonferonni's correction was used to minimize the possibility of chance significance as a result of multiple comparisons. For the effects of rolipram on apoptosis, nonparametric analysis was used with Kruskall–Wallis test, followed by the Wilcoxon rank test (two-tailed). To assess the differences between responses of cells from normal or asthmatic individuals, unpaired t-tests were used. A P value of less than 0.05 was considered to be significant.
Results
Effect on progenitor cell proliferation and differentiation
Colony formation by progenitor cells was assessed by culturing these cells from normal subjects (n=35) and patients with mild intermittent asthma (n=40). There was a significantly higher number of CD34+ cells expressed as a percent of progenitor cells from asthmatic patients (3.62±0.79%, n=40) than that of normal subjects (0.71±0.12%, n=35, P<0.01). There were significantly greater number of colonies grown from progenitor cells in asthmatics, cultured in methylcellulose in the presence of growth factors, GM-CSF, stem cell factor, IL-3 and IL-5 in asthmatics (146±11 colonies, n=40, P<0.0001) than from normal subjects (60±6 colonies, n=35). Histochemical staining of cytospin preparations of the cultured progenitor cells revealed a significantly higher percentage of cells of eosinophil lineage in asthmatics (13.4±0.8%, n=40; P<0.001) than that from normal subjects (2.8±0.3%, n=35).
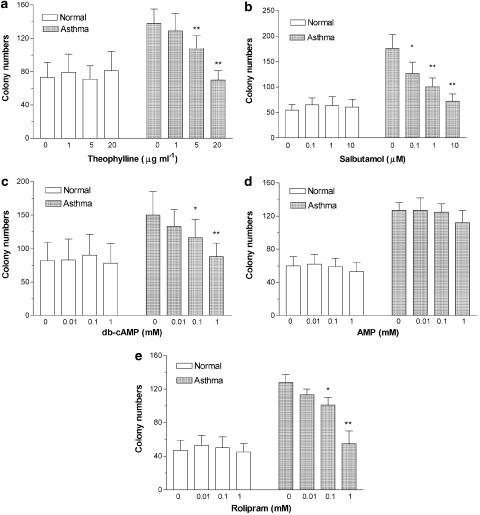
The effects of theophylline, salbutamol, db-cAMP, AMP and rolipram on the colony formation of progenitor cells are shown in Figure 1. Colony formation of progenitor cells from patients with asthma was suppressed by theophylline in a dose-dependent manner in patients with asthma with significant effects at 5 and 20 μg ml−1 (Figure 1a). In contrast, there was no significant effect of theophylline (5–20 μg ml−1) on the colony formation from progenitor cells of normal subjects (Figure 1a). In a smaller study, a high concentration of theophylline (100 μg ml−1) decreased colony numbers in both normal (28±7 colonies, P<0.001) and asthmatic (43±5, P<0.001) cell cultures (n=4 for both groups). Salbutamol, db-cAMP and rolipram also dose-dependently inhibited colony formation of progenitor cells from asthmatics (Figure 1b, c and e). There was no significant effect of salbutamol or db-cAMP or rolipram on the colony formation in normal subjects (Figure 1b, c and e). In contrast, addition of various concentrations of AMP did not affect colony growth either in asthmatics or in normal subjects (Figure 1d).
Figure 1.
Effects of theophylline, salbutamol, db-cAMP, rolipram and AMP on the colony formation of progenitor cells cultured for 14 days from normal subjects (n=7, in each group) and patients with mild intermittent asthma (n=8, in each group). Data shown as mean±s.e.m. *P<0.05, **P<0.01 compared with unstimulated controls in patients with asthma.
There was no significant change in the percentage of eosinophil or other colony-forming units with theophylline, salbutamol, db-cAMP, rolipram or AMP in progenitor cells either in asthmatics or in normal subjects. The percentage (median and range) of various precursors and cells were in asthmatics: blast 55.4% (50.2–62.5), promyelocytes 1.8% (0–3.9), myelocytes 7.0% (2.6–10), metamyelocytes 2.7% (0–7.2), band 2.3% (0–6.6), segment 0.7% (0–1.5), eosinophils 11.9% (5.7–18.1), basophils 0.7% (0–3.6) and macrophages 17.6% (10.7–30.3)] and in normal subjects: blast 71.6% (64.5–74.8), promyelocytes 0.5% (0–3.3), myelocytes 5.6% (1.3–7.4), metamyelocytes 1.2% (0–2.7), band 0.4% (0–1.0), segment 0.6% (0–2.3), eosinophils 2.7% (0.7–5.7), basophils 1.0% (0–3.6) and macrophages 14.4% (8.4–19.6), respectively.
Effects on progenitor cell apoptosis
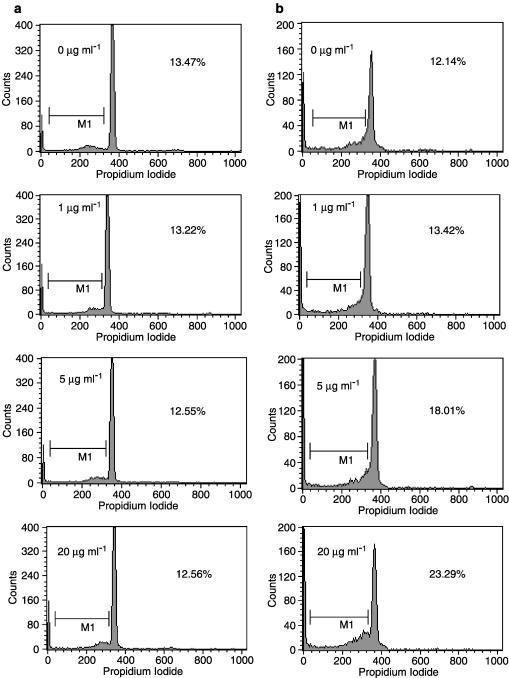
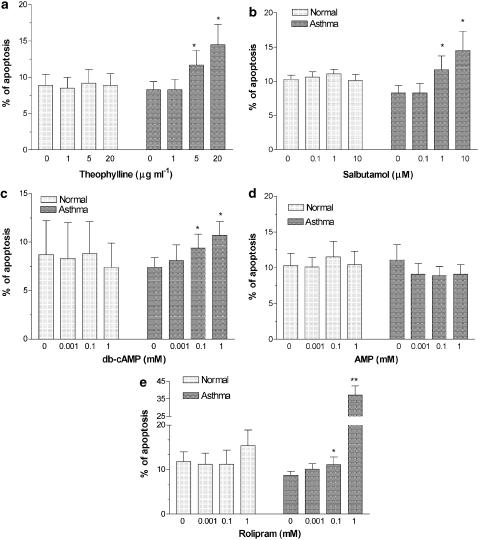
Cellular apoptosis with DNA fragmentation induced by theophylline was confirmed by PI staining and analyzed by flow cytometry (Figure 2). In the presence of haematopoietic factors, such as SCF, GM-CSF, IL-3, and IL-5, addition of theophylline (5 and 20 μg ml−1) induced apoptosis of progenitor cells in a dose-dependent manner (Figure 3a). In asthmatics, salbutamol (Figure 3b), db-cAMP (Figure 3c) and rolipram (Figure 3e) also dose-dependently increased progenitor cell apoptosis. Salbutamol (1 and 10 μM), db-cAMP (0.1 and 1 mM) and rolipram (0.1 and 1 mM) induced a significant increase in the proportion of apoptotic progenitor cells in asthmatic patients when compared to those from normal subjects. AMP had no significant effect on progenitor cell apoptosis (Figure 3d). Theophylline, rolipram, db-cAMP, salbutamol or AMP did not affect apoptosis in progenitor cells from normal subjects (Figure 3).
Figure 2.
Detection of apoptosis of progenitor cells induced by theophylline plus growth factors (GM-CSF, IL-3, SCF and IL-5) using PI staining by flow cytometric analysis. Progenitor cells from normal subjects (a) or patients with asthma (b) were treated with various concentrations of theophylline, collected after 72-h culture period and stained with PI to detect DNA fragmentation by flow cytometry. Percentages indicate the percentage of apoptotic cell nuclei/total cell nuclei.
Figure 3.
Effect of theophylline, salbutamol, db-cAMP, rolipram and AMP on the apoptosis of progenitor cells from normal subjects (n=7, in each group) and patients with mild intermittent asthma (n=8, in each group). The progenitor cells were incubated for 72 h in the presence of SCF, GM-CSF, IL-3 and IL-5. Data are shown as mean±s.e.m. *P<0.05, *ast;P<0.01 compared to controls in patients with asthma.
Bcl-2 expression in CD4+ progenitor cells
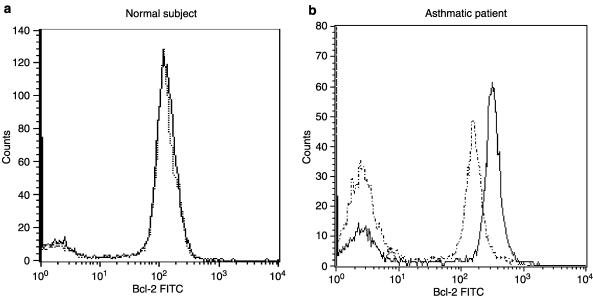
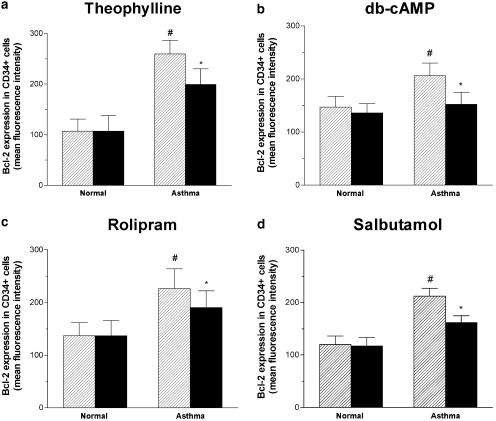
Bcl-2 protein expression in progenitor cells was analyzed by flow cytometry, and its expression was found to be higher in cells from asthmatics compared to normals. Theophylline at the concentration of 5 μg ml−1 significantly reduced the expression of Bcl-2 compared to control in CD34+ cells of asthmatics (Figure 5). Either db-cAMP (0.1 mM) or rolipram (0.1 mM) or salbutamol (1 μM) also inhibited the expression of Bcl-2 in the CD34+ progenitor cells of asthma compared to the controls (Figure 5). In contrast, neither the theophylline nor rolipram affected the expression of Bcl-2 in normal subjects (Figure 4 and Figure 5).
Figure 4.
Direct immunofluorescence flow cytometry was performed to detect intracellular expression of Bcl-2 on CD34+ cells. Cells from normal subjects and asthmatic patients were incubated with or without 5 μg ml−1 theophylline for 24 h. The graph shows data expressed in the form of histogram depicting specific Bcl-2 staining with (dashed line) or without (solid line) theophylline treatment for a normal subject (a) and for an asthmatic (b).
Figure 5.
Direct immunofluorescence flow cytometry was performed to detect intracellular expression of Bcl-2 on CD34+ cells. The mean fluorescence intensity (MFI) of Bcl-2 expression in cells treated with (solid bars) or without (hatched bars) 5 μg ml−1 theophylline (a) or 0.1 mM db-cAMP (b) or 0.1 mM rolipram (c) or 1 μM salbutamol (d) for 24 h in normal subjects (normal, n=7) or patients with mild intermittent asthma (asthma, n=8). *P<0.05 compared with the progenitor cells from normal subject in the absence of treatment. **P<0.05 compared with the progenitor cells in the absence of treatment. Data are means±s.e.m.
Discussion
In the present study, we have demonstrated that theophylline inhibits colony formation by increasing apoptosis of progenitor cells obtained from peripheral blood of patients with asthma. This effect is partly attributable to an increase in intracellular cAMP since the PDE type IV inhibitor, rolipram, the β2 receptor adrenoceptor agonist salbutamol and db-cAMP significantly inhibited colony growth and induced apoptosis of progenitor cells in asthmatics in a dose-dependent manner. AMP did not affect cell apoptosis or growth. In contrast, neither theophylline nor salbutamol nor db-cAMP, nor rolipram significantly induced apoptosis or affected colony formation of progenitor cells from normal subjects. The inhibitory effect of theophylline, salbutamol, db-cAMP and rolipram on colony growth and development is not limited to the eosinophil lineage alone.
Haematopoietic cells appear to be more vulnerable to cAMP-mediated signalling than mature granulocytes. Theophylline induces apoptosis of chronic lymphocytic leukemia cells via increased levels of intracellular cAMP by the type IV PDE inhibitor, rolipram (Mentz et al., 1995a; Kim & Lerner, 1998). Myeloid cell colony formation mediated by M-CSF can be inhibited by increasing intracellular cAMP levels (Kurland, 1978). Theophylline-induced mature granulocytes including eosinophil apoptosis is not mediated through the elevation of intracellular cAMP levels (Yasui et al., 1997), but mediated via adenosine A2A receptor antagonism rather than a cAMP-dependent pathway (Yasui et al., 2000). In contrast, cAMP is a ubiquitous secondary messenger that inhibits B and T lymphocyte proliferation (Kizaki et al., 1990) and induces the death of thymocytes through internucleosomal DNA cleavage (McConkey et al., 1990; Mentz et al., 1995b). cAMP has been shown to induce G1 cell arrest by inducing the cyclin-dependent kinase inhibitor p27Kip1 (Kato et al., 1994). The important role of the cAMP pathway in regulating large numbers of genes through cAMP-responsive element binding protein (CREB) (Lalli & Sassone-Corsi, 1994) may explain the effects of theophylline- or rolipram-induced apoptosis in progenitor cells from patients with asthma.
cAMP can induce cell cycle arrest at G1 phase in a human eosinophilic leukemia cell line (Uenoyama et al., 1991), an effect associated with a decrease in Bcl-2 expression (Tai et al., 1996). Bcl-2 plays an important role in cell growth/death and differentiation. Over-expression of Bcl-2 decreases cAMP-induced inhibition of cell proliferation and induction of apoptosis in SH-SY5Y human neuroblastoma cells (Kim et al., 2000). In contrast, cAMP does not modulate the expression of Bcl-2 expression in mature cells (Wilson et al., 1996). Thus, the resistance to cAMP-mediated apoptosis in mature granulocytes as shown in previous reports may result from alterations in the ability of cAMP to activate apoptotic signalling pathways between proliferating and mature cells. Our present study reveals that theophylline, salbutamol and db-cAMP downregulate the expression of Bcl-2 on progenitor cells, suggesting that theophylline induces progenitor cell apoptosis via a cAMP-dependent pathway, which may be different from mature eosinophils.
The reasons why these cAMP-elevating agents do not affect progenitor cells from normal subjects but only asthmatic cells are not clear from our study but several possibilities exist. PDE activity, which inactivates cAMP, is significantly increased in monocytes and eosinophils from asthmatics compared to those from healthy control subjects (Landells et al., 2000; Takeuchi et al., 2002). Either a heightened response of apoptotic pathways to cAMP or a greater increase in cAMP level in response to cAMP-elevating agents in asthmatic progenitor cells than that in normal cells may underlie the increased apoptosis observed in asthmatic cells. Secondly, cAMP-elevating agents inhibit the expression of several pro-inflammatory cytokines, such as GM-CSF (Seldon & Giembycz, 2001), IL-5, and IL-4 (Crocker et al., 1996), that promote proliferation of progenitor cells. We have recently shown that, in progenitor cells from asthmatics but not in those from normals, IL-5 autoregulates the proliferation and differentiation of eosinophilic colony formation from progenitor cells which express greater levels of IL-5Rα receptor (Kuo et al., 2001). Therefore, asthmatic progenitor cells may be more sensitive to regulation by proliferative cytokines. Haematopoietic cells with high proliferation capacity appear to be more vulnerable to cAMP-mediated signalling pathway (Myklebust et al., 1999). Augmentation of intracellular cAMP by cAMP-elevating agents significantly contributes to downregulation of Bcl-2 expression and acceleration of cell apoptosis in asthmatics, but not in normal subjects. Further studies are required to resolve the differences between progenitor cells from normal and asthmatic subjects.
Theophylline has been used worldwide in the treatment of bronchial asthma for several decades mainly as a bronchodilator (Weinberger, 1984), but has been reevaluated as an anti-inflammatory agent for the treatment of asthma (Chung, 1996). Previous studies have shown that doses of theophylline that achieve low serum therapeutic levels (5–10 μg ml−1) attenuates eosinophil cell numbers in the peripheral blood (Sullivan et al., 1994). The immunomodulatory activities of theophylline in bronchial asthma include an induction of eosinophil apoptosis (Adachi et al., 1996; Lim et al., 2000) and an inhibition of T-cell proliferation and activation (Kidney et al., 1995; Rabe & Dent, 1998). The present study extends the scope of the anti-inflammatory effects of theophylline. At a concentration as low as 5 μg ml−1, theophylline induces a significant apoptosis of bone marrow precursor cells and inhibits the colony formation of progenitor cells from patients with asthma. Therefore, the suppressing effect of theophylline on peripheral blood eosinophilia and airway submucosal eosinophil infiltration (Sullivan et al., 1994; Lim et al., 2000) may occur at the level of circulating progenitor cells. However, it is not known whether the degree of increased apoptosis and of decreased colony formation in incubations with theophylline or rolipram in vitro, is of importance when patients are treated with clinical doses of these agents.
The inhibitory effect of either theophylline or the phosphodiesterase IV inhibitor on the colony-forming assay was not selective towards eosinophils since there was also inhibition of basophil and macrophage lineage. The effect on cells of neutrophilic lineage could not be determined from these experiments since the cytokine milieu of the study did not encourage colony-forming units of neutrophils. However, in chronic obstructive pulmonary diseases, neutrophils recovered in induced sputum from patients were reduced by treatment with theophylline (Culpitt et al., 2002).
In conclusion, we have demonstrated that theophylline inhibits the proliferation of progenitor cells obtained from the peripheral blood of patients with asthma; this is associated with an increase of apoptosis of progenitor cells. We suggest that this could have resulted from an elevation of intracellular cAMP and a downregulation of Bcl-2 protein expression. However, further studies are needed to establish a definitive causal link. Our study is the first report to indicate that circulating progenitor cells could be a therapeutic target of theophylline in the treatment of bronchial asthma.
Acknowledgments
This study was supported by the Chang Gung Medical Research Project, CMRP-985, and the ROC National Science Council, NSC-89-2314-B-182A-028.
Abbreviations
- AMP
adenosine monophosphate
- cAMP
adenosine 3′,5′-cyclic monophosphate
- CFU
colony-forming units
- CFU-GM
granulocyte-macrophage CFU
- db-cAMP
dibutyryl-cAMP
- FEV1
forced expiratory volume in 1 s
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IL-3
interleukin-3
- IL-5
interleukin-5
- MFI
mean fluorescence intensity
- PE
phycoerythrin
- SCF
stem cell factor
References
- ADACHI T., MOTOJIMA S., HIRATA A., FUKUDA T., KIHARA N., KOSAKU A., OHTAKE H., MAKINO S. Eosinophil apoptosis caused by theophylline, glucorticoids, and macrolides after stimulation with IL-5. J. Allergy Clin. Immunol. 1996;98:S207–S215. doi: 10.1016/s0091-6749(96)70068-4. [DOI] [PubMed] [Google Scholar]
- CHAO D.T., KORSMEYER S.J. Bcl-2 family: regulators of cell death. Annu. Rev. Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- CHOU C.L., WANG C.H., KUO H.P. Upregulation of IL-5 receptor expression on bone marrow-derived CD34+ cells from patients with asthma. Chang Gung Med. J. 1999;22:416–422. [PubMed] [Google Scholar]
- CHUNG K.F. Theophylline in chronic asthma: evidence for disease-modifying properties. Clin. Exp. Allergy. 1996;26:22–27. doi: 10.1111/j.1365-2222.1996.tb01139.x. [DOI] [PubMed] [Google Scholar]
- CROCKER I.C., TOWNLEY R.G., KHAN N.M. Phosphodiesterase inhibitors suppress proliferation of peripheral blood mononuclear cells and interleukin-4 and interleukin-5 secretion by human T-helper type-2 cells. Immunopharmacology. 1996;31:223–235. doi: 10.1016/0162-3109(95)00053-4. [DOI] [PubMed] [Google Scholar]
- CULPITT S.V., DE MATOS C., RUSSELL R.E., DONNELLY L.E., ROGERS D.F., BARNES P.J. Effect of theophylline on induced sputum inflammatory indices and neutrophil chemotaxis in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;165:1371–1376. doi: 10.1164/rccm.2105106. [DOI] [PubMed] [Google Scholar]
- GIBSON P.G., DOLOVICH J., GIRGIS-GABARDO A., MORRIS M.M., ANDERSON M., HARGREAVE F.E., DENBERG J.A. The inflammatory response in asthma exacerbation: changes in circulating eosinophils, basophils and their progenitors. Clin. Exp. Allergy. 1990;20:661–668. doi: 10.1111/j.1365-2222.1990.tb02705.x. [DOI] [PubMed] [Google Scholar]
- GIBSON P.G., MANNING P.J., O'BYRNE P.M., GIRGIS-GABARDO A., DOLOVICH J., DENBURG J.A., HARGREAVE F.E. Allergen-induced asthmatic responses: relationship between increases in airway responsiveness and increases in circulating eosinophils, basophils and their progenitors. Am. Rev. Respir. Dis. 1991;143:331–335. doi: 10.1164/ajrccm/143.2.331. [DOI] [PubMed] [Google Scholar]
- GLEICH G.J. The eosinophil and bronchial asthma: current understanding. J. Allergy Clin. Immunol. 1990;85:422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- HOCKENBERY D.M., ZUTTER M., HICKEY W., NAHAM M., KORSMEYER S.J. Bcl-2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc. Natl. Acad. Sci. U.S.A. 1991;88:6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEFFERY P.K., WARDLAW A.J., NELSON F.C., COLLINS J.V., KAY A.B. Bronchial biopsies in asthma. An ultrastructural, qualitative study and correlation with hyperreactivity. Am. Rev. Respir. Dis. 1989;140:1745–1753. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- KATO J.Y., MATSUOKA M., POLYAK K., MASSAGUE J., SHERR C.J. Cyclic AMP-induced G1-phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- KIDNEY J., DOMIGUEZ M., TAYLOR P.M., ROSE M., CHUNG K.F., BARNES P.J. Immunomodulation by theophylline in asthma: demonstration by withdrawal of therapy. Am. J. Respir. Crit. Care Med. 1995;151:1907–1914. doi: 10.1164/ajrccm.151.6.7767539. [DOI] [PubMed] [Google Scholar]
- KIM D.H., LERNER A. Type 4 cyclic adenosine monophospate phosphodiesterase as therapeutic target in chronic lymphocytic leukemia. Blood. 1998;92:2484–2494. [PubMed] [Google Scholar]
- KIM S.N., KIM S.G., PARK J.H., LEE M.A., PARK S.D., CHO-CHUNG Y.S., HONG S.H. Dual anticancer activity of 8-Cl-cAMP: inhibition of cell proliferation and induction of apoptotic cell death. Biochem. Biophys. Res. Commun. 2000;273:404–410. doi: 10.1006/bbrc.2000.2949. [DOI] [PubMed] [Google Scholar]
- KIZAKI H., SUZUKI K., TADAKUMA T., ISHIMURA Y. Adenosine receptor-mediated accumulation of cyclic AMP-induced T-lymphocyte death through internucleosomal DNA cleavage. J. Biol. Chem. 1990;265:5280–5284. [PubMed] [Google Scholar]
- KUO H.P., WANG C.H., LIN H.C., HWANG K.S., LIU S.L., CHUNG K.F. Interleukin-5 in growth and differentiation of blood eosinophil progenitors in asthma: effect of glucocorticoids. Br. J. Pharmacol. 2001;134:1539–1547. doi: 10.1038/sj.bjp.0704389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURLAND J.I. Role for monocyte-macrophage-derived colony-stimulating factor and prostaglandin E in the positive and negative feedback control of myeloid stem cell proliferation. Blood. 1978;52:388–407. [PubMed] [Google Scholar]
- LAITINEN L.A., HEINO M., LAITINEN A., KAVA T., HAAHTELA T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am. Rev. Respir. Dis. 1985;131:599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- LAITINEN L.A., LEITINEN A., HAAHTELA T. Airway mucosal inflammation even in patients with newly diagnosed asthma. Am. Rev. Respir. Dis. 1993;147:697–704. doi: 10.1164/ajrccm/147.3.697. [DOI] [PubMed] [Google Scholar]
- LALLI E., SASSONE-CORSI P. Signal transduction and gene regulation. The nuclear response to cAMP. J. Biol. Chem. 1994;269:17359–17362. [PubMed] [Google Scholar]
- LANDELLS L.J., SPINA D., SOUNESS J.E., O'CONNOR B.J., PAGE C.P. A biochemical functional assessment of monocyte phosphodiesterase activity in healthy and asthmatic subjects. Pulmonary Pharmal. Therap. 2000;13:231–239. doi: 10.1006/pupt.2000.0248. [DOI] [PubMed] [Google Scholar]
- LIM S., TOMITA K., CARAMORI G., JATAKANON A., OLIVER B., KELLER A., ADCOCK I., CHUNG K.F., BARNES P.J. Low dose theophylline reduces eosinophilic inflammation but not exhaled nitric oxide in mild asthma. Am. J. Respir. Crit. Care Med. 2000;162:1912–1918. doi: 10.1164/ajrccm.164.2.2006043. [DOI] [PubMed] [Google Scholar]
- MCCONKEY D.J., ORRENIUS S., JONDAL M. Agents that elevate cAMP stimulate DNA fragmentation in thymocytes. J. Immunol. 1990;145:1227–1230. [PubMed] [Google Scholar]
- MENTZ F., MERLE-BERAL H., OUAAZ E., BINET J.L. Theophylline, a new inducer of apoptosis in B-CLL: role of cyclic nucleotides. Br. J. Hematol. 1995a;90:957–959. doi: 10.1111/j.1365-2141.1995.tb05225.x. [DOI] [PubMed] [Google Scholar]
- MENTZ F., MOSSALAYI M.D., OUAAZ F., DEBRE P. Involvement of cAMP in CD3/T cell receptor complex- and Cd2-mediated apoptosis of human thymocytes. Eur. J. Immunol. 1995b;25:1798–1801. doi: 10.1002/eji.1830250648. [DOI] [PubMed] [Google Scholar]
- MYKLEBUST J.H., JOSEFSEN D., BLOMHOFF H.K., LEVY F.O., NADERI S., REED J.C., SMELAND E.B. Activation of the cAMP signaling pathway increases apoptosis in human B-precursor cells and is associated with downregulation of Mcl-1 expression. J. Cell Physiol. 1999;180:71–80. doi: 10.1002/(SICI)1097-4652(199907)180:1<71::AID-JCP8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- NICOLETTI I., MIGLIORATI G., PAGLIACCI M.C., GRIGNANI F., RICCARDI C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- OHTA K., YAMASHITA N. Apoptosis of eosinophils and lymphocytes in allergic inflammation. J. Allergy Clin. Immunol. 1999;104:14–21. doi: 10.1016/s0091-6749(99)70107-7. [DOI] [PubMed] [Google Scholar]
- RABE K.F., DENT G. Theophylline and airway inflammation. Clin. Exp. Allergy. 1998;28:35–41. [PubMed] [Google Scholar]
- ROBINSON D.S., DAMIA R., ZEIBECOGLOU K., MOLET S., NORTH J., YAMADA T., KAY A.B., HAMID Q. CD34+/ interleukin-5R messenger RNA+ cells in the bronchial mucosa in asthma: potential airway eosinophil progenitor cells. Am. J. Respir. Cell Mol. Biol. 1999;20:9–13. doi: 10.1165/ajrcmb.20.1.3449. [DOI] [PubMed] [Google Scholar]
- RYAN J.J., PROCHOWNIK E., GOTTLIEB C.A., APEL I.J., MERINO R., NUNEZ G., CLARKE M.F. C-myc and bcl-2 modulate function by altering p53 subcellular trafficking during the cell cycle. Proc. Natl. Acad. Sci. U.S.A. 1994;91:5878–5882. doi: 10.1073/pnas.91.13.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELDON P.M., GIEMBYCZ A.M. Suppression of granulocyte/macrophage colony-stimulating factor release from human monocytes by cyclic AMP-elevating drugs: role of interleukin-10. Br. J. Pharmacol. 2001;134:58–67. doi: 10.1038/sj.bjp.0704238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIH L.Y., LEE C.T. Identification of masked polycythemia vera from patients with idiopathic marked thrombocytosis by endogenous erythroid colony assay. Blood. 1994;83:744–748. [PubMed] [Google Scholar]
- SULLIVAN P., BEKIR S., JAFFAR Z., PAGE C., JEFFERY P., COSTELLO J. Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet. 1994;343:1006–1008. doi: 10.1016/s0140-6736(94)90127-9. [DOI] [PubMed] [Google Scholar]
- TAI G., EUN-YOUNG J., YUJI H., MASAHIKO K., TOSHIO H., KENJI K., KENSHI F., MITSUFUMI M. Different effects of cyclic AMP and butyrate on eosinophilic differentiation, apoptosis and bcl-2 expression of human eosinophilic leukemia cell line, EoL-1. Hematol. Oncol. 1996;14:181–192. doi: 10.1002/(SICI)1099-1069(199612)14:4<181::AID-HON589>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI M., TATSUMI Y., KITAICHI K., BABA K., SUZUKI R., SHIBATA E., TAKAGI K., MIYAMOTO K., HASEGAWA T., TAKAGI K. Selective phosphodiesterase type 4 inhibitors reduce the prolonged survival of eosinophils stimulated by granulocyte-macrophage colony-stimulating factor. Biol. Pharm. Bull. 2002;25:184–187. doi: 10.1248/bpb.25.184. [DOI] [PubMed] [Google Scholar]
- UENOYAMA Y., OHSHIMA Y., MORITA M., AKUTAGAWA H., NAMBU M., KIM K.M., MAYUMI M., MIKAWA H. Dibutyryl cyclic AMP induces formyl peptide receptor expression and chemotactic response in a human eosinophilic cell line EoL-1. Exp. Hematol. 1991;19:823–828. [PubMed] [Google Scholar]
- WANG C.H., SHEIH W.Y., SHIH L.Y., LIN H.C., LIU C.Y., CHUNG K.F., KUO H.P. Increased progenitor cell proliferation in peripheral blood of patients with bronchial asthma: role of nitric oxide. J. Allergy Clin. Immunol. 1999;104:803–810. doi: 10.1016/s0091-6749(99)70291-5. [DOI] [PubMed] [Google Scholar]
- WEINBERGER M. The pharmacology and therapeutic use of theophylline. J. Allergy Clin. Immunol. 1984;73:525–540. doi: 10.1016/0091-6749(84)90505-0. [DOI] [PubMed] [Google Scholar]
- WILSON B.E., MOCHON E., BOXER L.M. Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol. Cell Biol. 1996;16:5546–5556. doi: 10.1128/mcb.16.10.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YASUI K., AGEMATSU K., SHINOZATCI K., HOKIBARA S., NAGUMO H., NAKAZAWA T., KOMIYAMA A. Theophylline induces neutrophil apoptosis through adenosine A2A receptor antagonism. J. Leukoc. Biol. 2000;67:529–535. doi: 10.1002/jlb.67.4.529. [DOI] [PubMed] [Google Scholar]
- YASUI K., HU B., NAKAZAWA T., AGEMATSU K., KOMIYAMA A. Theophylline accelerates human granulocyte apoptosis not via phosphodiesterase inhibition. J. Clin. Invest. 1997;100:1677–1684. doi: 10.1172/JCI119692. [DOI] [PMC free article] [PubMed] [Google Scholar]