Abstract
Aim of this study was to gain insight into the mechanism of action of CHF3381, a novel putative antiepileptic and neuroprotective drug.
CHF3381 blocked NMDA currents in primary cultures of cortical neurons: maximal effect was nearly −80% of the NMDA-evoked current, with EC50 of approximately 5 μM. This effect was selective, reversible, use-dependent and elicited at the concentrations reached in the rodent brain after peripheral administration of therapeutic doses.
CHF3381 also inhibited voltage-gated Na+ currents in an apparently voltage-dependent manner. However, this effect could be obtained only at relatively high concentrations (100 μM).
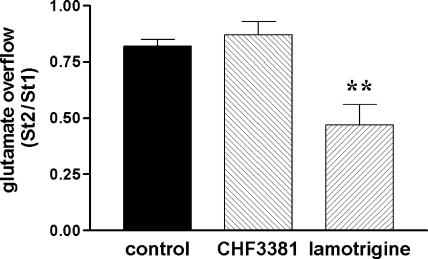
Consistent with the mild effects on voltage-gated Na+ channels, CHF3381 (100 μM) failed to affect electrical stimulation-evoked glutamate overflow in hippocampal slices. In contrast, the anti-convulsant agent and Na+ channel blocker lamotrigine (100 μM) inhibited stimulation-evoked glutamate overflow by approximately 50%.
CHF3381 reduced kindled seizure-induced c-fos mRNA levels within the same brain regions, and to a similar level, as the selective NMDA receptor antagonist MK801, providing circumstantial evidence to the idea that CHF3381 blocks NMDA receptors in vivo.
The present mechanistic studies suggest that the primary mechanism of action of CHF3381 in the forebrain is blockade of NMDA receptors. On this basis, this compound may have a potential use in other diseases caused by or associated with a pathologically high level of NMDA receptor activation.
Keywords: CHF3381, N-methyl-D-aspartate (NMDA), Na+ channel, glutamate, hippocampus, cortex
Introduction
Neurological disorders are very common, affecting more than 25% of all people at some time during their lives. These diseases have a large impact on individuals, families and communities. Effective treatments would certainly reduce this impact. However, many neurological disorders remain or become resistant to available drug treatments. Therefore, there is great interest in research devoted to identifying new drugs, with action mechanisms different from those currently in use.
CHF3381 [n-(2-indanyl)-glycinamide hydrochloride] has been recently described as a novel anticonvulsant and antiepileptic agent possessing a good therapeutic window, that is, a scant propensity to cause neurological side-effects (Villetti et al., 2001). Even more recently, it has been shown that the compound also possesses robust neuroprotective activity (Zucchini et al., 2002). Based on initial binding studies, CHF3381 has been proposed as a putative N-methyl-D-aspartate (NMDA) receptor antagonist (Gandolfi et al., 2001; Villetti et al., 2001). If this were the case, CHF3381 may demonstrate therapeutic properties not only in epilepsy, but also in hypoxic–ischemic brain injury, trauma, chronic pain and degenerative disorders like Huntington's disease, Parkinson's disease, Alzheimer's disease and AIDS dementia (Kohl & Dannhardt, 2001; Palmer, 2001; Parsons, 2001).
The aim of this study was to gain more insight into the mechanism of action of CHF3381. Thus, we examined (1) its potential for interaction with voltage- and receptor-gated ion channels using whole-cell patch-clamp electrophysiology techniques with primary neuronal cell cultures, (2) its effects on electrical stimulation-evoked glutamate release from hippocampal slices, and (3) its effects on kindled seizure-induced c-fos expression using in situ hybridisation.
Methods
Animals
Sprague–Dawley rats were used for all experiments: 1-day-old pups for cell cultures and adult male rats (300–350 g) for the release and gene expression studies. Animals were kept under standard conditions: constant temperature (22–24°C) and humidity (55–65%), 12 h dark–light cycle, free access to food and water. Procedures involving animals were carried out in accordance with European Community and national laws and policies.
Patch-clamp recordings on transmitter-gated currents
Whole-cell voltage-clamp recordings obtained from rat cortical neurons in primary culture were used to characterise the possible interaction of CHF3381 with glutamate NMDA, glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid–kainic acid (AMPA-KA) and GABAA receptors. Cortical neuronal cultures were prepared from 1-day-old Sprague–Dawley rats. The neurons were plated either in 35 mm culture dishes (NUNC) coated with 20 μg ml−1 poly-L-lysine and cultured in modified neurobasal medium by adding gentamycin sulphate 50 mg ml−1, B27 2% and GlutaMAXTM-I 500 μM (GibcoBRL). After 24–48 h, 5 μM cytosine arabinoside (ARA-C) was also added to prevent glial cell replication.
Whole-cell voltage-clamp recordings were carried out at room temperature (22°C), according to Hamill et al. (1981) in a control bathing solution containing 142 mM NaCl, 1.5 mM KCl, 1 mM CaCl2, 10 mM HEPES, 10 mM glucose and 20 mM sucrose (320 mOsm, pH 7.4). For glutamate receptor current experiments, the bathing solution contained 1 μM glycine (coagonist at the NMDA receptor) and 10 μM picrotoxin (to block GABAA channels). For experiments in which γ-aminobutyric acid (GABA) receptor currents were examined, the bathing solution also contained 1 mM MgCl2 to block NMDA receptor currents. Patch-clamp recordings were obtained with an Axopatch 1D amplifier (Axon instruments) using patch electrodes (2–3 MΩ) filled with an intracellular solution containing 153 mM CsCl, 10 mM EGTA, 10 mM HEPES and 4 mM MgCl2 (290 mOsm, pH 7.38). Currents were filtered at 5 kHz, digitally sampled at 10 kHz and acquired on computer using the CLAMPEX software (Axon Instruments). Currents were also recorded on a chart recorder.
For glutamate receptor current experiments, unless otherwise stated, cells were voltage clamped at −60 mV. The test compound was bath-applied, while receptor agonists were applied using a gravity-fed U-tube, positioned 200–300 μm away from the cell. Solenoid valves controlled solution exchange with a computer interface controller. Ionotropic glutamate receptor currents were evoked using 10 μM NMDA, 30 μM AMPA or 50 μM glutamate. Agonists were applied as 1 s pulses separated by 2 min wash periods. With this protocol, glutamate receptor currents were relatively stable for the duration of the recording period. The effects of CHF3381 on glutamate currents were evaluated using a paradigm that allows measurement of the direct effect of the compound. Following at least two control applications of agonist, the cell was perfused with CHF3381 beginning 1 min prior to new application of the agonist. Following the combined agonist and drug application, the perfusate was then switched back to drug alone. The agonist application was then repeated every 2 min until a steady-state current was observed. Finally, the perfusate was returned to the control bathing solution, and additional agonist responses were obtained to monitor return of the glutamate response to control levels (wash-out). This protocol allowed measurement of both the direct and modulatory actions of the test compound at glutamate receptors.
The use-dependence of CHF3381 effects on NMDA currents was assessed using two distinct experimental protocols. The first protocol was essentially identical to the one described above, that is, NMDA was applied using the U-tube, as 1 s pulses separated by either 30 s or 2 min wash periods, and CHF3381 was bath-applied. In the second protocol (Sobolevsky & Yelshansky, 2000), NMDA and CHF3381 were coapplied through the U-tube as 2 s pulses separated by 2 min wash periods.
The effects of CHF3381 on GABA currents were evaluated using a pretreatment paradigm similar to the one described above for glutamate. Briefly: following at least two control applications of GABA, cells were perfused with CHF3381 (100 μM) for 20–30 s prior to application of GABA plus the drug; the perfusate was than switched back to drug alone; this sequence was repeated until at least two consistent currents were observed; finally, the perfusate was returned to the control bathing solution and additional GABA responses were obtained to monitor return to control levels. This protocol allowed measurement of both the direct and modulatory actions of the test compound at GABAA receptors.
The agonist-evoked current values were measured using the Clampfit software (Axon Instruments). The effect of CHF3381 on agonist-evoked currents was determined by comparing the agonist currents in the presence of drug with control agonist responses in the absence of drug, and expressed as percent of control current. EC50 values for the effects observed have been calculated using the GraphPad Prism software.
Patch-clamp recordings on voltage-gated Na+ currents
The effect of CHF3381 on voltage-gated Na+ channels was assessed using N1E-115 murine neuroblastoma cells and patch-clamp recording techniques. We used this cell line for these experiments because the morphology of these cells allows achievement of adequate space clamp conditions while using whole-cell patch-clamp techniques. In contrast, cortical neurons maintained in primary culture have extensive neurite outgrowth, which can result in additional access resistance to the membrane occurring beyond the branch points, such that regions of the cell distal to the cell body will not be clamped to the voltage being applied at the tip of the electrode, distorting the time course of large regenerative (such as Na+) currents.
The N1E-115 murine neuroblastoma cell line was maintained at 35°C in Dulbecco's modified Eagle's Medium supplemented with 5% fetal calf serum, 20 mM HEPES, 80 μg ml−1 gentamicin and 4 mM glutamine. Prior to electrophysiological studies, cells were plated and incubated for 3–5 days in a differentiation medium similar to above with reduced (2.5%) fetal calf serum and 2% dimethyl sulphoxide (DMSO). Recordings were carried out at room temperature in a bathing solution containing 130 mM NaCl, 5 mM CaCl2, 1 mM MgCl2, 5 mM glucose, 5 mM HEPES. The buffer also contained 0.1 mM CdCl2 and 25 mM tetraethylammonium chloride to block voltage-gated Ca2+ and K+ channels, respectively. Whole-cell recordings were obtained using patch electrodes (1–2 MΩ) filled with the intracellular solution described above. The currents were filtered at 10 kHz and acquired on computer using pCLAMP 6 (Axon Instruments). Series resistance and capacitive currents were compensated using the internal clamp circuitry. The series resistance was 3–5 MΩ, and 75–85% of the series resistance was compensated.
Cells were voltage clamped at −60 mV, unless otherwise noted, and the test compound applied using the perfusion system described above. To activate voltage-gated sodium channels, cells were hyperpolarised to −90 mV for 90 ms and then depolarised to 0 mV for five trials in control solution and then again in the solution containing the test compound. This protocol was then repeated, but from a holding potential of −60 mV instead of −90 mV. Drugs were applied via a gravity-fed perfusion system coupled to a piezo-electric stepper controlled electronically.
Glutamate release experiments
The experimental procedures employed for release experiments have been previously described (Muzzolini et al., 1997). In brief, hippocampi were quickly removed and sectioned into 400 μm thick transverse slices using a McIlwain tissue chopper. Slices were allowed to re-equilibrate at room temperature in Krebs solution (mM NaCl 118.5; KCl 4.4; CaCl2 1.2; MgSO4 1.2; KH2PO4 1.2; NaHCO3 25; glucose 10) bubbled with 95% O2 and 5% CO2 for 30 min, then transferred into superfusion chambers according to Beani et al. (1978). Four slices (approximately 0.2 mg protein each) were placed together in superfusion chambers (volume 0.3 ml). Chambers were maintained at 33°C in a thermostatic bath, connected by tubing to a peristaltic pump and perfused at a flow rate of 0.5 ml min−1 with a preoxygenated (95% O2, 5% CO2) Krebs solution containing 100 μM L-trans-pyrrolidine-2,4-dicarboxylic acid (tPDC), a competitive inhibitor of glutamate reuptake. Slices were electrically stimulated by pulses of alternate polarity (30 mA cm−2). Two 5 min trains of impulses at 20 Hz were applied 60 and 90 min after starting the superfusion. Under these experimental conditions, electrically evoked glutamate overflow (see below) is tetrodotoxin-sensitive and calcium-dependent by approximately 80% (Muzzolini et al., 1997). Drug treatments were applied between the first (St1) and the second (St2) stimulation, in order to have an internal control for each experimental condition.
The perfusate was collected at 5 min intervals, and endogenous glutamate concentration was measured analysing an aliquot (15 μl) of the same sample by high-pressure liquid chromatography (HPLC) coupled to fluorimetric detection after precolumn derivatisation with o-phthalaldehyde. The system consisted of a Beckman 125 pump, a Triathlon autosampler (Spark Holland), a Chromsep analytical column (Chrompack) and a fluorescence spectrophotometer RF-551 (Shimatzu). The acquisition and analysis of the chromatograms were performed by a computer-controlled system (Beckman Gold System). Protein content in the preparation was determined by the method of Lowry et al. (1951).
Electrically evoked overflow of glutamate was calculated by subtracting the presumed basal outflow from the total glutamate found in the sample at stimulus application and in the following sample. The presumed basal outflow was obtained by interpolation between the preceding and the following sample. Data were expressed as the ratio between the overflow induced by St2 (in the presence of drug) and the overflow induced by St1 (in the absence of drug). Controls were slices never treated during the experiments (no drug added between St1 and St2).
Kindling
A twisted bipolar electrode was stereotaxically implanted into the right amygdala under ketamine (100 mg kg−1 i.p.) anaesthesia. Stereotaxic coordinates were: 4.8 mm lateral and 0.8 mm posterior to bregma, 8.3 mm deep from dura; the incisor bar was 5 mm above the intra-aural line (Pellegrino et al., 1979). After 7 days postoperative recovery, animals received once daily a single 1 s train of bipolar pulses (1 ms, 60 Hz, amplitude 25% above the afterdischarge threshold). Kindling criteria (three consecutive stage 4 or 5 seizures according to Racine, 1972) were reached after 18±1 stimulations. Rats were left unstimulated for 7 days and killed by decapitation under light diethyl-ether anaesthesia, 30 min after a last stimulation eliciting a tonic-clonic (stage 4 or 5) seizure, either without any pretreatement (control rats), or after CHF3381 (120 mg kg−1, administered i.p. 30 min before stimulation) or dizocilpine maleate (MK801, 1 mg kg−1 i.p., 60 min before stimulation). Brains were rapidly removed, frozen in isopentane cooled in a dry ice/methanol bath, and stored at −70°C until use.
In situ hybridisation
In situ hybridisation experiments were performed as previously described (Simonato et al., 1996). Briefly, antisense and sense c-fos riboprobes were prepared from a full-length cDNA insert (kindly supplied by Dr T. Curran). The full-length cDNA inserts were cloned in two SP65 plasmids containing the SP6 promoter and a single Xho1 restriction site. Plasmids were therefore linearised with Xho1 and transcribed with SP6 RNA polymerase. Riboprobes were obtained by running the transcription assays in the presence of α[33P]rUTP (New England Nuclear Dupont, Germany), and hydrolysed to fragments of approximately 200 bp with sodium carbonate at 60°C.
Coronal sections (20 μm) at the level of the ventral hippocampus (plate 39, Pellegrino et al., 1979) were thaw-mounted onto polylysine-coated slides, fixed in paraformaldehyde 4%, soaked in phosphate-buffered saline (PBS) for 10 min at room temperature, then rinsed in a graded ethanol series and dried. Sections were stored at −70°C until use. Immediately before in situ hybridisation, they were pretreated with proteinase K (1 μg μl−1 in Tris-ethylenediaminetetraacetic acid (EDTA) buffer) for 10 min at 37°C and with acetic anhydride 0.25% v v−1 for 10 min at room temperature.
The in situ hybridisation mixture contained 50% deionised formamide, 0.6 M NaCl, 2 mM EDTA, 20 mM Tris (2 × NTE), 5 × Denhart's solution, 100 μg ml−1 ssDNA, 100 μg ml−1 tRNA, 0.05% sodium pyrophosphate and 60 μg ml−1 [33P] riboprobe. Sections were incubated overnight at 52°C with 40 μl hybridisation mixture. Following hybridisation, they were rinsed twice for 10 min in 4 × saline-sodium citrate (SSC: 0.6 M NaCl, 0.06 M citric acid) and treated with RNAase A (20 μg ml−1) for 30 min at 37°C. Sections were then rinsed in 1 × SSC for 10 min, 0.1 × SSC at 52°C for 30 min, 0.1 × SSC at room temperature for 10 min, dehydrated through an ethanol series, and apposed to Hyperfilm β-max (Amersham Italia, Milano, Italy) for 30–40 days. Films were developed in Kodak D-19 (5 min) and fixed in Kodak rapid fixer (5 min). The mean total optical density within an area of interest was calculated by multiple sampling of that area in four sections taken from each animal using a digital analysis system (RBR Altair, Firenze, Italy). Background (nonspecific) hybridisation was estimated using other sections incubated with the sense probe, and subtracted from the total optical density. Data have been expressed as percent of the mean optical density measures in the right piriform cortex of untreated animals.
Results
Effect of CHF3381 on receptor- and voltage-gated currents
To probe the effects of CHF3381 on GABA and glutamate ionotropic receptors, we first tested a single (100 μM) concentration in neuronal primary cultures. Results are summarised in Table 1 . At this concentration, no significant mean effect was observed on GABA-evoked currents, although there was a subset of cells (three of the nine examined) where a 60% current increase was noted.
Table 1.
Effect of 100 μM CHF3381 on whole-cell currents of primary cultures of cortical neurons
| Agonist | Holding potential (mV) | Number of cells | % Control (mean±s.e.m.)a |
|---|---|---|---|
| GABA (1 μM) | −70 | 9 | 118±4 |
| Glutamate (50 μM) | −60 | 5 | 38±2** |
| kainate (100 μM) | −60 | 5 | 96±3 |
| AMPA (30 μM) | −60 | 9 | 103±5 |
| NMDA (10 μM) | −60 | 8 | 44±5** |
| NMDA (10 μM) | +40 | 5 | 72±2* |
Results are expressed as percent of control peak current (pA).
P<0.05
P<0.01 compared with control (saline); Student's t-test for paired data.
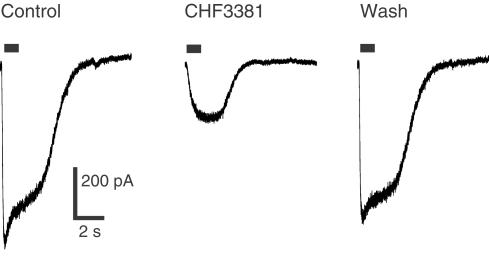
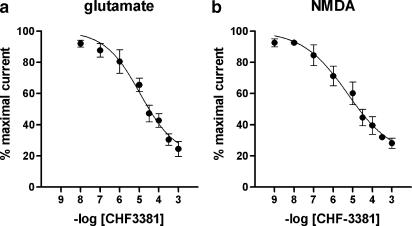
Regarding ionotropic glutamate receptors, 100 μM CHF3381 reduced the glutamate-evoked peak current by approximately 60% at −60 mV. Thus, this effect was further analysed using agonists selective for the ionotropic glutamate receptor subtypes. CHF3381 did not produce significant effects on kainate- and AMPA-evoked currents, while it highly significantly blocked the NMDA-evoked current (approximately −60%, Table 1 and Figure 1). This effect was fully evident only 5 min after CHF3381 application (i.e. on the third NMDA pulse applied in the presence of the test compound, indicating use-dependency), and completely washed-out within 5 min (Figure 1). When the cells were voltage-clamped at +40 mV, only a 28% block of the NMDA-mediated current was observed (Table 1). Concentration-response curves have been generated for the effects of CHF3381 on glutamate- and NMDA-evoked currents at −60 mV (Figure 2). Currents were inhibited with an estimated EC50 of approximately 11 and 5 μM, respectively.
Figure 1.
Effect of CHF3381 on whole-cell NMDA currents in primary cultures of rat cortical neurons. Cell cultures were prepared and whole-cell patch recordings were made as described in the Methods section. Cells were maintained at a holding potential of −60 mV, and 1 s pulses of NMDA (bar above traces) were applied. Left trace: representative control NMDA-evoked current. Middle trace: NMDA-evoked current in the presence of CHF3381. The NMDA pulse in this trace was applied 5 min after onset of 100 μM CHF3381 bath perfusion. Right trace: NMDA-evoked current after wash-out of CHF3381. The NMDA pulse in this trace was applied 5 min after withdrawal of CHF3381 from perfusion.
Figure 2.
Inhibition of glutamate- (a) and NMDA-evoked (b) currents by CHF3381. Cells were maintained at a holding potential of −60 mV. Each point represents the mean±s.e.m. of normalised data from six to nine separate cells. EC50 value for the effect on glutamate-evoked currents (with 95% confidence limits in parenthesis) was 11.0 μM (6.5–18.7). EC50 value for the effect on NMDA-evoked currents was 5.3 μM (2.6–11.1).
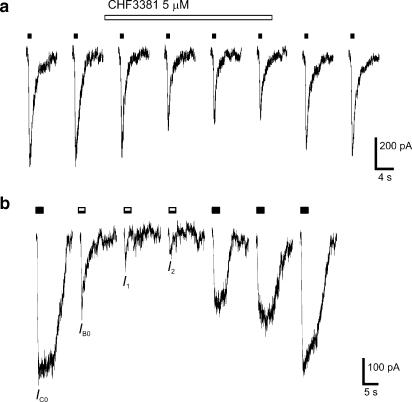
The use-dependence of the effect on the NMDA current was further analysed using the EC50 (5 μM) and the fully effective (300 μM) concentrations of CHF3381. First, we compared the effect of CHF3381 when using 30 s or 2 min interpulse intervals in the above protocol. Independent of the time interval between NMDA pulses, and at both CHF3381 concentrations tested, we found that the effect was complete only at the third NMDA application (Figure 3a). Second, NMDA and CHF3381 were coapplied in 2 s pulses. A representative example of currents obtained in response to consecutive pulses is shown in Figure 3b. Again, at both CHF3381 concentrations tested, the initial current in response to the first coapplication (IB0)–which was smaller than the initial control current (IC0)–was always greater than the initial current in response to the second coapplication (I1). In turn, maximal inhibitory effects were obtained only at the third coapplication. The level of stationary current (Ibs) for all responses was the same, indicating that, during the 2 s coapplication, CHF3381 inhibition of the NMDA channel reached saturation. In both protocols, recovery from the CHF3381 block was obtained by repetitive application of NMDA (Figure 3).
Figure 3.
Use-dependence of the CHF3381 inhibition of NMDA currents. (a) Responses to consecutive 1 s applications of NMDA (10 μM, solid bars, 120 s interpulse interval) in the presence of bath-applied CHF3381 (5 μM, open bar). Note the progressive inhibition of the NMDA current produced by CHF3381, which reached a maximum only after the third NMDA application in the presence of the antagonist. Similar results were obtained with 300 μM CHF3381 and/or using a 30 s interpulse interval. (b) Responses to consecutive 2 s applications of: one NMDA (10 μM, solid bar) alone, three NMDA plus CHF3381 (10 and 300 μM, respectively, black and white bars) coapplications and three NMDA alone (10 μM, solid bars). The interpulse interval was 120 s. Note that the progressive reduction in the initial current (compare IC0, Ib0, I1 and I2). Similar, smaller effects were obtained coapplying 5 μM CHF3381.
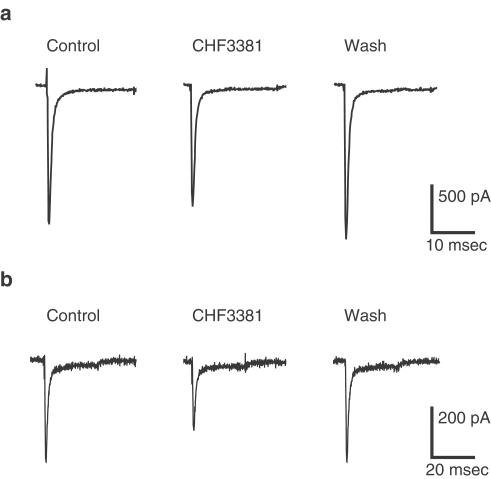
CHF3381 (again at 100 μM) was also tested on voltage-activated Na+ currents, at holding potentials of −90 and −60 mV (Table 2 and Figure 4). A 17% reduction of Na+ currents was observed at −90 mV, and a slightly greater block (28%) was obtained when the holding potential was held at −60 mV.
Table 2.
Effect of 100 μM CHF3381 on voltage-dependent sodium currents in NIE-115 neuroblastoma cells
| Holding potential (mV) | Number of cells | % Control (mean±s.e.m.)a |
|---|---|---|
| −90 | 4 | 83±2* |
| −60 | 4 | 72±2* |
Results are expressed as percent of control peak current (pA).
P<0.05 compared with control; Student's t-test for paired data.
Figure 4.
Effect of 100 μM CHF3381 on whole-cell Na+ currents in neuroblastoma cells. Cell cultures were prepared and whole-cell patch recordings were made as described in the Methods section. CHF3381 was tested holding cells to −90 (a) or to −60 (b) mV for 90 ms and then depolarising to 0 mV. Left trace (control): representative control Na+ current. Middle trace (CHF3381): Na+ current in the presence of CHF3381. Right trace (wash): Na+ current after wash-out of CHF3381.
Release experiments
To examine the effect of CHF3381 on a Na+ channel-dependent phenomenon, we performed experiments on glutamate release from hippocampal slices. Lamotrigine has been employed as a reference drug in these experiments, because it has been reported to inhibit glutamate and GABA release from cortical slices stimulated with the Na+ channel opener veratrine (Leach et al., 1986). These effects have been attributed to blockade of Na+ channels, and are thought to contribute to the antiepileptic effects of the drug (Leach et al., 1986). When applied at a concentration of 100 μM, lamotrigine highly significantly reduced (approximately −50%) electrically evoked glutamate overflow from hippocampal slices (Figure 5). In contrast, CHF3381, up to 100 μM, was devoid of any effect (Figure 5).
Figure 5.
Effect of 100 μM CHF3381 and 100 μM lamotrigine on electrical stimulation-evoked glutamate overflow from hippocampal slices. Slices were prepared and experiments performed as described in the Methods section. Each point represents the mean±s.e.m. of five to seven separate experiments. **P<0.01, ANOVA and post hoc Newman–Keuls test.
c-fos expression
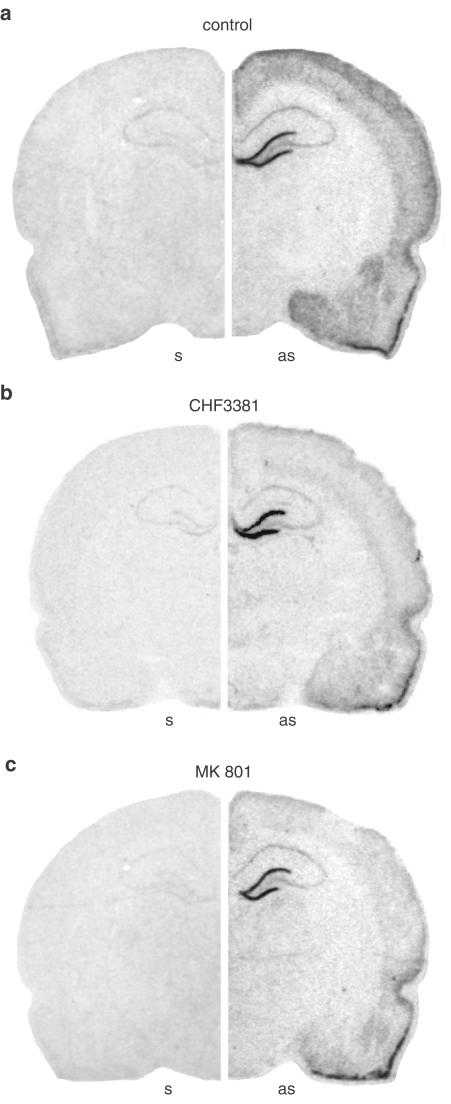
c-fos mRNA is not constitutively found in the brain under normal conditions. However, seizures induce its expression in an activity-dependent manner: it is thought that c-fos expression ‘marks' disrhythmic neurons, although not all dysrhythmic neurons will necessarily express c-fos (Labiner et al., 1993). Areas expressing c-fos in a kindled seizure-induced manner include: piriform cortex, amygdala (medial and anterior cortical nuclei), dentate gyrus of the hippocampus, neocortex (Hosford et al., 1995). Indeed, in untreated animals killed 30 min after a fully kindled seizure, these areas were found to express c-fos mRNA (Figure 6a).
Figure 6.
Anatomical distribution of c-fos mRNA expression 30 min after induction of amygdala stimulation-induced fully kindled (class 5) seizures, under control, that is, no treatment, conditions (a, control), after pretreatment with 120 mg kg−1 CHF3381, administered i.p. 30 min before stimulation (b, CHF3381), and after pretreatment with 1 mg kg−1 MK801, administered i.p. 60 min before stimulation (c, MK801). Shown are autoradiograms of representative coronal brain sections at the level of the dorsal hippocampus (plate 39, Pellegrino et al., 1979), exhibiting hybridisation of the sense (s, left panels) and of the antisense (as, right panels) [33P]labelled c-fos probes. These representative sections will not fully correlate with the mean changes in c-fos mRNA levels shown in Figure 7, because of slight differences in gene expression in the six animals of each group.
Kindled seizure-induced c-fos expression is also partly dependent upon NMDA receptor activation (Labiner et al., 1993). Thus, in order to verify whether CHF3381 can block NMDA receptors in vivo, we sought to investigate whether it can inhibit kindled seizure-induced c-fos expression in a manner similar to the one of a selective NMDA receptor antagonist, MK801. We used MK801 and CHF3381 under conditions (dose, time and route of administration) in which they exert equipotent effects on kindled seizures (Villetti et al., 2001). MK801 (1 mg kg−1 i.p., administered 60 min before amygdala stimulation in kindled animals) only slightly attenuated seizures (Table 3 ), but caused a general reduction of c-fos expression (Figure 6c), which reached levels of significance in the medial and anterior cortical nuclei of the right amygdala and in the neocortex (Figure 7). CHF3381 (120 mg kg−1 i.p., administered 30 min before amygdala stimulation in kindled animals) also produced a slight attenuation of seizures (Table 3) and a pattern of effects on kindled seizure-induced c-fos expression that, anatomically and quantitatively, matched perfectly the one produced by MK801: general attenuation with significant inhibition in the medial and anterior cortical nuclei of the right amygdala and in the neocortex (Figures 6b and 7).
Table 3.
Effects of CHF3381 and of MK801 on kindled seizures
| Treatment | ADD duration (s) | Rats with class four or five seizures | Forelimb clonus duration (s) |
|---|---|---|---|
| Control (vehicle) | 69±7 | 6/6 | 40±4 |
| CHF3381 (120 mg kg−1 i.p.) | 55±11 | 5/6 | 23±7* |
| MK801 (1 mg kg−1 i.p.) | 54±7 | 4/6 | 17±5* |
<0.05, compared with control (vehicle); Student's t-test for nonpaired data.
Figure 7.
Relative optical density values of c-fos mRNA hybridisation to riboprobes in the rat amygdala (a) and neocortex (b) 30 min after amygdala stimulation-induced fully kindled (class 5) seizures, under control conditions (no treatment), and after pretreatment with CHF3381 (120 mg kg−1 i.p., 30 min before stimulation) or with MK801 (1 mg kg−1 i.p., 60 min before stimulation). Optical density values have been calculated as described in the Methods section, and expressed as percent of the mean specific optical density over the right piriform cortex of controls. Data are the means±s.e.m. of six animals per group, each run in quadruplicate. *P<0.05, **P<0.01 vs the corresponding region in controls; ANOVA and post hoc Newman–Keuls test.
Discussion
The mechanistic studies reported in this article assess the effect of CHF3381 on voltage-sensitive Na+ channels, on glutamate ionotropic and on GABAA receptors. The main findings can be summarised as follows: (1) CHF3381 blocks NMDA receptors and (less effectively) Na+ channels in cell cultures; (2) the latter effect does not translate into inhibition of excitatory neurotransmission in hippocampal slices, because CHF3381 does not affect the electrically evoked release of glutamate, while, (3) the former effect appears to take place in vivo, because peripheral administration of CHF3381 reduces kindled seizure-induced c-fos expression in a manner that closely resembles the one of selective NMDA receptor antagonists. These findings will be separately discussed below.
The greatest and most consistent effect of CHF3381 was observed on NMDA-evoked currents. The compound was found to dramatically block the NMDA current. This effect displayed a series of characteristics. First, it was selective for the NMDA receptor subtype, since kainate- and AMPA-evoked currents were not affected. This observation is in line with previous binding studies indicating that CHF3381 selectively inhibited [3H]1-[1-(2-thienyl)ciclohexyl] piperidine (TCP; Villetti et al., 2001) and [3H]MK801 (Gandolfi et al., 2001) binding to brain membranes, in the absence of significant binding to other NMDA receptor complex subsites and/or to other glutamate receptor subtypes. Based on previous studies (Villetti et al., 2001), it is likely that the effect of CHF3381 is also specific, because it does not display significant activities in an array of receptor and ion channel assays. Furthermore, the compound does not modify the uptake of [3H]dopamine (Gandolfi et al., 2001), [3H]noradrenaline and [3H]serotonin (unpublished data) to striatal and cortical synaptosomes, respectively. The absence of significant effects on GABA-evoked currents (i.e. at the GABAA receptor) observed in the present study integrates these specificity data (but see below). Second, the effect of CHF3381 on NMDA currents was produced at the concentrations that are estimated to be reached in the rodent brain after peripheral administration of therapeutic doses (Villetti et al., 2001). These data are in line with (1) the anticonvulsant profile of CHF3381 (Villetti et al., 2001); (2) its efficacy against the behavioural effects and the lethality of systemically administered NMDA (Villetti et al., 2001); (3) its neuroprotective profile (Zucchini et al., 2002). Thus, CHF3381 could have a potential use in many diseases caused by or associated with a pathologically high level of NMDA receptor activation.
Other characteristics of NMDA receptor blockade emerge from this study. The effect of CHF3381 is rapidly and fully reversible. Furthermore, it is use-dependent, since maximal inhibitory effects are observed only on the third NMDA pulse, and voltage-dependent, since clamping the membrane potential to +40 mV greatly attenuated the current block. Finally, previous binding studies indicate that CHF3381 acts as a noncompetitive antagonist (Gandolfi et al., 2001). Therefore, CHF3381 appears to possess NMDA receptor blocking characteristics closely related to those of memantine (Parsons et al., 1993).
It is interesting that CHF3381 potentiated GABA currents in a subset of cells. This may reflect differences in action on the different types of neurons in the cortical culture (that contains inhibitory interneurones and excitatory pyramidal cells). Although this is a potential anticonvulsant effect, the present data do not sufficiently substantiate it, and further studies will be required to examine its relevance.
CHF3381 also possesses some ability to inhibit voltage-gated Na+ currents. In this respect, CHF3381 may at least partly share a similar mechanism of action to that of the prototype anticonvulsants phenytoin, carbamazepine and lamotrigine (McNamara, 2001). However, while phenytoin and carbamazepine block [3H]batrachotoxin binding (Willow & Catterall, 1982; Willow et al., 1984) and deeply inhibit Na+ currents (Willow et al., 1984; 1985) in a concentration range correlated with the brain levels achieved during therapy, CHF3381 did not significantly affect [3H]batrachotoxin binding (Villetti et al., 2001) and was less effective than these anticonvulsants at blocking voltage-sensitive Na+ channels (present data). Thus, it seems unlikely that inhibition of Na+ channels will significantly contribute to the therapeutic actions of CHF3381 at a forebrain level (for example, to its anticonvulsant effects).
This idea has been assessed by studying CHF3381 effects on glutamate release, because the effects on voltage-gated Na+ currents suggest that the compound may possess the ability to modulate excitatory neurotransmission. In fact, the reference drug lamotrigine reduced stimulus-evoked glutamate overflow in a highly significant manner. Taking into account that lamotrigine should not affect the tetrodotoxin-insensitive, Ca2+-independent component of electrically evoked glutamate overflow (approximately 20%, Muzzolini et al., 1997), it can be estimated that it inhibits 60–70% of the Ca2+-dependent component. This estimate is in line with data in the literature (Leach et al., 1986). However, CHF3381, applied at concentrations that nearly fully inhibit NMDA currents in primary cultures, failed to affect electrical stimulation-evoked glutamate overflow in hippocampal slices. This observation further supports the notion of a postsynaptic site of action.
Thus, the data collected suggest that, at the concentrations achieved in the brain during treatment (approximately 10 μM, Villetti et al., 2001), CHF3381 may exert its therapeutic (for example, anticonvulsant) actions mainly through blockade of NMDA receptors. To assess whether this is the case in vivo is not trivial. We attempted to approach this problem by studying modulation of seizure-induced c-fos expression. c-fos is an immediate-early gene encoding a transcription factor that is not constitutively expressed in the adult CNS (Hughes et al., 1999). Seizures are sufficient to induce rapid, dramatic and fleeting c-fos expression in discrete neuronal populations of the rodent forebrain (Morgan et al., 1987; Saffen et al., 1988; Shin et al., 1990; Simonato et al., 1991; Hosford et al., 1995). Kindled seizure-induced c-fos mRNA expression is thought to correlate with sustained neuronal depolarisation and subsequent rise in intracellular calcium evoked by multiple factors, one of which is NMDA receptor activation (Lerea et al., 1992; Labiner et al., 1993).
We thought to take advantage of this property of kindled seizure-induced c-fos expression. We argued that, if CHF3381 would produce a reduction in kindled seizure-induced c-fos mRNA levels within the same brain regions, and to a similar level, as the one produced by the selective NMDA receptor antagonist MK801, this would provide at least circumstantial evidence to the idea that CHF3381 blocks NMDA receptors in vivo, and that this phenomenon contributes to its anti-convulsant effects. Indeed, this proved to be the case.
In conclusion, the present initial mechanistic studies on CHF3381 suggest that its primary mechanism of action in the forebrain is blockade of NMDA receptors. This effect can interpret its known therapeutic properties, being consistent with its anticonvulsant and neuroprotective profiles, which strikingly overlap the one of NMDA receptor antagonists (Villetti et al., 2001; Zucchini et al., 2002). On this basis, this compound may have a potential use in other diseases caused by or associated with a pathologically high level of NMDA receptor activation. CHF3381 also possesses some ability to inhibit voltage-gated Na+ currents. However, it seems unlikely that inhibition of Na+ channels will significantly contribute to the therapeutic actions of CHF3381 at a forebrain level.
Acknowledgments
This work was supported by a grant from Chiesi Farmaceutici S.p.a. The authors thank Anna Binaschi, Manuela Mazzuferi and Luca Marani for technical assistance.
Abbreviations
- AMPA-KA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid–kainic acid
- ARA-C
cytosine arabinoside
- CHF3381
n-(2-indanyl)-glycinamide hydrochloride
- DMSO
dimethyl sulphoxide
- EDTA
ethylenediaminetetraacetic acid
- GABA
γ-aminobutyric acid
- HPLC
high-pressure liquid chromatography
- MK801
dizocilpine maleate
- NMDA
N-methyl-D-aspartate
- PBS
phosphate-buffered saline
- SSC
saline-sodium citrate
- TCP
1-[1-(2-thienyl)ciclohexyl] piperidine
- tPDC
L-trans-pyrrolidine-2,4-dicarboxylic acid
References
- BEANI L., BIANCHI C., GIACOMELLI A., TAMBERI F. Noradrenaline inhibition of acetylcholine release from guinea-pig brain. Eur. J. Pharmacol. 1978;48:179–193. doi: 10.1016/0014-2999(78)90327-8. [DOI] [PubMed] [Google Scholar]
- GANDOLFI O., BONFANTE V., VOLTATTORNI M., DALL'OLIO R., POLI A., PIETRA C., VILLETTI G. Anticonvulsant preclinical profile of CHF 3381: dopaminergic and glutamatergic mechanisms. Pharmacol. Biochem. Behav. 2001;70:157–166. doi: 10.1016/s0091-3057(01)00591-3. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HOSFORD D.A., SIMONATO M., CAO Z., GARCIA-CAIRASCO N., SILVER J.M., BUTLER L., SHIN C., MCNAMARA J.O. Differences in the anatomic distribution of immediate-early gene expression in amygdala and angular bundle kindling development. J. Neurosci. 1995;15:2513–2523. doi: 10.1523/JNEUROSCI.15-03-02513.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES P.E., ALEXI T., WALTON M., WILLIAMS C.E., DRAGUNOW M., CLARK R.G., GLUCKMAN P.D. Activity and injury-dependent expression of inducible transcription factors, growth factors and apoptosis-related genes within the central nervous system. Prog. Neurobiol. 1999;57:421–450. doi: 10.1016/s0301-0082(98)00057-4. [DOI] [PubMed] [Google Scholar]
- KOHL B.K., DANNHARDT G. The NMDA receptor complex: a promising target for novel antiepileptic strategies. Curr. Med. Chem. 2001;8:1275–1289. doi: 10.2174/0929867013372328. [DOI] [PubMed] [Google Scholar]
- LABINER D.M., BUTLER L.S., CAO Z., HOSFORD D.A., SHIN C., MCNAMARA J.O. Induction of c-fos mRNA by kindled seizures: complex relationship with neuronal burst firing. J. Neurosci. 1993;13:744–751. doi: 10.1523/JNEUROSCI.13-02-00744.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEACH M.J., MARDEN C.M., MILLER A.A. Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: II. Neurochemical studies on the mechanism of action. Epilepsia. 1986;27:490–497. doi: 10.1111/j.1528-1157.1986.tb03573.x. [DOI] [PubMed] [Google Scholar]
- LEREA L.S., BUTLER L.S., MCNAMARA J.O. NMDA and non-NMDA receptor-mediated increase of c-fos mRNA in dentate gyrus neurons involves calcium influx via different routes. J. Neurosci. 1992;12:2973–2981. doi: 10.1523/JNEUROSCI.12-08-02973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MCNAMARA J.O.Drugs effective in the treatment of the epilepsies The Pharmacological Basis of Therapeutics 2001New York: McGraw-Hill; 521–547.10th edn., ed. Hardman, J.G., Limbird, L.E. & Goodman Gilman, A. pp [Google Scholar]
- MORGAN J.I., COHEN D.R., HEMPSTEAD J.L., CURRAN T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- MUZZOLINI A., BREGOLA G., BIANCHI C., BEANI L., SIMONATO M. Characterization of glutamate and [3H]D-aspartate outflow from various in vitro preparations of the rat hippocampus. Neurochem. Int. 1997;31:113–124. doi: 10.1016/s0197-0186(96)00129-5. [DOI] [PubMed] [Google Scholar]
- PALMER G.C. Neuroprotection by NMDA receptor antagonists in a variety of neuropathologies. Curr. Drug Targets. 2001;2:241–271. doi: 10.2174/1389450013348335. [DOI] [PubMed] [Google Scholar]
- PARSONS C.G. NMDA receptors as targets for drug action in neuropathic pain. Eur. J. Pharmacol. 2001;429:71–78. doi: 10.1016/s0014-2999(01)01307-3. [DOI] [PubMed] [Google Scholar]
- PARSONS C.G., GRUNER R., ROZENTAL J., MILLAR J., LODGE D. Patch clamp studies on the kinetics and selectivity of N-methyl-D-aspartate receptor antagonism by memantine (1-amino-3,5-dimethyladamantan) Neuropharmacology. 1993;32:1337–1350. doi: 10.1016/0028-3908(93)90029-3. [DOI] [PubMed] [Google Scholar]
- PELLEGRINO L.J., PELLEGRINO A.S., CUSHMAN A.J. A Stereotaxic Atlas of the Rat Brain. New York: Plenum Press; 1979. [Google Scholar]
- SAFFEN D.W., COLE A.J., WORLEY P.F., CHRISTY B.A., RYDER K., BARABAN J.M. Convulsant-induced increase in transcription factor messenger RNAs in rat brain. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7795–7799. doi: 10.1073/pnas.85.20.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIN C., MCNAMARA J.O., MORGAN J.I., CURRAN T., COHEN D.R. Induction of c-fos mRNA expression by afterdischarge in the hippocampus of naive and kindled rats. J. Neurochem. 1990;55:1050–1055. doi: 10.1111/j.1471-4159.1990.tb04595.x. [DOI] [PubMed] [Google Scholar]
- SIMONATO M., BREGOLA G., DONATINI A., BIANCHI C., BEANI L., FERRI S., ROMUALDI P. Kindled seizure-induced c-fos and prodynorphin mRNA expressions are unrelated in the rat brain. Eur. J. Neurosci. 1996;8:2064–2067. doi: 10.1111/j.1460-9568.1996.tb00726.x. [DOI] [PubMed] [Google Scholar]
- SIMONATO M., HOSFORD D.A., LABINER D.M., SHIN C., MANSBACH H.H., MCNAMARA J.O. Differential expression of immediate early genes in the hippocampus in the kindling model of epilepsy. Mol. Brain Res. 1991;11:115–124. doi: 10.1016/0169-328x(91)90113-c. [DOI] [PubMed] [Google Scholar]
- SOBOLEVSKY A.I., YELSHANSKY M.V. The trapping block of NMDA receptor channels in acutely isolated rat hippocampal neurones. J. Physiol. 2000;526:493–506. doi: 10.1111/j.1469-7793.2000.t01-2-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLETTI G., BREGOLA G., BASSANI F., BERGAMASCHI M., RONDELLI I., PIETRA C., SIMONATO M. Preclinical evaluation of CHF3381 as a novel antiepileptic agent. Neuropharmacology. 2001;40:866–878. doi: 10.1016/s0028-3908(01)00026-0. [DOI] [PubMed] [Google Scholar]
- WILLOW M., CATTERALL W.A. Inhibition of binding of [3H]batrachotoxinin A 20-alpha-benzoate to sodium channels by the anticonvulsant drugs diphenylhydantoin and carbamazepine. Mol. Pharmacol. 1982;22:627–635. [PubMed] [Google Scholar]
- WILLOW M., GONOI T., CATTERALL W.A. Voltage clamp analysis of the inhibitory actions of diphenylhydantoin and carbamazepine on voltage-sensitive sodium channels in neuroblastoma cells. Mol. Pharmacol. 1985;27:549–558. [PubMed] [Google Scholar]
- WILLOW M., KUENZEL E.A., CATTERALL W.A. Inhibition of voltage-sensitive sodium channels in neuroblastoma cells and synaptosomes by the anticonvulsant drugs diphenylhydantoin and carbamazepine. Mol. Pharmacol. 1984;25:228–234. [PubMed] [Google Scholar]
- ZUCCHINI S., BUZZI A., BERGAMASCHI M., PIETRA C., VILLETTI G., SIMONATO M. Neuroprotective activity of CHF3381, a putative N-methyl-D-aspartate receptor antagonist. NeuroReport. 2002;13:2071–2074. doi: 10.1097/00001756-200211150-00016. [DOI] [PubMed] [Google Scholar]