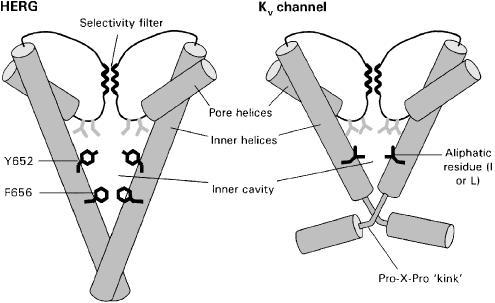
Figure 1.
The structures of two of the four subunits that form the pore and inner cavity of HERG and Kv channels are shown. The inner helices and loops extending from the pore helices to the selectivity filter form the inner cavity and drug-binding site of HERG. Several structural features that help explain the nonspecific drug-binding properties of HERG are illustrated. The inner cavity of HERG is long, creating a relatively large space for trapping drugs and for channel–drug interactions. Aromatic residues (black) not found in Kv channels are critical sites for interaction for most compounds, but not for fluvoxamine. Other sites for drug interaction are polar residues (grey) located close to the selectivity filter. Kv channels have a proline-X-proline motif that is proposed to insert a ‘kink' in the inner helices, resulting in a relatively small inner cavity. The inner cavity is lined by aliphatic rather than aromatic residues.