Abstract
In guinea-pig ileal longitudinal muscle, muscarinic partial agonists, 4-(N-[3-chlorophenyl]-carbomoyloxy)-2-butynyl-trimethylammonium (McN-A343) and pilocarpine, each produced parallel increases in tension and cytosolic Ca2+ concentration ([Ca2+]c) with a higher EC50 than that of the full agonist carbachol. The maximum response of [Ca2+]c or tension was not much different among the three agonists. The Ca2+ channel blocker nicardipine markedly inhibited the effects of all three agonists
The contractile response to any agonist was antagonized in a competitive manner by M2 receptor selective antagonists (N,N′-bis[6-[[(2-methoyphenyl)methyl]amino]hexyl]-1,8-octanediamine tetrahydrochloride and 11-[[2-[(diethlamino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3-b][1,4] benzodiazepine-6-one), and the apparent order of M2 antagonist sensitivity was McN-A343>pilocarpine>carbachol. M3 receptor selective antagonists, 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide and darifenacin, both severely depressed the maximum response for McN-A343, while darifenacin had a similar action in the case of pilocarpine. Both M3 antagonists behaved in a competitive manner in the case of the carbachol response.
McN-A343 failed to release Ca2+ from the intracellular stores, and the Ca2+-releasing action of pilocarpine was very weak compared with that of carbachol. All three agonists were capable of increasing Ca2+ sensitivity of the contractile proteins.
McN-A343 rarely produced membrane depolarization, but always accelerated electrical spike discharge. Pilocarpine effect was more often accompanied by membrane depolarization, as was usually seen using carbachol.
The results suggest that muscarinic agonist-evoked contractions result primarily from the integration of Ca2+ entry associated with the increased spike discharge and myofilaments Ca2+ sensitization, and that Ca2+ store release may contribute to the contraction indirectly via potentiation of the electrical membrane responses. They may also support the idea that an interaction of M2 and M3 receptors plays a crucial role in mediating the contraction response.
Keywords: M2 and M3 receptors, intestinal smooth muscle, cationic current, Ca2+-store release, Ca2+ sensitization, membrane potential, carbachol, pilocarpine, McN-A343
Introduction
In intestinal smooth muscle, acetylcholine and its related stimulants produce contraction by activating muscarinic receptors. Although the muscle has the M2 and the M3 muscarinic receptors with a preponderance of the former subtype (4 : 1 or 5 : 1), the contractions evoked are generally regarded as mediated via the minor M3 subtype (see Caufieled, 1993; Eglen et al., 1996). The M3 receptor links via Gq type of the GTP-binding protein (G-protein) to stimulation of phospholipase C (PLC), formation of inositol-1, 4, 5-trisphosphate (InsP3), and release of intracellular Ca2+ stores, resulting in a rise in cytosolic Ca2+ concentration ([Ca2+]c) (Somlyo & Himpens, 1989; Komori & Bolton, 1991; Komori et al., 1992; Prestwich & Bolton, 1995; Morel et al., 1997). Thus, the M3-mediated Ca2+ store release might be supposed as the major cause of the muscarinic agonist-evoked contractions. However, the contractile response to muscarinic agonists, regardless of the full or partial type, is severely inhibited by voltage-dependent Ca2+ channel blockers (Brading & Sneddon, 1980; Takayanagi et al., 1990; Blackwood & Bolton, 1993; Hishinuma et al., 1997). This means that the muscarinic contraction depends largely on Ca2+ entry via Ca2+ channels opened by membrane depolarization. The questions then arise whether or not, and if so, how M3 receptor activation leads to the voltage-dependent Ca2+ entry, and what role the M3-mediated Ca2+ release has in determining the contractile response.
Electrophysiological studies have suggested the possible participation of the M2 receptors in the voltage-dependent Ca2+ entry underlying the muscarinic contraction. This is based on the following lines of evidence: first, a full agonist such as carbachol produces membrane depolarization leading to the activation of voltage-dependent Ca2+ channels (Bolton, 1972, 1979; Kuriyama et al., 1998); secondly, this membrane depolarization results from the opening of receptor-operated cationic channels via pertussis toxin-sensitive Go-type G-protein (Benham et al., 1985; Inoue & Isenberg, 1990a; Komori et al., 1992; Kim et al., 1998); and finally, M2 receptors primarily mediate cationic channel opening (Bolton & Zholos, 1997; Zholos & Bolton, 1997; Komori et al., 1998; Rhee et al., 2000). From these findings, one would expect the possible involvement of the M2 receptor in voltage-dependent Ca2+ entry, and so in mediating the contractile response. However, no direct role of the M2 receptor has been detected in many contractile studies. This remains as an enigma to be explained. It should be noted that there have been many pieces of evidence suggesting that the M2-mediated cationic channel opening is strongly potentiated by simultaneous activation of M3 receptors through both Ca2+-dependent and -independent mechanisms (Inoue & Isenberg, 1990b; Pacaud & Bolton, 1991; Komori et al., 1993; Zholos & Bolton, 1997; Kohda et al., 1998; Okamoto et al., 2002). The M3-mediated amplifications might make it obscure to reveal the M2 receptor participation in the contractile response.
4-(N-[3-chlorophenyl]-carbamoyloxy)-2-butynyl-trimethylammonium (McN-A343) and AHR-602, referred to as muscarinic partial agonists for the contractile response of intestinal smooth muscle, cause neither significant stimulation of phosphoinositide metabolism, nor any appreciable contraction because of the release of intracellularly stored Ca2+ (Gardner et al., 1988; Hishinuma et al., 1997). McN-A343 is also described as being ineffective in eliciting Ca2+-activated K+ current because of a massive Ca2+ store release (Okamoto et al., 2002). Nonetheless, these agonists are not so much different in the ceiling activity for contraction from the full agonist carbachol (Eglen et al., 1987; Gardner et al., 1988; Hishinuma et al., 1997). Analogous profiles have been described for another partial agonist pilocarpine (Takayanagi et al., 1991; Wang et al., 1992; Okamoto et al., 2002). Therefore, it seems likely that the mechanisms by which the partial agonists evoke contraction are relatively simple, so that systematic comparison of their pharmacological profiles with those of the full-type agonists may provide important information to resolve the above-mentioned questions and enigma.
In this report, using McN-A343 and pilocarpine as the partial agonist, we have investigated their effects on tension generation, [Ca2+]c, intracellular Ca2+ stores, myofilament Ca2+ sensitivity, and electrical membrane activity, comparing with those of the full agonist carbachol most characterized so far, in guinea-pig ileal longitudinal smooth muscle. We also investigated how their contractile effects were affected by the M2 or M3 selective antagonists. Our data suggest that the muscarinic contractile response results primarily from the integration of Ca2+ entry associated with accelerated electrical activity and of Ca2+ sensitization of the contractile proteins, and that Ca2+ store release contributes to the contraction indirectly by influencing electrical membrane events. The present results may also support the hypothesis that an interaction between M2 and M3 receptors plays a crucial role in mediating the contractile response. The possible M2/M3 interaction will be discussed.
Methods
Male guinea-pigs, weighing 300–400 g, were euthanized, and a 10- to 15-cm length of the ileum except the terminal 5 cm segment was removed and placed in a physiological medium. The isolated intestine was cut into 1.5- to 2.0-cm segments. The longitudinal muscle layer of the segments was peeled from the underlying tissue, and then subjected to procedures for use in various experiments.
All procedures described above were performed according to the guidelines approved by a local animal ethics committee of the Faculty of Agriculture, Gifu University.
Recording of the contractile responses
The longitudinal muscle strips (ca. 15 mm long) were vertically mounted in a 5-ml organ bath filled with Tyrode solution (in mM; NaCl 137, KCl 2.9, CaCl2 1.8, MgCl2 2.1, NaHPO4 0.4, NaHCO3 11.9, glucose 5.6) bubbled with air and kept at 36–37°C. The tissues were equilibrated under a tension of 0.4 g for 60 min, during which 1 μM carbachol was repeatedly applied until tissue responsiveness became reproducible. The contractile responses to the muscarinic agonist McN-A343, pilocarpine, and carbachol were measured with an isotonic transducer (JD-112S, Nihon Kohden, Tokyo, Japan) and expressed as percentage of the contractile response to the prior application of 1 μM carbachol. Concentration–response curves of the agonists were obtained by means of application at ascending concentrations spaced by three- or 3.3-fold. When the pA2 values of the muscarinic antagonists were determined, the agonist concentration–response curve was repeatedly measured at 20–25 min intervals in the presence of an antagonist at three to five different concentrations which were changed with three- or 3.3-fold increments. Antagonists used were methoctramine and AF-DX116 (both M2 selective), 1,1-dimethyl-4-diphenylacetoxypiperidiniumiodide (4-DAMP) and darifenacin (M3 selective), and atropine (nonselective muscarinic antagonist).
Simultaneous measurements of [Ca2+]c and tension
Changes in [Ca2+]c and tension produced by the muscarinic agonists were simultaneously measured in a muscle tissue loaded with the fluorescent Ca2+ indicator fura 2, as described (Kwon et al., 1993). Briefly, longitudinal muscle strips (8–10 mm long) were preincubated with fura 2-AM (10 μM) in the presence of 0.02% cremophor EL for 3–4 h at room temperature. The fura 2-loaded tissue was then transferred to an 8-ml organ bath integrated in the fluorometer (CAF-100, Japan Spectroscopic, Tokyo, Japan). The organ bath was filled with a solution that had the following composition (mM): NaCl 126, KCl 6, CaCl2 2, MgCl2 1.2, glucose 14, and HEPES 10.5 (pH adjusted to 7.2 with NaOH), aerated with a mixture of 95% O2 and 5% CO2, and kept at 36–37°C. The tissue was illuminated alternately (48 Hz) with 340- and 380-nm light. The lights emitted from the tissue (F340 and F380) were collected by a photomultiplier through a 500-nm filter, and the ratio of F340/F380 was used to measure changes in [Ca2+]c. Concomitant changes in tension were isometrically measured with a force–displacement transducer. Both the F340/F380 and tension signals were recorded on a two-channel pen recorder (TA-240, Gould, U.S.A.). Experiments were started after the changes in [Ca2+]c and tension caused by high-K+ external solution (70 mM K+) became reproducible.
Agonist concentration–response curves for the changes in [Ca2+]c and tension were obtained by cumulative application as described above. The Ca2+ and tension responses to the agonists were expressed as percentage of the respective corresponding responses to 70 mM K+.
Tension measurements in permeabilized preparations
A muscle strip (0.1 mm wide and 3 mm long) was dissected from the separated longitudinal muscle layer, and mounted horizontally in a 1-ml organ bath with its one end fixed to the siliconized bottom of the bath with a pin and the other attached to the thin lever of the force–displacement transducer with a thread. The tissue was treated with 80 mg ml−1 α-toxin for 20–30 min to permeabilize muscle cells, as described (Kwon et al., 2000). The skinned preparation was then equilibrated in a Ca2+-free solution under a tension of 50 mg for 60–90 min, during which the bath solution was replaced with a Ca2+-containing solution at intervals until the resulting rises in tension became constant. The Ca2+-free solution consisted of (mM) K+ propionate 130, MgCl2 4, Na2-ATP 5, creatine phosphate 10, creatine phosphokinase 2, Tris-maleate 20, and ethyleneglycol-bis (b-aminoethyl ether)N, N, N′, N′,-tetraacetic acid (EGTA) 2 (pH 6.8). Solutions containing various Ca2+ concentrations (pCa 6.5, 6.0, 5.5, and 5.0) were prepared by adding appropriate amounts of CaCl2 to the Ca2+-free solution. The apparent binding constant of EGTA for Ca2+ was considered to be 1 μM at pH 6.8 and at 25°C (Itagaki et al., 1995).
Agonist activity in releasing Ca2+ from the internal stores was examined as follows: the skinned preparation was exposed to pCa 5 solution for 10 min in order for the Ca2+ stores to be filled, and after reintroduction of the Ca2+-free solution, a muscarinic agonist and then the potent Ca2+-releasing drug caffeine were applied. Caffeine served to check the amount of stored Ca2+ remaining after the application of the muscarinic agonist (Komori et al., 1995). When agonist activity in modulating the Ca2+ sensitivity of the contractile proteins was examined, the skinned preparations used were previously treated with the Ca2+ ionophore A23187 (10 μM) for 10 min to eliminate Ca2+ store function (Itagaki et al., 1995). Tension changes produced by application of ascending Ca2+ concentrations (pCa 6.5–5.0) were measured in the absence and presence of an agonist. The sizes of the tension responses were expressed as percentage of the sustained tension increase by pCa 5 applied before starting the experiments. Experiments were carried out at room temperature (23–25°C).
Recording of the membrane potential
The separated longitudinal muscle layer was folded in thirds at right angle to the longitudinal axis, and pinned over a labor block in an organ bath. The bath had a volume of 1.5 ml, and was irrigated at a rate of 4–5 mlmin−1 with Tyrode solution kept at 32°C and bubbled with air. For the intracellular recordings of the membrane potential, smooth muscle cells were penetrated with 3M KCl-filled glass microelectrodes of 40–60 MΩ resistance (Komori and Ohashi, 1988). Before starting experiments, the tissue was treated with the myosin light-chain kinase inhibitor wortmannin (10 μM) for 30 min, since this procedure was effective for long in weakening smooth muscle movement with no significant alteration in spontaneous electrical activity or in acetylcholine-evoked depolarization (Burke et al., 1996). It was also reported that 10 μM wortmannin causes neither direct blockade of voltage-dependent Ca2+ channels nor the muscarinic cationic channel in ileal muscle cells (Unno et al., 1998). In the present experiments, the pretreatment with wortmannin allowed a long-lasting microelectrode impalement of the cell. A muscarinic agonist was applied to the tissue by changing the bathing solution to another of identical composition but containing the drug. Membrane potential changes were measured via a microelectrode amplifier (MEZ-8101, Nihon Kohden, Tokyo, Japan), displayed on an oscilloscope and a thermal array recorder (Nihon Kohden, RTA-1100), and also stored on a PCM data recorder (RD-111T, TEAC, Tokyo, Japan).
Single cells isolated enzymatically from the longitudinal muscle layer were also used to measure changes in the membrane potential (Unno et al., 2000). The single cells were placed in a 0.5-ml organ bath filled with a solution consisting of (mM) NaCl 134, KCl 6, CaCl2 2, MgCl2 1.2, glucose 14, and HEPES 10.5 (titrated to pH 7.2 with NaOH) at room temperature (23–26°C), and held under the current clamp mode with the nystatin perforated patch-clamp technique. Patch pipettes with a tip resistance of 4–6 MΩ were filled with a solution that had the following composition (mM): KCl 134 and HEPES 10.5 (adjusted to pH 7.4 with KOH), containing nystatin at 0.2 mg ml−1. These conditions rarely allowed the cells to exhibit spontaneous electrical activity, whereby the depolarizing effect of muscarinic agonists could be evaluated without complications involved in action potential discharge (Unno et al., 2000). A muscarinic agonist was applied by replacing the bath solution with another of the identical composition but containing the drug. When electrotonic potentials were evoked, current pulses with varied strengths and polarities were delivered by a stimulator (DPS-1100D, Dia Medical System, Tokyo, Japan). Changes in the membrane potential were measured via a patch-clamp amplifier (CEZ-2400, Nihon Kohden, Tokyo, Japan), stored on a PCM data recorder and replayed onto a thermal array recorder for analysis and illustration.
Data analysis
The agonist concentration–response curves were analyzed using computer software (SPSS Inc., DeltaGraph 4.0) that fits the data directly with a logistic function, providing the EC50 value (the concentration required for an agonist to produce a half-maximal response), the maximum response (Emax), and Hill coefficient for the curve. The pA2 values of the muscarinic antagonists were obtained by Schild regression analysis, where the EC50 values in the presence of an antagonist at varied concentrations were divided by that in the absence of the antagonist to obtain the dose ratio. The pCa–tension curves were also analyzed by means of curve fitting, providing the pCa required to produce half-maximal increase in tension and Emax.
Values in the text are given as means±s.e.m. of the number of the experiments on tissues or cells (n). Student's unpaired t-test was used to determine the statistical significance of differences between two group means. For statistical comparison between multiple group means, one-way analysis of variance (ANOVA) followed by a post hoc Bonferroni test to compare between two of multiple groups were used. The differences were judged to be statistically significant when P<0.05.
Drugs
The following drugs were used: acetylcholine chloride, N,N′-bis [6-[[(2-methoyphenyl)methyl]amino]hexyl]-1,8-octanediamine tetrahydrochloride (methoctramine), McN-A343, nicardipine, nystatin, physostigmine, pilocarpine hydrochloride, α-toxin (all purchased from Sigma, St Louise, MO, U.S.A.), carbachol chloride (CCh; from Tokyo kasei, Tokyo, Japan), atropine sulfate, caffeine, tetrodotoxin (TTX), wortmannin (from Wako, Tokyo, Japan), 11-[[2-[(diethlamino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3-b][1,4] benzodiazepine-6-one (AF-DX116), 4-DAMP (from Tocris, Ballwin, MO, U.S.A.), darifenacin (kindly given as a gift from Pfizer, Kent, U.K.), ω-conotoxin GVIA (from Bachem, Budendorf, Switerland), EGTA, fuar-2/AM (from Dojin Kagaku, Kumamoto, Japan). All other chemicals of the highest grade commercially available were obtained from Sigma or WAKO.
Results
Contraction
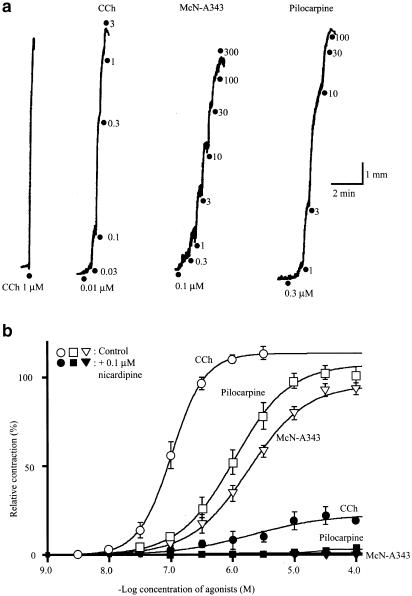
In ileal longitudinal muscle strips, ascending concentrations of McN-A343 (0.1–100 or 300 μM) or pilocarpine (0.1–30 or 100 μM), as well as carbachol (0.01–1 or 3 μM), produced contractions in a concentration-dependent manner (Figure 1a). The averaged concentration–response curves of the three agonists are shown in Figure 1b, in which the amplitude of contractions was expressed as percentage of that of a contraction evoked by 1 μM carbachol beforehand. The cumulative concentration–response data obtained in each experiment were subjected to curve-fitting analysis to determine the EC50, Emax, and Hill coefficient. Statistical analysis by ANOVA showed that there were significant differences between the three agonists in each parameter (P<0.05). The EC50 values of McN-A343 and pilocarpine were 2.29±0.25 and 1.83±0.62 μM (n=16 each), respectively, higher (P<0.05, Bonferroni test) than the corresponding value for carbachol (0.11±0.02 μM, n=13). The Emax values for McN-A343 and pilocarpine were 98±3% and 106±3% (n=16 each), respectively; the former mean value, but not the latter, differed significantly (P<0.05, Bonferroni test) from the corresponding value for carbachol (111±2%, n=13). These Emax values gave the relative efficacies of McN-A343 and pilocarpine to carbachol as the respective values of 0.88 and 0.95. The Hill coefficients for McN-A343 and pilocarpine were 1.0±0.1 and 1.4±0.1 (each n=16), respectively. The mean values for both the agonists were significantly smaller than that for carbachol (2.2±0.3, n=13) (P<0.001, Bonferroni test).
Figure 1.
The contractile effects of McN-A343, pilocarpine, and carbachol (CCh) in the guinea-pig ileal longitudinal muscle. (a) The contractile responses produced by the application of ascending concentrations of each agonist as indicated were isotonically recorded. (b) Averaged concentration–response curves for the agonist-evoked contractions in the absence (open symbols) and presence of 0.1 μM nicardipine (closed symbols). Each point is the mean±s.e.m. of the size of contractions expressed as percentage of the initial response to 1 μM CCh-evoked contraction beforehand; n=13–16 without nicardipine and n=3–5 in its presence.
Submaximal effects of McN-A343 (10 μM) and pilocarpine (3 μM) were almost unaffected by the neuronal blocker TTX. Actually, in the presence of 1 μM TTX applied 10 min beforehand, the McN-A343 and pilocarpine evoked contractions corresponding to 107±3% (n=3) and 105±3% (n=4) of control, respectively. Similarly, they evoked, respectively, 104±1 and 109±8% contractions (each n=3) in the presence of 1 μM ω-conotoxin GIVA that was shown to inhibit acetylcholine release in ileal muscle tissues (Cousins et al., 1993). In the presence of the choline esterase inhibitor physostigmine (30 nM) effective enough to double or treble the size of 10 nM acetylcholine-evoked contractions (n=3), McN-A343 and pilocarpine evoked 101±1 and 106±6% contractions of control (n=3 each), respectively. Therefore, there was no evidence for the involvement of neuronal factors in the contractile effects of both agonists, as described for carbachol.
As shown in Figure 1b (see closed symbols), the concentration–response curves of McN-A343 and pilocarpine were almost flattened after a 10-min treatment with the voltage-dependent Ca2+ channel blocker nicardipine (0.1 μM). The corresponding curve of carbachol was also markedly depressed in respect of the Emax but not flattened. Thus, the contractile response to any agonist was largely dependent on the activation of voltage-dependent Ca2+ channels that admit Ca2+ into the cell.
Antagonism by muscarinic antagonists
M2 and M3 receptors are found as the major subtypes of the muscarinic receptors in the guinea-pig ileal smooth muscle (Caufieled, 1993; Eglen et al., 1996). Therefore, we investigated the effects of the M2 or M3 selective antagonists on the concentration–response curves for contractions evoked by McN-A343, pilocarpine, and carbachol.
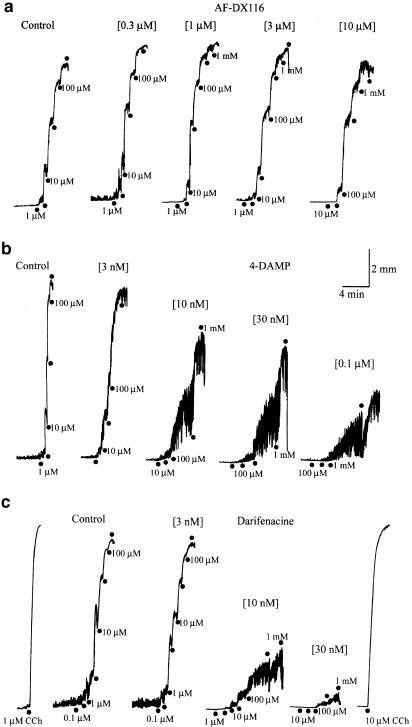
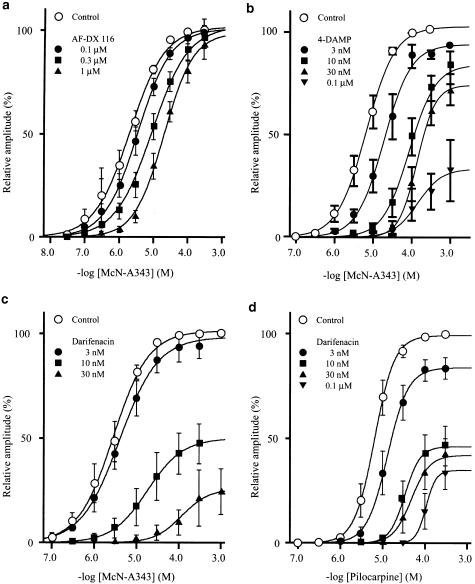
The M2 selective antagonist methoctramine (0.1–3 μM) produced a rightward parallel shift of the agonists' curves, that is it increased the EC50 value of each agonist in a concentration-dependent manner without notable depression of the Emax. Another M2 selective antagonist AF-DX116 (0.1–3 or 10 μM) behaved similarly. Its actual behaviors on the contractile response to cumulative applications of McN-A343 are illustrated in Figure 2a (also see Figure 3a). The pA2 values of these antagonists against the individual agonists were determined by Schild plot regression analysis. As presented in Table 1, the pA2 value of methoctramine against McN-A343 (6.92) or pilocarpine (6.60) was significantly (P<0.05, Bonferroni test) greater than that against carbachol (5.87). For AF-DX116, the pA2 value against McN-A343 (7.14) was significantly (P<0.001, Bonferroni test) greater than that against pilocarpine (6.32) or carbachol (6.41). The results suggested that the apparent order of the agonists for the M2 antagonist sensitivity is McN-A343> pilocarpine> carbachol.
Figure 2.
Contractions produced by McN-A343 in the absence (control) and presence of the muscarinic antagonist AF-DX116 (a), 4-DAMP (b) and darifenacin (c), applied at various concentrations as indicated in the parentheses. McN-A343 was applied at stepwise increasing (three or 3.3-fold) concentrations at points marked by the closed circles. In (c), carbachol (CCh) was applied in the absence and presence of 30 nM darifenacin, at concentrations as indicated. Note the change in the response pattern and weak overcoming of McN-A343 in the presence of 4-DAMP and darifenacin.
Figure 3.
Averaged concentration–response curves for contraction evoked by McN-A343 (a–c) and pilocarpine (d), in the absence (control) and presence of AF-DX 116 (a), 4-DAMP (b), or darifenacin (c and d). The size of contractions was expressed as percentage of that of a maximum contraction in the absence of antagonists. Each point indicates the mean±s.e.m. of five to eight measurements. Note severe depression of the maximum response for McN-A343 and pilocarpine by 4-DAMP or darifenacin. See text for details.
Table 1.
pA2 values of muscarinic antagonists against the contractile responses to McN-A343, pilocarpine, and carbachol in guinea-pig ileal longitudinal muscle
| Antagonist | McN-A343 | Pilocarpine | Carbachol | |
|---|---|---|---|---|
| AF-DX 116 | pA2 | 7.14±0.12*,** | 6.32±0.13 | 6.41±0.08 |
| Slope | 1.00±0.10 | 1.22±0.15 | 0.97±0.07 | |
| n=7 | n=5 | n=7 | ||
| Methoctramine | pA2 | 6.92±0.25* | 6.60±0.19* | 5.87±0.08 |
| Slope | 0.97±0.16 | 0.94±0.09 | 0.99±0.07 | |
| n=6 | n=6 | n=7 | ||
| 4-DAMP | pA2 | ND | 8.70±0.08 | 8.68±0.11 |
| Slope | ND | 1.02±0.15 | 1.10±0.16 | |
| n=6 | n=5 | n=5 | ||
| Darifenacin | pA2 | ND | ND | 8.50±0.13 |
| Slope | ND | ND | 0.82±0.11 | |
| n=7 | n=6 | n=6 |
Each value represents mean±s.e.m. of the number of experiments, (n). ND: not determined. The slope indicates that of regression line obtained by Schilds plot analysis. One-way analysis of variance (ANOVA) showed that there were significant differences between the group means for AF-DX 116 (P<0.0002) and methoctramine (P<0.005). * and ** represent significantly different (P<0.05) from the corresponding value for carbachol and that for pilocarpine, respectively, which were evaluated by a post hoc Bonferroni test.
The M3 selective antagonists, 4-DAMP (3–100 nM) and darifenacin (3–30 nM), each shifted the McN-A343 concentration–response curve to the right and depressed the Emax, in a concentration-dependent manner (Figure 3b and c). The depression of the Emax was more severe with darifenacin, since the Emax was decreased to 21±3% (n=7) of control by 30 nM darifenacin, and to 51±5% (n=6) by 100 nM 4-DAMP. Adachi et al. (1996) previously observed Emax depression by 4-DAMP in the McN-A343 concentration–response curve that was isometrically obtained in this tissue. Thus, our observations appeared not to be attributable to the method of isotonic recording. The pattern of the contractile response to McN-A343 was also altered by 4-DAMP and darifenacin. As shown in Figure 2b and c, in the presence of either antagonist at certain concentrations, the tissue responded to a concentration of McN-A343 with repetitions of brief contractions which progressively increased in size and partially fused into a slowly developing tonic contraction. In the presence of 30 nM darifenacin where McN-A343 had been little effective, carbachol could evoke a full size of contraction (Figure 2c), implying that the insurmountable antagonism of McN-A343 was not ascribed to deterioration of the tissues. Severe depression of the Emax and changes in the response pattern did not allow pA2 values of the M3 antagonists to be determined. In some experiments, we made an attempt to use atropine, a nonselective muscarinic antagonist. The antagonist at 0.1 and 0.3 μM reduced the Emax for McN-A343 to 65 and 15% of control, respectively, whereas even at 10 μM, the Emax for carbachol remained unaffected.
When pilocarpine was used to generate concentration–response curves, 4-DAMP behaved as a competitive antagonist within the concentration range of 3–100 nM; it produced a rightward parallel shift of the pilocarpine's curve in a concentration-dependent manner with a pA2 value of 8.70±0.08 (n=5) (Table 1). The mean value was close to its published affinity constant for the M3 receptor (8.9–9.3; see Table 1 in Caufieled, 1993). When 4-DAMP was used at a higher concentration (300 nM or 1 μM), a depression of the Emax of pilocarpine's curve by 30–40% was observed. On the other hand, darifenacin (3–100 μM) behaved as a noncompetitive antagonist; it not only shifted the pilocarpine's curve to the right, but also depressed the Emax, in a concentration-dependent manner (Figure 3d). With 100 nM darifenacin, the Emax was decreased to 34±8% (n=6) of control. Darifenacin also altered the pattern of the response to pilocarpine, as observed for the McN-A343 response. Therefore, its pA2 value against pilocarpine was not determined. Either 4-DAMP or darifenacin (3–300 nM) produced rightward parallel shift of the carbachol's curve with the pA2 values of 8.68±0.11 (n=5) and 8.50±0.13 (n=6), respectively. These values were consistent with the idea that carbachol contraction is an M3-mediated response (Eglen et al., 1996).
Increases in [Ca2+]c and tension
Using fura-2-loaded muscle strips, we examined the effects of McN-A343 and pilocarpine on [Ca2+]c and tension, comparing with those of carbachol.
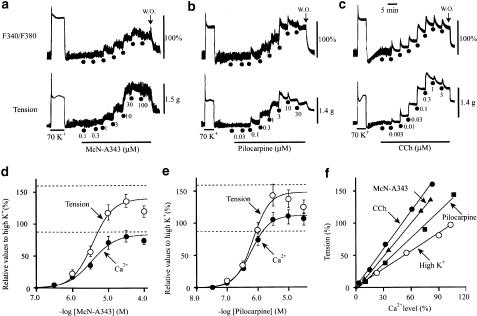
The cumulative application of McN-A343 (0.1–100 μM) or pilocarpine (0.01–30 μM) increased [Ca2+]c in a concentration-dependent manner with a parallel rise in tension (Figure 4a and b). Similar results were obtained with carbachol (0.001–3 μM; Figure 4c). The increases in [Ca2+]c and tension were expressed as percentage of the sustained component of the respective corresponding responses to 70 mM K+. The averaged concentration–response curves for McN-A343 obtained from seven preparations are shown in Figure 4d, and the corresponding curves for pilocarpine (n=7) in Figure 4e. From curve fitting of the averaged data points, the EC50 values of McN-A343 for the Ca2+ and tension responses were estimated to be 2.5 and 3.8 μM, respectively, and the corresponding respective values of pilocarpine to be 0.6 and 0.8 μM. The EC50 value of each agonist for either response was greater than the corresponding EC50 value of carbachol estimated similarly (0.05 μM for Ca2+ response and 0.04 μM for tension response; n=8). The Emax's of the tension response for McN-A343 and pilocarpine were 140 and 148% of the 70 mM K+ response, respectively, and these values were smaller than the corresponding value for carbachol (165%: see the dashed lines in Figure 4d and e). For the Emax value of the Ca2+ response, McN-A343 and pilocarpine were 83 and 113% of the 70 mM K+ response, respectively, and these values were similar to or rather greater than the value estimated for carbachol (86%).
Figure 4.
Increases in cytosolic Ca2+ level ([Ca2+]c) (upper traces) and in tension (lower traces) produced by McN-A343 (a), pilocarpine (b) and carbachol (CCh) (c) applied at ascending concentrations, as indicated, in fura-2-loaded muscle strips. The concentration–response curves of McN-A343 (d) and pilocarpine (e) for the [Ca2+]c and tension increases, which were expressed as percentage of those evoked by 70 mM K+ (see a–c). Each point indicates the mean±s.e.m. of four or five measurements. In (d and e), the dashed lines indicate the mean maximum response to CCh (n=8) for the tension (the upper) and [Ca2+]c (the lower). (f) Relations between [Ca2+]c and tension levels, in which the points were originated from the mean values for the Ca2+ and tension responses in (d and e). The slope of regression lines calculated from the data points was 1.66 for McN-A343, and 1.31 for pilocarpine. The regression line for CCh (slope=1.97) was similarly obtained from the cumulative concentration–response data (n=8), and the corresponding line for high K+ (slope=1.00), obtained at ascending concentrations of 15, 25, 40, and 70 mM (n=10). Note that the regression lines for McN-A343 and pilocarpine are steeper than the line for high K+, but less steep than that for CCh. See text for details.
Figure 4f shows relation between [Ca2+]c and tension levels in the averaged concentration–response curves for McN-A343, pilocarpine, and carbachol, and in the corresponding curves for high K+ (n=10) obtained by cumulative applications at 15, 25, 40, and 70 mM. The slope of the regression line calculated using each set of data points was 1.66 for McN-A343 and 1.31 for pilocarpine, less steep than that for carbachol (1.97), but steeper than the reference slope (high K+; 1.00), indicating that McN-A343 and pilocarpine as well as carbachol can elicit greater contractions than high K+ at a given [Ca2+]c. This implied that all the agonists cause an increase in Ca2+ sensitivity of the contractile proteins (see below).
Ca2+ store release and Ca2+ sensitization
Using α-toxin-permeabilized muscle strips, we studied the effects of McN-A343 and pilocarpine on intracellular Ca2+ stores and myofilament Ca2+ sensitivity.
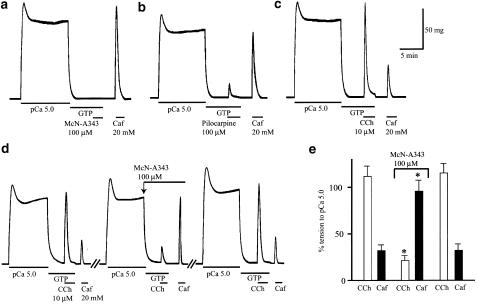
The Ca2+ stores in the permeabilized tissues were loaded with Ca2+ by means of a 10-min exposure to pCa 5 solution, during which a rise in tension occurred that peaked within 2 min and then declined to a sustained elevated level. At 5 min after reintroduction of Ca2+-free relaxing solution, a maximally effective concentration of McN-A343 (100 μM) was applied in the presence of GTP (100 μM). However, as shown in Figure 5a, no appreciable rise in tension was elicited (n=5). Subsequent application of 20 mM caffeine evoked a marked rise in tension, indicating that the Ca2+ store retained an adequate amount of releasable Ca2+. Even at a higher concentration of 300 μM, McN-A343 was almost ineffective (n=4). Application of pilocarpine (100 μM) after Ca2+ loading resulted in a rise in tension corresponding to 23±3% (n=5) of the sustained tension increase by pCa 5, which was followed by a greater tension generation because of 20 mM caffeine (Figure 5b). The efficacy of pilocarpine was given as the value 0.12 relative to 10 μM carbachol that produced a rise in tension corresponding to 113+11% (n=5) of the pCa 5-evoked tension increase (Figure 5c). As shown in Figure 5d, the effect of 10 μM carbachol was inhibited by 100 μM McN-A343 applied following Ca2+ loading, and instead the effect of subsequent 20 mM caffeine increased, in a reversible manner. The quantified data (n=6) are shown in Figure 5e. The results suggested that McN-A343 can bind to the muscarinic receptors mediating Ca2+ store release without activating them.
Figure 5.
Effects of the muscarinic agonists on the intracellular Ca2+ stores in α-toxin-permeabilized muscle strips. In (a–c), following the loading of the stores with Ca2+ by exposure to pCa 5 solution as indicated, 100 μM McN-A343 (a), 100 μM pilocarpine (b) and 10 μM carbachol (CCh) (c) were applied in the absence of extracellular Ca2+ but in the presence of 100 μM GTP, which was followed by application of 20 mM caffeine (Caf). The records in (a–c) are from different preparations. In (d), a series of applications of pCa 5, 10 μM CCh, and then 20 mM Caf were repeated three times in a preparation, except in the second trial, the CCh was applied in the presence of 100 μM McN-A343. (e) The summarized results from experiments as in (d). The sizes of CCh- and Caf-evoked tension responses were expressed as percentage of the sustained tension increase by pCa 5 applied for Ca2+ loading. Each column indicates mean±s.e.m. of six measurements. *Significantly different (P<0.05) from the corresponding value for control.
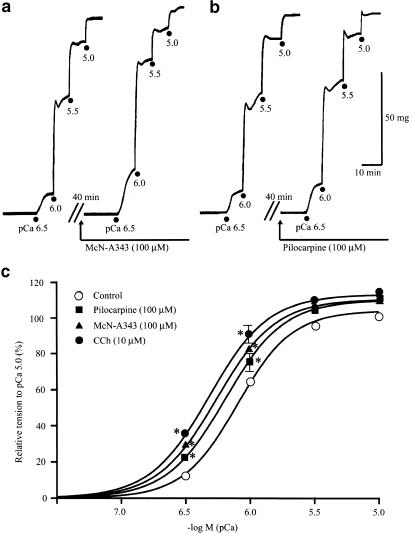
Figure 6a and b exemplify the respective experiments with McN-A343 and pilocarpine, in which their effects on pCa–tension curve were studied. Application of either agonist (100 μM) caused increases in pCa-induced tension development without changing the maximum tension level. Consequently, as summarized in Figure 6c, the pCa–tension curve was shifted to the left in parallel along the pCa axis after the application of McN-A343 or pilocarpine. Similar effects were obtained with 10 μM carbachol (Figure 6c). The mean value of pCa required to produce half-maximum tension increase was 6.35±0.03 (n=5) for McN-A343, 6.30±0.05 (n=4) for pilocarpine, and 6.38±0.03 (n=8) for carbachol, each mean value was significantly greater (P<0.05) than the control (6.21±0.06, n=18). The difference between any pair of the mean values for the three agonists was statistically insignificant.
Figure 6.
The effects of the muscarinic agonists on myofilament Ca2+ sensitivity in α-toxin-permeabilized preparations. (a and b) Tension responses to ascending concentrations of Ca2+ (pCa 6.5–5) before and after application of 100 μM McN-A343 (a) or 100 μM pilocarpine (b). (c) pCa–tension curves in the absence (control) and presence of McN-A343, pilocarpine, and carbachol (CCh). Each point indicates mean±s.e.m. of eights measurements for control, the fives for McN-A343, the fours for pilocarpine, and the eights for carbachol. *Significantly different (P<0.05) from the corresponding value for control.
Effects on the membrane potential
To examine the effect of McN-A343 and pilocarpine on the membrane potential, we used single ileal muscle cells held in the current clamp mode using nystatin-perforated patch-clamp techniques (Unno et al., 2000). Most of the cells studied were electrically quiescent and had a resting potential of −52.7±4.3 mV (n=15). From the size of electrotonic potentials evoked by injection of a hyperpolarizing current of 5–10 pA, the input resistance of the cell was estimated to be 1.8±0.2 GΩ (n=11).
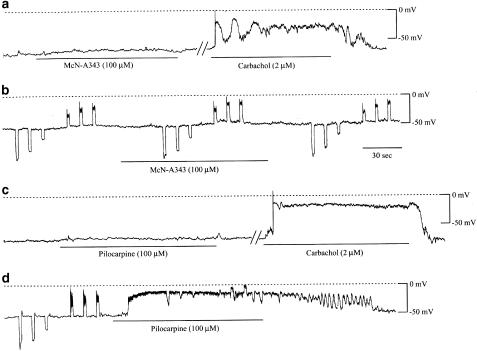
Application of the maximally effective concentrations of McN-A343 (100 or 300 μM) produced no or little appreciable change in the membrane potential in five out of six cells (Figure 7a and b). Electrotonic potentials also remained unchanged (Figure 7b). The one remaining cell, which was generating spike potentials spontaneously, responded to 100 μM McN-A343 with a sustained depolarization of 6–8 mV during which spikes were discharged at an increased frequency. Pilocarpine (100 μM) produced no noticeable change of the membrane potential in four out of nine cells (Figure 7c), but did produce a sustained depolarization in the five remaining cells (Figure 7d). Its size varied from 10 to 40 mV in different cells, giving a mean value of 16.4±2.9 mV (n=5). A greater depolarization was accompanied by the reduction of electrotonic potentials (Figure 7d). In the cells with no or little sensitivity to McN-A343 or pilocarpine, subsequent application of carbachol (2–10 μM) invariably elicited sustained or oscillatory depolarizations (see Figure 7a and c), as reported previously (Unno et al., 2000).
Figure 7.
The effects of McN-A343 and pilocarpine on the membrane potential in single ileal muscle cells. Changes in the membrane potential were measured by nystatin-perforated patch-clamp techniques. In this cell (a), McN-A343 produced no appreciable change of the membrane potential, whereas subsequent carbachol application did produce an oscillatory depolarization. In another cell (b), in which hyperpolarizing and depolarizing current pulses (10, 20 and 30 pA; for 2 s each) were applied to evoke electrotonic potentials, McN-A343 hardly had any effect on the electrotonic potentials or resting potential. Pilocarpine produced no appreciable depolarization in a cell (c), which subsequently responded to carbachol with a large depolarization, and in another cell (d), it produced a large depolarization of 40 mV with marked decreases of the evoked electrotonic potentials.
To examine the effects of the agonists on the electrical spike activity, we used tissue preparations that were usually active in generating action potentials spontaneously (Bolton, 1972). The tissues used had been pretreated with 10 μM wortmannin for 30 min to minimize the mechanical activity (Burke et al., 1996). The resting membrane potential was −44.5±1.4 mV (n=25) with cell-to-cell variations from −32 to −58 mV.
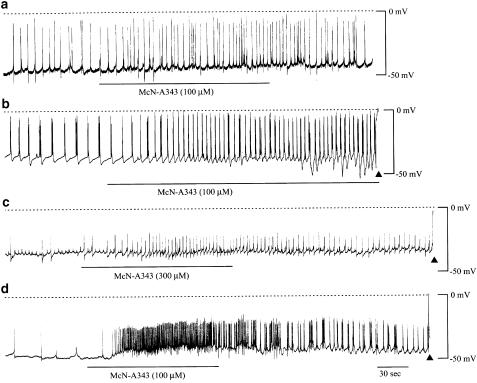
Figure 8 shows traces recorded from four different tissues, in all of which McN-A343 (100 or 300 μM) produced an increased frequency of action potential discharge. Similar effects were observed in nine other tissue preparations. However, as seen from Figure 8a–c, in many cases the increase in the spike frequency occurred without noticeable depolarization. In these cases, the increase in the spike frequency was associated with a steeper rise of the initial depolarizing phase of the action potentials (see Figure 8b), suggesting that McN-A343 produces a net inward current to trigger action potential generation. It was also noted that a burst discharge of several spikes was followed by a brief, profound hyperpolarization (Figure 8b), so that the burst discharge failed to become longer in its duration (cf. Figure 9a and b). In four preparations, a sustained depolarization with a size of 5–10 mV occurred that had an increased frequency of spike discharge superimposed. The most pronounced depolarization elicited is shown in Figure 8d.
Figure 8.
The effects of McN-A343 on the electrical membrane activity in ileal longitudinal muscle tissues. Changes of the membrane potential were recorded by the intracellular microelectrode technique under conditions where smooth muscle movements were suppressed by 30-min pretreatment with wortmannin. McN-A343 was applied by changing the bath solution to another containing the drug at 100 or 300 μM for a period as indicated by the bars. Application of McN-A343 produced an increased frequency of action potential discharge without noticeable depolarization (a–c) or with a pronounced depolarization (d).
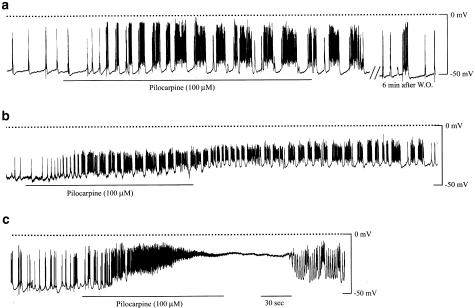
Figure 9.
The effects of pilocarpine on the electrical membrane activity in ileal longitudinal muscle tissues. Membrane potential recordings and drug applications were performed in the same way as described in Figure 8. Application of pilocarpine (100 μM) produced burst-type discharges of the action potentials with a slight sustained depolarization (a) or with a larger sustained depolarization (b). In some preparations, pilocarpine elicited a strong depolarization which caused depolarization block of the spike discharge following an initial increase of the discharge frequency (c).
Application of pilocarpine (100 μM) caused repetitions of the burst-type discharge. The duration of each burst discharge, or the number of spikes within it, increased gradually with time for a while after the beginning of the agonist application (Figure 9a and b). In the individual bursts, spikes arose on a slow wave-like depolarization. Furthermore, the slow waves were often superimposed on a sustained depolarization of 5–20 mV (Figure 9a and b). In three preparations, pilocarpine (100 μM) evoked a greater depolarization that initially carried an increased frequency of the spike discharge and then resulted in a cessation of this (depolarization block), as shown in Figure 9c. When carbachol was applied at 1–3 μM, burst-type discharges of spike potentials were elicited that resembled those seen with pilocarpine. At a higher concentration of 10 μM, a profound depolarization was evoked with an initial increase and subsequent cessation of the spike discharge (n=3) (data not shown, but very similar to Figure 9c obtained with pilocarpine). The depolarization reached a level near −10 mV reported for the equilibrium potential of the muscarinic receptor-operated cationic channel (Benham et al., 1985).
Discussion
In the present study, we systematically investigated various effects of the muscarinic partial agonist McN-A343 and pilocarpine on guinea-pig ileal longitudinal muscle, comparing with those of the full agonist carbachol. The results may provide some insights into understanding of receptor signaling mechanisms underlying the muscarinic contractile response.
A primary mechanism of the muscarinic contraction
The present result indicated that McN-A343 has no or little activity in releasing Ca2+ from intracellular stores (Figure 5), as reported by other studies (Hishinuma et al., 1997; Okamoto et al., 2002). Nonetheless, the agonist produced both rises in tension and [Ca2+]c with a maximum efficacy not much different from carbachol, although higher concentrations were required to produce a maximum response (Figure 4). This also held true for pilocarpine the Ca2+-releasing activity of which was very poor compared to carbachol (Figure 4 and Figure 5). The contractions evoked by all three agonists were severely reduced by the voltage-dependent Ca2+ entry blocker nicardipine (Figure 1). These findings are difficult to reconcile with the general thought that the release of stored Ca2+ is the major cause of the muscarinic contractions (Eglen et al., 1996; Sawyer & Ehlert, 1999). Instead, we consider that the acceleration of action potential discharge is primarily important for the muscarinic contraction. This comes from the microelectrode experiments in which McN-A343 and pilocarpine, not to mention carbachol, surely increased the frequency of action potential discharges (Figure 8 and Figure 9). The action potential is the most important mechanism by which a rise in [Ca2+]c is produced in smooth muscles that normally generate action potentials (Bolton, 1979). Kohda et al. (1997) observed in single ileal muscle cells that when action potentials were evoked repeatedly by current injection, associated Ca2+ signals fused into a greater rise in [Ca2+]c. Such fusion of the action potential-triggered Ca2+ signals in each individual cell may account for the agonist-induced [Ca2+]c increases that were observed in multicellular tissue preparations in the present study (Figure 4).
Apart from acceleration of the spike discharge, another common effect of the three agonists that favors contraction is to increase the Ca2+ sensitivity of the contractile proteins. This comes from both observations that the regression line representing the relation between tension and [Ca2+]c levels in the concentration-dependent responses was steeper for all three agonists than for high K+ (Figure 4f), and that any agonist produced a significant leftward shift of the pCa–tension curve (Figure 6c). Therefore, it is likely that the muscarinic contractile response results primarily from the integration of a rise in [Ca2+]c because of accelerated spike discharge and an increase in the Ca2+ sensitivity of the contractile proteins.
The role M3 receptors in the contractile response
In intestinal smooth muscle, muscarinic receptor activation produces depolarization that triggers and accelerates spike discharge, except when it is so extreme that discharge ceases (Bolton, 1972; the present study, Figure 9). The muscarinic depolarization is known to occur because of the opening of cationic channels that is mediated by M2 receptors (Zholos & Bolton, 1997; Komori et al., 1998) and potentiated by M3 activation through two parallel pathways: one involves Ca2+ store release mediated by the PLC/InsP3 system (Pacaud & Bolton, 1991; Komori et al., 1993; Zholos et al., 1994) and the other, a more direct interaction with M2 receptors in which it is assumed that a massage generated by M3 activation acts at the receptor level to potentiate M2-initiated cationic channel opening (Zholos and Bolton, 1997; Okamoto et al., 2002).
In the present experiments, the order of agonist efficacy for depolarization was the same as for Ca2+ store release that represents M3 activation, that is, carbachol>pilocarpine≫ McN-A343. The finding suggests that M3 activation may contribute to voltage-dependent Ca2+ entry into the cell by potentiating the M2-mediated cationic current through both the indirect (Ca2+ store release) and direct pathways, and so in turn by increasing the size of depolarization and the frequency of spike discharges. The idea is supported by our previous observation that depletion of Ca2+ stores attenuated carbachol-evoked depolarizations in single ileal muscle cells (Kohda et al., 1998; Unno et al., 2000). The present observation that pilocarpine produced oscillatory depolarizations (Figure 9a and b) may also indicate that its electrical effects have some contribution from M3 activation, since carbachol-evoked oscillations in depolarization occur as a result of cyclical Ca2+ release from the store (Komori et al., 1993; Kohda et al., 1998). To the contrary, the electrical effects of McN-A343 seem likely to hardly benefit from M3 activation, since it rarely produced an appreciable depolarization, and the depolarizations evoked were relatively small and lacked the oscillatory component.
One might expect a more straightforward function of M3 activation, for example, the Ca2+ released from the store directly activates the contractile proteins. The direct function would be rather important for an initial brief phase of contractions to high concentrations of a full agonist such as carbachol, or tension generation by agonists in the absence of extracellular Ca2+ (Brading & Sneddon, 1980; Blackwood & Bolton, 1993).
Functional interaction of M2 and M3 receptors
A major finding in the present analyses using muscarinic antagonists is that M2 antagonists (methoctramine and AF-DX116) produced rightward parallel shift of the concentration–response curves of all three agonists, whereas M3 antagonists (darifenacine and 4-DAMP) both depressed the Emax for McN-A343, one of which, darifenacine, did so for pilocarpine, and neither of which did so for carbachol (Figure 2 and Figure 3). This finding may provide evidence for the idea that the muscarinic contractile response represents an allosteric interaction of M2 and M3 receptors, in which it is assumed that M3 activation acts to potentiate the M2-induced electrical events that are primarily important for contraction (see above). The assumption may predict that the greater is amplification by M3 activation, the M2-mediated component becomes the less pronounced in the relative contribution and the contraction approaches apparently to M3 mediation.
Based on the agonist profiles as described so far, it seems likely that the contractile mechanism for McN-A343 is such a representative as that involving only a weak amplification by M3 activation, and so a high fractional receptor occupancy is required to evoke a maximal contraction. Thus, it is plausible that the McN-A343-evoked contraction tends to approach an M2-mediated response, and the Emax will be depressed by an M3 antagonist from the outset because M3 blockade results in a severe reduction of the M2 effect. To the contrary, the contractile mechanism for carbachol seems to be another representative that involves a strong amplification by M3 activation, and so requires only a low fractional receptor occupancy to evoke the Emax. Thus, carbachol contraction apparently exhibits the profiles in consistent with M3 mediation, and the agonist's curve will be rightwardly shifted with no depression of the Emax by an M3 antagonist even at a high concentration. It is interesting to note that Sawyer and Ehlert (1999), analyzing the contractile response of guinea-pig colon to the full agonist oxotremorine-M, showed that selective inactivation of most of the M3 receptors uncovered M2 participation in the agonist response. For pilocarpine, the observed M2 and M3 antagonism profiles may be explained by assuming that amplification by M3 activation is intermediate between the cases for McN-A343 and carbachol. The possible variations in the extent of the M3-mediated amplification among the three agonists might reflect their differences in the Hill coefficient of the concentration–response curve for the isotonic contraction (Figure 1b).
A possible functional array of M2 and M3 receptors
How are M2 and M3 receptors then arrayed to provide the contractile mechanisms as described above? A conventional array that comprises discrete M2 and M3 receptors alone might be unlikely, because it is difficult to explain the mechanism by which M3 activation potentiates directly the M2-mediated cationic current. Zholos & Bolton (1997) have then hypothesized the existence of hetero-oligomers of M2 and M3 receptors (M2/M3 complexes) to explain the direct amplification by M3 activation. Our recent study (Okamoto et al., 2002) suggested that two kinds of M2 receptor may exist, one of which links via Gi protein to adenylate cyclase inhibition and the other via Go protein to cationic channel opening, and that the latter kind of M2 receptor may interact directly with the Gq-coupled M3 receptors to form the M2/M3 complex. Meanwhile, Takayanagi et al. (1990) have subclassified M3 receptors into two kinds depending on differences in the sensitivity to propylbenzilylcholine mustard, a muscarinic receptor-alkylating drug. Supposing that either kind of M3 receptors take part in the M2/M3 complex, these circumstances may allow a functional receptor array that comprises the M2/M3 complex and independent M2 and M3 receptors to be tentatively depicted.
In this model, the M2/M3 complex enables M3 activation to potentiate the M2-initiated channel opening through both a direct but unidentified, and an indirect (Ca2+ store release-dependent) pathway (Okamoto et al., 2002). The independent M3 receptor also serves to do so via the indirect pathway, while the independent M2 receptor seems to make no or little contribution, since muscarinic inhibition of adenylate cyclases causes no significant contraction under standard conditions (Ehlert et al., 1999). Considering agonist-to-agonist variations in efficacy for M3 and/or M2 activation, the model may account for the interaction between M2 and M3 receptor signal transduction and yield results similar to the profiles of the three agonists that were observed in the present study. At present there is no direct evidence for expression of the M2/M3 complex in intact smooth muscles, so that the model proposed could be premature. However, it should be noted that recent coexpression experiments with M2- and M3-encoding genes have suggested heterodimerization of both gene products (Chiacchio et al., 2000; Devi, 2001). Further studies are needed to see whether M2 and M3 receptors heterodimerize in intestinal smooth muscle and to evaluate the present model and other likely ones of functional array of muscarinic receptors.
Acknowledgments
We thank Dr A.V. Zholos (St George's Hospital Medical School, London) for his critical reading of the manuscript. This work was supported by a Grant-in-Aid Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (nos. 13460141 and 14760188).
Abbreviations
- AF-DX116
11-[[2-[(diethlamino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3-b][l,4]benzodiazepine-6-one
- [Ca2+]c
cytosolic Ca2+ concentration
- CCh
carbachol chloride
- 4-DAMP
1,1-dimethyl-4-diphenylacetoxypiperidinium iodide
- EGTA
ethyleneglycol-bis(b-aminoethyl ether) N,N,N′,N′,-tetraacetic acid
- G protein
GTP-binding protein
- InsP3
inositol-1,4,5-trisphosphate
- McN-A343
4-(N-[3-chlorophenyl]-carbamoyloxy)-2-butynyl-trimethylammonium chloride
- methoctramine
N,N′-bis.[6-[[(2-methoyphenyl)methyl]amino]hexyl]-1,8-octanediamine tetrahydrochloride
- PLC
phospholipase C
- TTX
tetrodotoxin
References
- ADACHI S., KOIKE K., TAKAYANAGI I. Pharmacological characteristics of indoline derivatives in muscarinic receptor subtypes. Pharmacology. 1996;53:250–258. doi: 10.1159/000139437. [DOI] [PubMed] [Google Scholar]
- BENHAM C.D., BOLTON T.B., LANG R.J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985;316:345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- BLACKWOOD A.M., BOLTON T.B. Mechanism of carbachol-evoked contractions of guinea-pig ileal smooth muscle close to freezing point. Br. J. Pharmacol. 1993;109:1029–1037. doi: 10.1111/j.1476-5381.1993.tb13725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTON T.B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J. Physiol. 1972;220:647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTON T.B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol. Rev. 1979;59:647–671. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- BOLTON T.B., ZHOLOS A.V. Activation of M2 muscarinic receptors in guinea-pig ileum opens cationic channels modulated by M3 muscarinic receptors. Life Sci. 1997;60:1121–1128. doi: 10.1016/s0024-3205(97)00056-8. [DOI] [PubMed] [Google Scholar]
- BRADING A.F., SNEDDON P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig Taenia coli on stimulation with carbachol. Br. J. Pharmacol. 1980;70:229–240. doi: 10.1111/j.1476-5381.1980.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKE E.P., GERTHOFFER W.T., SANDERS K.M., PUBLICOVER N.G. Wortmannin inhibits contraction without altering electrical activity in canine gastric smooth muscle. Am. J. Physiol. 1996;270:C1405–C1412. doi: 10.1152/ajpcell.1996.270.5.C1405. [DOI] [PubMed] [Google Scholar]
- CAUFIELED M.P. Muscarinic receptors–characterization, coupling and function. Pharmacol. Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- CHIACCHIO S., SCARSELLI M., ARMOGIDA M., MAGGIO R. Pharmacological evidence of muscarinic receptor heterodimerization. Pharm. Acta Helv. 2000;74:315–326. doi: 10.1016/s0031-6865(99)00041-2. [DOI] [PubMed] [Google Scholar]
- COUSINS H.M., EDWARDS F.R., HIRST G.D.S., WENDT I.R. Cholinergic neurotransmission in the longitudinal muscle of the guinea-pig ileum. J. Physiol. 1993;471:61–86. doi: 10.1113/jphysiol.1993.sp019891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVI L.A. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trend Pharmacol. Sci. 2001;22:532–537. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M., HEGDE S.S., WATSON N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- EGLEN R.M., KENNY B.A., MICHEL A.D., WHITING R.L. Muscarinic activity of McN-A-343 and its value in muscarinic receptor classification. Br. J. Pharmacol. 1987;90:693–700. doi: 10.1111/j.1476-5381.1987.tb11222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHLERT F.J., SAWYER G.W., ESQUEDA E.E. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal smooth muscle. Life Sci. 1999;64:387–394. doi: 10.1016/s0024-3205(98)00584-0. [DOI] [PubMed] [Google Scholar]
- GARDNER A.L., CHOO L.K., MITCHELSON F. Comparison of the effects of some muscarinic agonists on smooth muscle function and phosphatidylinositol turnover in the guinea-pig Taeniacaeci. Br. J. Pharmacol. 1988;94:199–211. doi: 10.1111/j.1476-5381.1988.tb11516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HISHINUMA S., HONGO I., MATSUMOTO Y., NARITA F., KUROKAWA M. Contracting effects of carbachol, McN-A-343 and AHR-602 on Ca2+-mobilization and Ca2+-influx pathways in Taenia caeci. Br. J. Pharmacol. 1997;122:985–992. doi: 10.1038/sj.bjp.0701467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE R., ISENBERG G. Acetylcholine activates nonselective cation channels in guinea-pig ileum through a G-protein. Am. J. Physiol. 1990a;258:C1–C6. doi: 10.1152/ajpcell.1990.258.6.C1173. [DOI] [PubMed] [Google Scholar]
- INOUE R., ISENBERG G. Intracellular calcium ions modulate acetylcholine-induced inward current in guinea-pig ileum. J. Physiol. 1990b;424:73–92. doi: 10.1113/jphysiol.1990.sp018056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITAGAKI M., KOMORI S., UNNO T., SYUTO B., OHASHI H. Possible involvement of a small G-protein sensitive to exoenzyme C3 of Clostridium botulium in the regulation of myofilament Ca2+ sensitivity in β-escin skinned smooth muscle of guinea-pig ileum. Jpn. J. Pharmacol. 1995;67:1–7. doi: 10.1254/jjp.67.1. [DOI] [PubMed] [Google Scholar]
- KIM Y.C., KIM S.J., SIM J.H., CHO C.H., JUHNN Y.S., SUH S.H., SO I., KIM K.W. Suppression of the carbachol-activated nonselective cationic current by antibody against α subunit of Go protein in guinea-pig gastric myocytes. Pflugers Arch. 1998;436:494–496. doi: 10.1007/s004240050663. [DOI] [PubMed] [Google Scholar]
- KOHDA M., KOMORI S., UNNO T., OHASHI H. Characterization of action potential-triggered [Ca2+]i transients in single smooth muscle cells of guinea-pig ileum. Br. J. Pharmacol. 1997;122:477–486. doi: 10.1038/sj.bjp.0701407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHDA M., KOMORI S., UNNO T., OHASHI H. Carbachol-induced oscillations in membrane potential and [Ca2+]i in guinea-pig ileal smooth muscle cells. J. Physiol. 1998;511:559–571. doi: 10.1111/j.1469-7793.1998.559bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., BOLTON T.B. Calcium release induced by inositol 1,4,5-trisphosphate in single rabbit intestinal smooth muscle cells. J. Physiol. 1991;433:495–517. doi: 10.1113/jphysiol.1991.sp018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., ITAGAKI M., UNNO T., OHASHI H. Caffeine and carbachol act on common Ca2+ stores to release Ca2+ in guinea-pig ileal smooth muscle. Eur. J. Pharmacol. 1995;277:173–180. doi: 10.1016/0014-2999(95)00072-s. [DOI] [PubMed] [Google Scholar]
- KOMORI S., KAWAI M., PACAUD P., OHASHI H., BOLTON T.B. Oscillations of receptor-operated cationic current and internal calcium in single guinea-pig ileal smooth muscle cells. Pflugers Arch. 1993;424:431–438. doi: 10.1007/BF00374905. [DOI] [PubMed] [Google Scholar]
- KOMORI S., KAWAI M., TAKEWAKI T., OHASHI H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J. Physiol. 1992;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., OHASHI H. Some membrane properties of the circular muscle of chicken rectum and its non-adrenergic non-cholinergic innervation. J. Physiol. 1988;401:417–435. doi: 10.1113/jphysiol.1988.sp017170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., UNNO T., NAKAYAMA T., OHASHI H. M2 and M3 muscarinic receptors couple, respectively, with activation of nonselective cationic channels and potassium channels in intestinal smooth muscle cells. Jpn. J. Pharmacol. 1998;76:213–218. doi: 10.1254/jjp.76.213. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H., KITAMURA K., ITOH T., INOUE R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol. Rev. 1998;78:811–920. doi: 10.1152/physrev.1998.78.3.811. [DOI] [PubMed] [Google Scholar]
- KWON S-C., OZAKI H., HORI M., KARAKI H. Isoproterenol changes the relationship between cytosolic Ca2+ and contraction in guinea-pig Taenia caecum. Jpn. J. Pharmacol. 1993;61:57–64. doi: 10.1254/jjp.61.57. [DOI] [PubMed] [Google Scholar]
- KWON S-C., OZAKI H., KARAKI H. NO donor sodium nitroprusside inhibits excitation–contraction coupling in guinea pig Taenia coli. Am. J. Physiol. 2000;279:G1235–G1241. doi: 10.1152/ajpgi.2000.279.6.G1235. [DOI] [PubMed] [Google Scholar]
- MOREL J.L., MACREZ N., MIRONNEAU J. Specific Gq protein involvement in muscarinic M3 receptor-induced phophatidylinositol hydrolysis and Ca2+ release in mouse duodenal myocytes. Br. J. Pharmacol. 1997;121:451–458. doi: 10.1038/sj.bjp.0701157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAMOTO H., PRESTWICH S.A., ASAI S., UNNO T., BOLTON T.B., KOMORI S. Muscarinic agonist potencies at three different effector systems linked to the M2 or M3 receptor in longitudinal smooth muscle of guinea-pig small intestine. Br. J. Pharmacol. 2002;135:1765–1775. doi: 10.1038/sj.bjp.0704642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACAUD P., BOLTON T.B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J. Physiol. 1991;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESTWICH S.A., BOLTON T.B. G-protein involvement in muscarinic receptor-stimulation of inositol phosphates in longitudinal smooth muscle from the small intestine of the guinea-pig. Br. J. Pharmacol. 1995;114:119–126. doi: 10.1111/j.1476-5381.1995.tb14915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHEE J.C., RHEE P.L., PARK M.K., SO I., UHM D.Y., KIM K.W., KANG T.M. Muscarinic receptors controlling the carbachol-activated nonselective cationic current in guinea pig gastric smooth muscle cells. Jpn. J. Pharmacol. 2000;82:331–337. doi: 10.1254/jjp.82.331. [DOI] [PubMed] [Google Scholar]
- SAWYER G.W., EHLERT F.J. Muscarinic M3 receptor inactivation reveals a pertussis toxin-sensitive contractile response in the guinea-pig colon: evidence for M2/M3 receptor interactions. J. Pharmacol. Exp. Ther. 1999;289:464–476. [PubMed] [Google Scholar]
- SOMLYO A.P., HIMPENS B. Cell activation and its regulation in smooth muscle. FASEB J. 1989;3:2266–2276. doi: 10.1096/fasebj.3.11.2506092. [DOI] [PubMed] [Google Scholar]
- TAKAYANAGI I., HARADA M., KOIKE K. A difference in receptor mechanisms for muscarinic full and partial agonists. Jpn. J. Pharmacol. 1991;56:23–31. doi: 10.1254/jjp.56.23. [DOI] [PubMed] [Google Scholar]
- TAKAYANAGI I., KIKUCHI Y., OHTSUKI H., HARADA M. Activation of propylbenzilylcholine mustard-sensitive muscarinic cholinoceptors more effectively utilizes cytosolic Ca2+ for contraction in guinea-pig smooth muscle. Eur. J. Pharmacol. 1990;187:139–142. doi: 10.1016/0014-2999(90)90352-7. [DOI] [PubMed] [Google Scholar]
- UNNO T., BEECH D.J., KOMORI S., OHASHI H. Inhibitors of spasmogen-induced Ca2+ channel suppression in smooth muscle cells from small intestine. Br. J. Pharmacol. 1998;125:667–674. doi: 10.1038/sj.bjp.0702112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNNO T., INABA T., OHASHI H., TAKEWAKI T., KOMORI S. Role of Ca2+ mobilization in muscarinic receptor-mediated membrane depolarization in guinea-pig ileal smooth muscle cells. Jpn. J. Pharmacol. 2000;84:431–437. doi: 10.1254/jjp.84.431. [DOI] [PubMed] [Google Scholar]
- WANG X-B., OSUGI T., UCHIDA S. Different pathways for Ca2+ influx and intracellular release of Ca2+ mediated by muscarinic receptors in ileal longitudinal smooth muscle. Jpn. J. Pharmacol. 1992;58:407–415. doi: 10.1254/jjp.58.407. [DOI] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. Br. J. Pharmacol. 1997;122:885–893. doi: 10.1038/sj.bjp.0701438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., KOMORI S., OHASHI H., BOLTON T.B. Ca2+ inhibition of inositol trisphosphate-induced Ca2+ release in single smooth muscle cells of guinea-pig small intestine. J. Physiol. 1994;481:97–109. doi: 10.1113/jphysiol.1994.sp020421. [DOI] [PMC free article] [PubMed] [Google Scholar]