Abstract
We have investigated the effects of specific PKC isoforms in TNF-α mediated cellular damage using a human intestinal cell line (SCBN).
TNF-α treatment induced a decrease in the extent of intestinal cellular viability as determined by a formazan-based assay and an increase in the apoptotic index as assessed by immunohistology. These changes in cellular integrity were found to be related to the degradation of I-κBα, mobilization of NF-κB and release of mitochondrial cytochrome c.
TNF-α treatment also induced the activation of selective PKC isoforms which were associated with the decrease in cellular viability and an increase of cellular apoptosis.
Nonselective PKC antagonists, such as GF109203X and Gö6976 as well as isoform-selective PKC-inhibiting peptides would reverse the cellular injury as well as reduce the degradation of I-κBα and mitochondrial cytochrome c release. These effects were most highly correlated with changes in PKCδ and ɛ primarily.
Intestinal cellular injury could be induced by treating cells with agonists selective for PKCδ and ɛ mainly.
In conclusion, this study has shown that TNF-α treatment can induce the activation of PKCδ and ɛ in the human intestinal cell line, SCBN, and this response is closely associated with an increase in cellular damage and apoptosis. PKCδ and ɛ primarily mediate the release of mitochondrial cytochrome c and degradation of I-κBα and hence mobilization of NF-κB, which are responsible for the pathway leading to cell injury.
Keywords: Intestinal, SCBN cells, tumour necrosis factor, PKC isoforms, apoptosis, NF-κB, I-κB, cytochrome c
Introduction
Tumour necrosis factor (TNF)-α is involved in the regulation of many processes including experimentally induced intestinal inflammation (Garside et al., 1993; Kojouharoff et al., 1997; Neurath et al., 1997; Brown et al., 1999). Furthermore, TNF levels have been shown to be increased in colonic tissue taken from Crohn's disease patients (Beil et al., 1995) and in mononuclear cells harvested from mice with experimentally induced intestinal inflammation (Schmitz et al., 1999). TNF-α has also been shown to initiate apoptotic events in isolated cells of the gastrointestinal tract including the colon (Kim et al., 1998; Csukai & Mochly-Rosen, 1999; Wright et al., 1999) and TNF treatment can impair epithelial barrier function in vitro (Beil et al., 1995; Schmitz et al., 1999; Wright et al., 1999).
The results of previous as well as recent studies from this laboratory have suggested that the inflammatory actions of TNF-α on the intestine are associated with activation of the intracellular signalling mediator, protein kinase C (PKC) (Chang & Tepperman, 2001). These studies have revealed that the intestinal cell damage and apoptosis associated with TNF-α challenge are related to the activation of specific PKC isoforms. As PKC is not a single entity but rather a family of related isoenzymes comprising at least nine different members (Nishizuka, 1992), it is important to determine which PKC isoform(s) mediate intestinal cell injury. Activation of discrete PKC isoforms might influence the susceptibility of cells exposed to challenges such as TNF-α.
Several lines of evidence suggest that individual PKC isozymes play distinct regulatory roles in cell growth, differentiation and apoptosis in the intestine. In HeLa and NIH3T3 cell lines, overexposure of constitutively active catalytic fragments of PKCδ causes apoptosis (Ghayuar et al., 1996). It has also been demonstrated that the PKC activator, phorbol myristate acetate (PMA) will result in apoptosis in colonic epithelial cells and that this event is closely associated with activation of PKCδ, whereas in other cell types activation of PKCɛ has been associated with cytotoxicity (O'Connell et al., 1997). In our previous study, TNF challenge resulted in translocation, and hence activation, of both PKCδ and ɛ primarily in rodent intestinal epithelial cells and this change was linked to cellular integrity. The activation of other isoforms could also be linked with the extent of cell integrity. The precise functional role of PKCδ and ɛ in the mediation of cytokine challenge to intestinal cells has not, as yet, been established.
In the present study, we have attempted to better define a role for these various PKC isoforms in intestinal cellular integrity in response to TNF-α, as well as examine their role in human cells. We have approached the problem by using peptides which are highly selective PKC inhibitors and activators. These recently developed important pharmacological tools have been shown to either stabilize the active state of that PKC isoform or to inhibit the same interaction with the isozyme-specific anchoring proteins termed RACKs (receptors for activated C kinases; Mochly-Rosen et al., 2001; Dorn & Mochly-Rosen, 2002). These isoform-selective peptides have been fully characterized with regard to their ability to enhance or antagonize the activation and cytotoxic effects of certain PKC isoforms in a variety of cell types including cardiomyocytes and thyroid cells (Jobin et al., 1997; Jones et al., 1997). We have also examined the role of the nuclear transcription factor NF-κB as a part of the signal transduction process along with PKC leading to cell injury after TNF-α treatment.
Methods
Cell culture and treatment
The human small intestinal epithelial cell line (SCBN) was used in these studies. These cells were generously provided by Dr A Buret (Gastrointestinal Research Group, University of Calgary, Calgary, Canada). SCBN is a nontransformed duodenal epithelial cell line. These cells do not form tumours when inoculated into nude mice, which contrasts with the considerable variation in colon cancer-derived intestinal epithelial cell lines (Pang et al., 1993). Furthermore, this cell line shows morphological and functional characteristics typical of small intestinal crypt cells, including microvilli, brush border enzyme, junctional complexes, defined apical and basolateral surfaces, apical Cl− secretion and constitutively expressed mRNA for IL-6, VCAM-1 and epidermal growth factors (Kim et al., 1998).
In our studies, SCBN cells were cultured routinely in DMEM complete medium (Sigma, Chemical Co., St Louis, MO, U.S.A.) with 2.8 mM glutamine, 100 U ml−1 penicillin G, 100 μg ml−1 streptomycin, 80 μg ml−1 tylosin and 10% fetal calf serum. The cells were transferred weekly by short incubation of monolayers with 0.25% trypsin–EDTA. All cells were kept in a humidified atmosphere containing 5% CO2, 95% air at 37°C, to reach 80–90% confluency. In these experiments, the cells were transferred to serum-free DMEM medium for a 30 min pretreatment with PKC inhibitors (Biomol, PA, U.S.A.), including GF109203X (5 μM), a nonspecific PKC inhibitor; Gö6976 (0.2 μM), an inhibitor of PKCα; rottlerin (6 μM), a selective PKCδ inhibitor and Myristolated PKCɛV1–2(4 μM), a PKCɛ translocation inhibitor. The concentration of inhibitors was chosen on the basis of preliminary experiments demonstrating effective antagonism of the effects of TNF-α in SCBN cells. Some groups of cells were also treated with PKC specific agonist and antagonist peptides (purchased from Dr Daria Mochly-Rosen, Department of Molecular Pharmacology, Stanford University, Stanford, CA, U.S.A.), including ψβRACK (receptors for activated C-kinase pp111, 0.5 μM), a classical PKC agonist; Beta C 2–4 (pp95), a classical PKC antagonist (0.5 μM); ψδRACK (pp114), a PKCδ agonist (0.75 μM); Delta V1-1 (pp101, 0.5 μM), a PKCδ antagonist; ψɛRACK2 (pp106, 0.75 μM), a PKCɛ agonist; Epsilon V1-2 (pp93, 0.5 μM), a PKCɛ antagonist. The isozyme selective inhibitors used were mainly derived from the RACK-binding site on individual PKCs (Mochly-Rosen, 1995; Souroujon & Mochly-Rosen, 1998). The doses of the antagonist and agonist peptides used in the studies were chosen based on findings that these peptides showed appropriate isozyme action in neonatal myocytes (Hu et al., 2000), as well as in our preliminary experiments using intestinal epithelial cells. Following this pretreatment, cells were challenged with TNF-α (10 ng ml−1) with addition of the transcription inhibitor actinomycin D (AMD; 2 μg ml−1) for periods ranging from 1 to 24 h.
Cytosolic and particulate extraction for PKC Western blot analysis
Cytosolic and particulate fractions of SCBN cells were obtained as described previously (Chang & Tepperman, 2001). Briefly, the cells were washed, scraped from the plates and homogenized in buffer containing 50 mM Tris-HCl, 2 mM EDTA, 1 mM EGTA, 50 μg ml−1 phenylmethylsulphonyl fluoride (PMSF), 25 μg ml−1 each of soybean trypsin inhibitor, leupeptin and aprotinin and 5% of mercaptoethanol, then centrifuged at 100,000 × g for 60 min at 4°C. The supernatant was collected as the cytosolic fraction. The resulting pellet was resuspended in the homogenization buffer containing 0.1% Triton X-100, mixed for 60 min and centrifuged again at 100,000 × g at 4°C to remove insoluble membrane components. The resultant supernatant was kept as the particulate fraction. The particulate and cytosolic fraction extracts (15 μg) were prepared for electrophoresis by boiling for 5 min in an equal volume of SDS sample buffer (125 mM Tris, pH 6.8, containing 20% glycerol and 10% mercaptoethanol). Protein content was determined using the BioRad assay (Bradford, 1994). Each fraction was subjected to 10% SDS–PAGE and transferred to nitrocellulose membrane at 100 V for 75 min. After blocking nonspecific binding sites with 10% nonfat milk in PBS-TWEEN buffer (80 mM Na2HPO4, 10 mM NaCl, 20 mM NaH2PO4 and 0.05% Tween-20), the membranes were incubated for 2 h with specific PKCα antibody (1 : 1500), 3 h with PKCδ, ɛ and ζ antibodies (1 : 1000) (Santa Cruz Biotechnology, CA, U.S.A.) at room temperature, followed by incubation with 1 : 6000 dilution of HRP-conjugated anti-rabbit IgG (Jackson Immuno Research Laboratories, Mississauga, Canada) for 1 h at room temperature and then detected with ECL reagents according to the manufacturer's instructions (Amersham, England). The peptide used to raise the antibody was used in competition studies to demonstrate specificity of the polyclonal antibody. Equal loading of proteins on the gel was verified by 10% SDS–PAGE stained with Coomassie Blue (R250). The results were determined by densitometric analysis of blots using the ImageMaster DTS software (Pharmacia Biotech, CA, U.S.A.).
PKC isoform translocation
To examine the translocation of the PKC isozymes in challenged SCBN cells, the cells were cultured to subconfluence on sterile glass coverslips and treated as described above. Cells were washed three times in ice-cold PBS and permeabilized for 45 min in 1 : 1 cold ethanol—acetone, followed by two washes with cold PBS. The cells were then incubated for 1 h in PBS with 1% normal goat serum and 0.1% of Triton X-100 to block the nonspecific binding sites, followed by overnight incubation with PKC isozyme-specific antibodies as detailed above and diluted 1 : 100–1 : 300 in PBS containing 2 mg ml−1 BSA, and 0.1% Triton X-100. The cells were again washed with PBS and incubated with FITC-conjugated anti-rabbit antibody at 1 : 500. The coverslips were mounted onto glass slides using Airvol (Doval, PA, U.S.A.) after washing three times and viewed with a Zeiss microscope equipped with appropriate optics and filter plates at × 63 oil-immersion objective. Images from the microscope were recorded by Sensican software and Adobe photoshop image-processing utilities to determine isoform translocation.
Cell viability analysis
The effects of PKC isoform nonspecific inhibitors as well as the PKC-specific agonist and antagonist peptides on viability of SCBN cells treated with TNF-α plus AMD were determined by a formazan-based assay (3-[4,5 dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; MTT), a sensitive procedure to measure cell metabolism in which MTT is reduced to an insoluble formazan dye by mitochondrial enzymes associated with metabolic activity (Twentyman & Luscombe, 1994). Briefly, SCBN cells were plated onto 96-well plates and left for 24 h at 37°C. After pretreatment with PKC nonselective or specific PKC peptide inhibitors or activators as detailed above, cells were incubated for 18 h with TNF-α and AMD and then washed in PBS and stained in freshly prepared MTT solution at a concentration of 0.5 mg ml−1 for 2 h at 37°C. A solution consisting of 90% isopropanol, 0.01 N HCl and 0.2% SDS was then added to dissolve the formazan crystals formed in the wells. The absorbance of the resultant solution was read at 570 nm on a spectrophotometer plate reader (SLT Instruments, Ges, Austria). The percent cellular viability was calculated as previously reported (Twentyman & Luscombe, 1994).
Microscopic determination of apoptosis
SCBN cells were grown on glass coverslips to subconfluence and treated with test components as described above in DMEM medium and then fixed with 4% paraformaldehyde. Nuclear condensation and fragmentation were visualized by fluorescent microscopy after a 15 min incubation with the cell-permeable flurochrome Hoechst 33258 (0.25 μg ml−1, Sigma), and were mounted onto slides using fluorescent mounting medium (Dako, Carpinteria, CA, U.S.A.). The proportion of cells undergoing apoptosis 18 h after initiation of treatment was determined by counting the total number of cells and the cells exhibiting two or more membrane blebs and brightly stained condensed and fragmented chromatin per high power field (× 40 oil immersion objective). Apoptotic index was calculated as the percentage of cells displaying the characteristics described above. A minimum of five different fields and at least 300 cells for each treatment were counted for each sample. All experiments were repeated three times to ensure reproducibility.
Determination of I-κBα protein
SCBN cells were challenged with 10 ng ml−1 of TNF-α for 10–90 min. Some groups of cells were pretreated with PKC-specific and nonspecific inhibitors or activators as described above. Cells were washed in cold PBS and lysed in ice-cold lysis buffer containing 50 mM Tris (pH 8.00; 110 mM NaCl, 5 mM EDTA, 1% Triton X-1000, 2 mM dithiothreitol (DTT) and 1 mM PMSF). Protein concentration was determined using the Bioford assay (Bio-Rad). Cell lysates were boiled in equal volumes of loading buffer and 15 μg protein loaded per lane on 12% Tris-glycine gel. The proteins were transferred to Hybond-C nitrocellulose membrane at 4°C for 75 min. The blots were blocked with a solution of 10% dry milk in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBS-T) for 1 h at room temperature. The I-κBα antibody (Sigma) was added at a dilution of 1 : 2000 and blots were incubated for 1 h followed by three rapid washes in TBS-T. The blots were incubated for 1 h in 5% milk containing anti-rabbit IgG conjugated with horseradish peroxidase (Amersham) at a dilution of 1 : 6000. Immunoreactive bands were visualized using the ECL detecting kit. The 36-kDa protein was confirmed to be I-κBα protein. Equal loading of proteins on the gel was verified by reprobing the same blot with monoclonal antibody against actin (Chemicon International, Temecula, CA, U.S.A.).
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) was used to measure the activation of NF-κB in our studies. Briefly, SCBN cells were cultured in 100 mm2 dishes to 80% confluence and the experimental treatment was conducted as described above. Nuclear extraction procedure was performed on ice with pre-cooled reagents as described previously (Cespinskas et al., 1999). Cells were washed twice with cold PBS and harvested by scraping into 1 ml of PBS and pelleted by brief centrifugation at 1000 × g. The pellet was then resuspended in ice-cold buffer A, consisting of 10 mM Tris, 60 mM NaCl, 1 mM EDTA, 10 mM KCl, 2 mM DTT, 1 mM PMSF, 0.5 μg ml−1 of leupeptin and aprotinin and 0.1% Triton X-100. After a 10 min incubation with occasional vortexing, nuclei were collected by centrifugation at 2000 × g for 10 min at 4°C and were rinsed with 1 ml ice-cold buffer A. Pellets were then resuspended in 30 μl of ice-cold buffer B (20 mM Tris, 20% glycerol, 0.4 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 2 mM EGTA, 2 mM DTT, 1 mM PMSF, 0.5 μg ml−1 of leupeptin, 0.5 μg ml−1 aprotinin), and incubated on ice for 40 min with occasional vortexing. The nuclear proteins were isolated by centrifugation at 14,000 × g for 15 min at 4°C, snap frozen in liquid nitrogen and stored in −80°C until EMSA analysis was performed. Protein concentrations were determined by Biorad Assay.
The double-strand oligonucleotide containing consensus (5′-AGGGACTTTCCGCTGGGGACTTT CC-3′) binding sites for NF-κB (Promega) were 5-end labelled (1.75 pmol μl−1) according to the manufacturer's instructions using T4 polynucleotide kinase (GIBCO, BRL) and γ-32P-ATP (Amersham), and purified on a Bio-Spin chromatography column (Bio-Rad, Hercules, CA, U.S.A.). For EMSA, 5 μg of nuclear extract was incubated with 1 pmol of the labelled oligonucleotide in binding buffer (40 mM HEPES pH 7.9, 0.32 M NaCl, 2 mM MgCl, 0.4 mM EDTA, 4 mM PMSF, 40% glycerol, 50 ng ml−1 poly(dI-dC) and BSA (1 mg ml−1)) for 30 min in room temperature. A 50-fold excess of cold oligonucleotide was added to duplicate samples to verify the specificity of the protein–DNA interaction. The reaction mixture was resolved using a nondenaturing polyacrylamide gel (29 : 1) and electrophoresed at 250 V in 0.5 × Tris-boric-EDTA (TBE) buffer (45 mM Tris-HCl, 45 mM boric acid, 1 mM EDTA). Gels were then transferred to Whatman 3M paper, dried under vacuum at 70°C for 1 h and exposed to X-ray film (Kodak) at −80° for over 16 h with an intensifying screen for analysis.
Detection of cytochrome c by Western blot analysis
Cytochrome c is a key mitochondrial-associated soluble intermembrane protein which is released into the cytosol upon stimulation, where it triggers the assembly of a complex of apoptosomes and facilitates apoptosis progression by activation of caspase-9 and -3. For detection of cytochrome c, SCBN cells were fractionated into mitochondrial and cytoplasmic compartments following the instructions of the ApoAlert cell fractionation kit (Clontech, CA, U.S.A.). The mitochondrial and cytoplasmic fractions were denatured in 5 × sample buffer at 95°C and fractionated by SDS electrophoresis on a 12% polyacrylamide gel and then were probed with a rabbit antibody (1 : 200) directed towards mitochondrial cytochrome c, followed by horseradish peroxidase-conjugated donkey anti-rabbit Ig at 1 : 6000. The bound antibody was detected by chemiluminescence using ECL reagent and viewed by a densitometer.
Statistical analysis
All data are expressed as means±s.e.m. Each experiment was performed in triplicate. Statistical analysis was performed using ANOVA and Dunnett's test. Differences resulting in P-values <0.05 were considered to be statistically significantly.
Results
Effects of TNF-α on PKC isoform translocation in SCBN cells
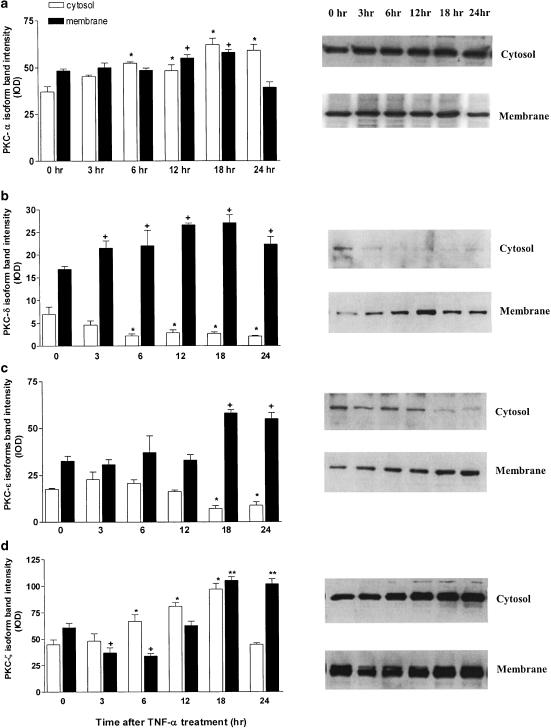
SCBN cells strongly expressed PKCα, δ, ɛ and ζ proteins as determined by Western blot analysis (Figure 1). Furthermore, treatment of SCBN cells with TNF-α in the presence of AMD resulted in changes in the subcellular distribution of these PKC isoforms. The amount of PKC-α protein increased significantly in both cytosolic and particulate fractions from 12 to 18 h after TNF-α challenge. The subcellular distribution of PKC-δ occurred as early as 3 h with particulate protein increasing and cytosolic protein decreasing in response to the treatment. The same translocation change was observed in PKC-ɛ protein by 18 h after TNF-α treatment, reflecting the activation of these isoforms. PKCζ isoform showed increased density in both cytosolic and membrane compartments in the incubation period with TNF plus AMD (Figure 1).
Figure 1.
PKC isoform protein levels from SCBN cells challenged with TNF-α (10 ng ml−1) in the presence of AMD (2 μg ml−1). Cells were collected at the times indicated after challenge, membrane and cytosolic fractions were obtained, and Western blotting was performed as described in Methods using specific isoform antibodies. Protein levels were quantified by densitometry. Values are means±s.e. of three separate experiments, each performed in triplicate. Representative Western blots are displayed in the right column. Isoform protein distributions for PKCα (a), PKCδ (b), PKCɛ (c) and PKCζ (d) are displayed. Analysis of variance (ANOVA) and Dunnett's test were used to determine significance. *P<0.05 from respective membrane control. +P<0.05 from respective cytosol control.
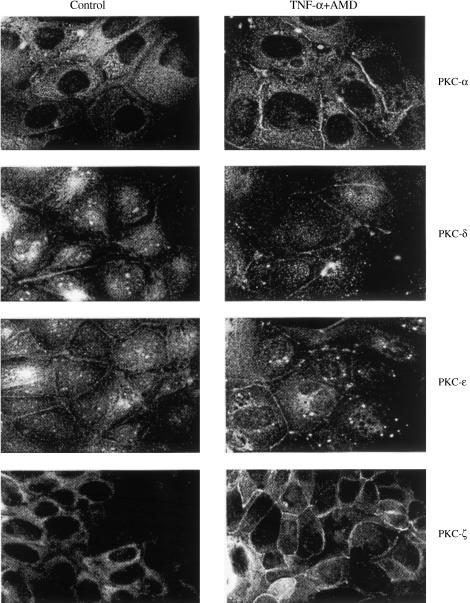
Immunostaining for various PKC isoforms using specific antibodies to these isozymes is shown in Figure 2. These studies revealed that PKCα, δ, ɛ and ζ were located mainly in the cytosolic fraction. The faint staining for PKCα, δ and ɛ could be observed around the cellular membrane. After exposure to the TNF-α and AMD combination for 18 h, PKCα staining appeared to be intensified within both cytosolic and membrane compartments. PKCδ and ɛ staining appeared to be more intense around the cellular membrane region. Similarly, the intensity of staining for PKCζ also increased in the region around the cell membrane (Figure 2).
Figure 2.
Immunofluorescent localization of PKC isoforms in SCBN cells treated with TNF-α in the presence of 0.2 μg ml−1 AMD or in untreated control cells. Cells were harvested 18 h after treatment. Control cells showed primary cytosolic distribution of PKCα, δ, ɛ and ζ and faint peripheral staining for PKCα, δ and ɛ. TNF-α-treated SCBN cells displayed intensified staining for PKC-α in both cytosolic and membrane fractions. PKC-δ and ɛ showed only increased peripheral staining (magnification, × 815).
Effect of PKC inhibitors and activators on SCBN cellular viability after TNF-α
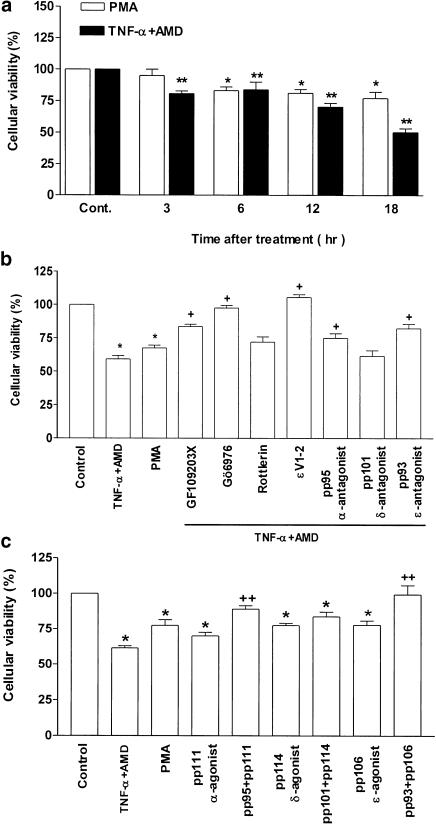
SCBN cells were incubated with TNF-α or PMA for a period of 18 h. The extent of cell injury was estimated by examining cellular metabolism using the MTT assay. Treatment with the PKC activator, PMA induced a significant decrease in cellular viability after 6 h of incubation, and the cellular viability decreased with prolongation of the incubation period. The same degree of cellular viability decrease occurred as early as 1 h after TNF-α treatment and became severe with the prolongation of treatment (Figure 3a). The effect of TNF-α was enhanced by addition of AMD to the incubation medium, while AMD treatment by itself did not significantly increase cell injury (data not shown). AMD did not enhance the effect of PMA on cell injury (data not shown).
Figure 3.
Effect of TNF-α or PMA treatment on SCBN cellular viability in the presence or absence of PKC isoenzyme inhibitors and activators. Cellular integrity was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay as indicated in Methods. The degree of viability was calculated as a percentage of living cells in the total number of cells examined in each group. (a) Cellular viability changes after TNF-α or PMA treatment. (b) Effect of a 30-min pretreatment with PKC isoform inhibitors on the cellular viability in response to treatment of TNF-α or PMA. (c) Comparison of the effect of specific PKC agonist peptides (pp111, pp114 and pp106) and antagonist peptides (pp93, pp95 and pp101) to that of TNF challenge alone. In all of the experiments described here, cells were examined 18 h after TNF-α (+AMD) treatment. Each bar of the histogram represents the mean±s.e. (n=6) from identically treated groups of cells. *P<0.05, significant difference compared to control groups of cells; +P<0.05, significant difference compared to TNF+AMD groups; ++P<0.05, significant difference compared to corresponding agonist groups, as assessed by ANOVA and Duncan's multiple range test.
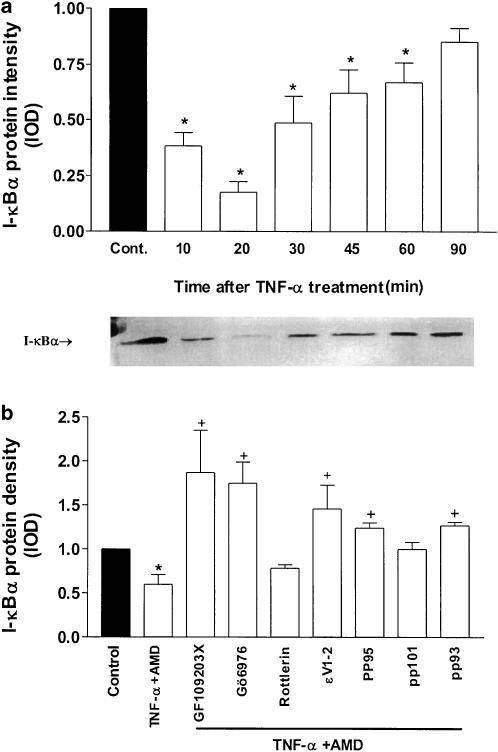
The damaging effect of TNF-α was reduced significantly by preincubation of the cells with PKC nonselective inhibitor GF-109203X and an α-selective inhibitor, Gö6976, as well as the selective PKC-ɛ translocation inhibitor PKCɛV1-2. Similar protective effects were observed by preincubation of cells with the specific cPKC antagonist (pp95) and PKCɛ antagonist peptide (pp93). Incubation with the PKCδ antagonists rottlerin and pp101, respectively, did not reverse the cellular injury in response to TNF-α treatment (Figure 3b).
To further delineate the actions of PKC isoform inhibition on the SCBN cells, the cells were treated with specific pp95, pp101 and pp93 peptides, respectively, before adding corresponding PKC agonists. Compared to the effects of TNF-α and PMA alone, cPKC agonist pp111, δPKC agonist pp114 and PKCɛ agonist pp106 induced nearly the same extent of cell injury as that seen with that of TNF-α and PMA treatment. Antagonist for specific cPKC activity via pp95 blocked this cell injury to some extent. The specific PKCɛ antagonist pp93 reversed completely TNF-α-induced cell injury. The specific PKCδ antagonist pp101 did not reverse the damaging effect of TNF-α (Figure 3c). The vehicle control group was treated with saline and the results of all groups were expressed as percentage of control.
Effect of selective PKC activators and antagonists on TNF-α-induced SCBN apoptosis
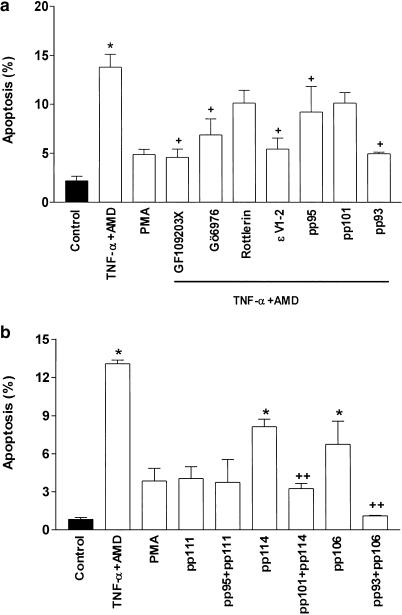
SCBN cell apoptosis was estimated by Hoechst 33258 nuclear staining to determine the incidence of nuclear condensation and fragments after TNF-α treatment with or without preaddition of PKC agonists and antagonists. Many cells were detached from the culture dish and significant apoptotic nuclear fragments were observed to occur by 18 h of TNF-α plus AMD treatment (data are not shown). This apoptotic effect was decreased by nonspecific PKC inhibitors such as GF109203X and Gö6976 and the specific ɛ-translocation inhibitor, ɛV1–2. The relatively specific PKCδ inhibitor, rottlerin, did not ameliorate the effect of TNF-α. Furthermore, the apoptosis was inhibited by pretreating the cells with the specific cPKC antagonist, pp95, and the ɛ antagonist pp93, but not the PKCδ antagonist pp101 (Figure 4a). To delineate the effects of these PKC-isoforms, we compared the action of cPKC agonist pp111, PKCδ agonist pp114 and the PKCɛ agonist pp106 on SCBN cell apoptosis with TNF-α. We observed that pp114 and pp106 alone resulted in apoptotic nuclear fragmentation. Their corresponding specific antagonist peptides blocked the appearance of apoptotic nuclear fragmentation (Figure 4b). The immunohistological changes associated with apoptosis in response to various treatments are shown in Figure 5.
Figure 4.
Effect of TNF-α on apoptotic index of SCBN cells in the presence or absence of PKC isoenzyme activators and inhibitors. Apoptotic index was assessed as the percentage of cells displaying nuclear condensation and fragmentation. A total of at least 300 cells were counted in each experiment. (a) Changes of apoptotic index in SCBN cells treated with TNF-α and the effect of a 30-min pretreatment with PKC isoform peptide inhibitors, pp95 (0.5 μM), cPKC antagonist; pp101 (0.5 μM), PKCδ antagonist; pp93 (0.5 μM), PKCɛ antagonist. Cells were examined 18 h after TNF-α treatment. (b) Comparison of the effect of PKC agonists, pp111 (0.5 μM), a cPKC agonist; pp114 (0.75 μM), a PKCδ agonist and pp106 (0.75 μM), a PKCɛ agonist and antagonist peptides with that of TNF-α challenge alone on apoptotic index. Data are means±s.e. of three separate experiments each performed in duplicate. *P<0.05, significant difference compared to control groups; +P<0.05, significant difference compared with TNF+AMD groups; ++P<0.05, significant difference compared with corresponding agonist peptides group, respectively, as determined by ANOVA and Dunnett's test.
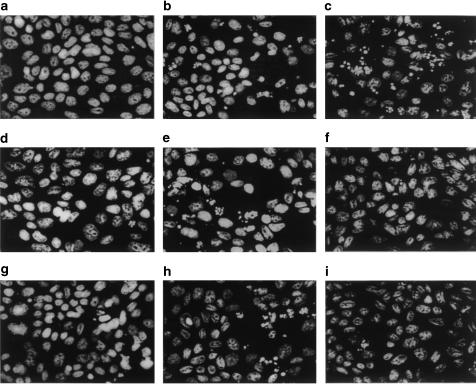
Figure 5.
Fluorescent micrographs of apoptosis of SCBN cells induced by TNF-α in the presence or absence of PKC isoenzyme peptide inhibitors and activators. Nuclear condensation and fragmentation were detected by staining with Hoescht 33258, and the cells were observed under a high-power fluorescent microscope (× 40) with optical filter. (a) Control; (b) the cells exposed to TNF-α in the presence of AMD; (c) the cells treated with TNF-α and PMA; (d) GF109203X-treated cells exposed to TNF-α; (e) Rottlerin-treated cells exposed to TNF-α; (f) ɛ-V1-2 translocation inhibitor-treated cells exposed to TNF-α; (g) cPKC specific antagonist pp95-treated cells exposed to TNF-α; (h) δ-PKC specific antagonist pp101-treated cells exposed to TNF-α; (i) ɛ-PKC (pp93) specific antagonist-treated cells exposed to TNF-α.
Effect of specific PKC-isoform peptide inhibitors and activators on I-κBα protein levels
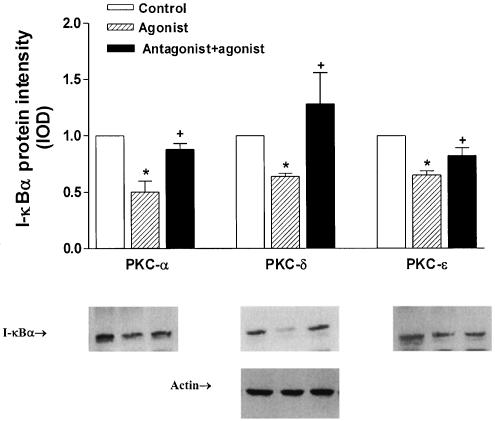
To elucidate the mechanism responsible for altered susceptibility of SCBN cells to TNF-α-induced cell injury and apoptosis, the role of the transcription factor NF-κB and its inhibitory protein I-κBα in this process was investigated. Data presented in Figure 6a showed a single 36 kDa protein band, identified as immunoreactive I-κBα, and the density band was observed to be decreased after 10 min of exposure to TNF-α. This decrease in I-κBα protein persisted until 60 min after treatment. Pretreatment of SCBN cells with GF109203X, Gö6976 and the ɛ translocation inhibitor, ɛV1–2, prevented the decrease of I-κBα protein caused by TNF-α. The specific PKC antagonist peptide pp95 and pp93 also partly blocked the decrease of I-κBα protein levels (Figure 6b). The cPKC agonist pp111, PKCδ agonist pp114 and the PKCɛ agonist pp106 also demonstrated the same effects in decreasing I-κBα levels in SCBN cells after 18 h of TNF-α treatment. Pretreatment of cells with the corresponding specific antagonists PKC pp95, pp93 and pp101 appeared to reverse the I-κBα decrease (Figure 7).
Figure 6.
Effect of TNF-α in combination with PKC antagonist peptides on I-κBα protein expression in SCBN cells. (a) I-κBα protein levels in SCBN cells treated 10–90 min with TNF-α. A representative autoradiogram of SCBN cells treated with TNF-α is displayed below the figure. (b) Effects of administration of selective and nonselective PKC inhibitors on I-κBα protein levels. Histogram values represent the means±s.e. of data from three separate experiments. *P<0.05, significant difference compared with control groups, +P<0.05, significant differences compared with TNFα+AMD-treated groups.
Figure 7.
Effect of PKC selective isoenzyme peptide activators and inhibitors on I-κBα protein levels in SCBN cells treated with TNF-α (10 ng ml−1) in the presence of AMD (2 μg ml−1). SCBN cells were pretreated for 1 h in the presence or absence of PKC inhibitors, before specific agonists are applied. Quantitative analysis of IκBα protein changes was derived from densitometric analysis of autoradiograms from the treated cells. Values are mean±s.e. of data from three separate experiments. *P<0.05 compared with control; +P<0.05 compared with agonist peptide-treated group. Reprobe of the same blot with actin monoclonal antibody showed equal loading.
Changes in NF-κB sequence-specific DNA-binding activity in SCBN cells challenged with TNF-α
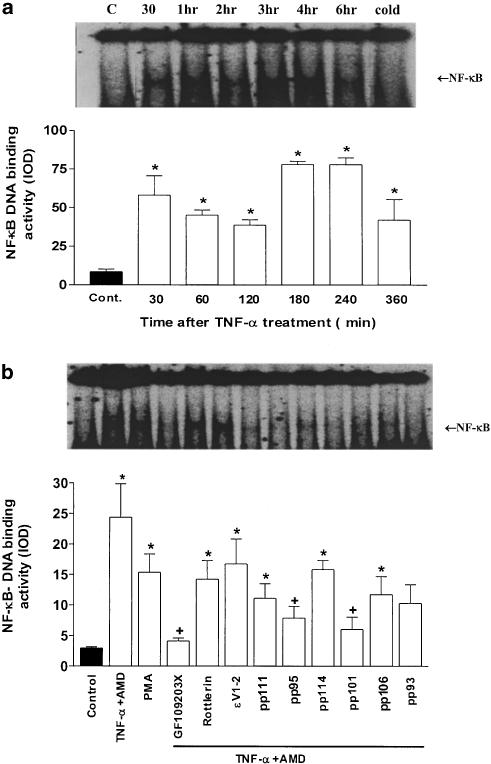
In these studies, NF-κB was directly measured by EMSA. A significant increase in NF-κB-binding activity was measured in SCBN cells after 30 min of TNF-α stimulation. The nuclear NF-κB activity peaked 3–4 h after treatment and remained elevated 6 h after TNF-α addition (Figure 8a). For comparison, treatment of SCBN cells with PMA and cPKC, δ and ɛ agonist peptides, respectively, also resulted in increased NF-κB-binding activity but with reduced intensity. Nonspecific PKC antagonism with GF109203X pretreatment blocked the increase in NF-κB-binding activity, whereas the PKC-δ inhibitor, rottlerin, and the PKCɛ translocation inhibitor, ɛV1-2, displayed no blocking action in this regard. Specific cPKC and δ antagonist peptides showed some inhibitory action in NF-κB-binding activity, but ɛ antagonist showed no statistical significance (Figure 8b).
Figure 8.
Effect of selective and nonselective PKC isoenzyme activators and inhibitors on NF-κB-binding activity in TNF-α-treated or control SCBN cells. (a) The mean±s.e. NF-κB activity as determined 0–360 min after the treatment. A representative autoradiogram of this experiment is shown above this figure. The effect of incubation of the cellular lysate with an excess of unlabelled (cold) oligonucleotide is also shown in the most right lane of this autoradiogram. (b) The mean (±s.e.) effect of PKC isoenzyme activators and inhibitors on NF-κB-binding density in response to TNF-α treatment. The representative autoradiogram for this experiment is displayed above the figure. Histogram values are the means±s.e. of data from three separate experiments, *P<0.05 compared to control group; +P<0.05 compared to group treated with TNF-α (+AMD) alone.
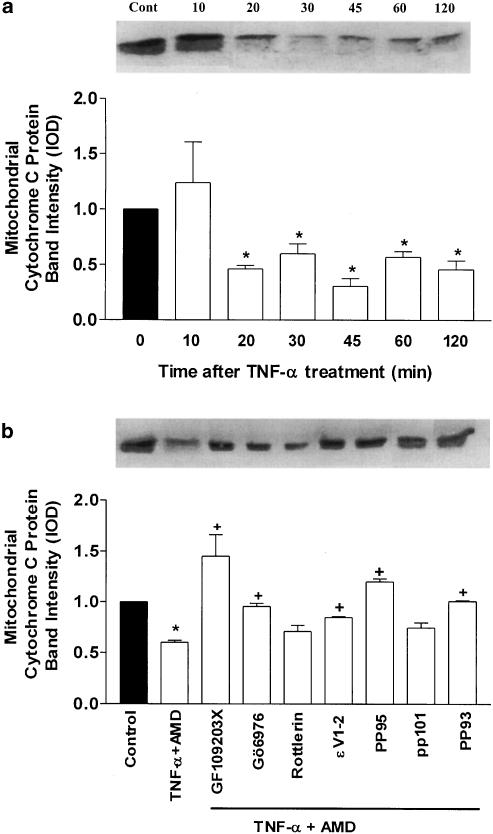
Effect of TNF-α treatment on cytochrome c
We examined cytochrome c release in SCBN cells subjected to TNF-α challenge as well as PKC activators and inhibitors. In contrast to control cells, a rapid and significant decrease in mitochondrial cytochrome c was detected in the mitochondrial fraction after TNF-α challenge (Figure 9a). The mitochondrial marker, cytochrome c oxidase subunit V was detected exclusively in the mitochondrial but not in the cytoplasmic fraction (bands not shown). This ensured that cytochrome c release was not due to mitochondrial contamination during the isolation procedure. Pretreatment of SCBN cells with PKC inhibitors could reverse the release of cytochrome c from the mitochondria (Figure 9b).
Figure 9.
Western blot analysis of mitochondrial cytochrome c protein levels in SCBN cells subjected to TNF-α challenge. (a) Cytochrome c protein changes after TNF-α treatment over a period of 120 min. A representative autoradiogram is shown above the figure. (b) Effect of pretreatment of the SCBN cells with selective and nonselective PKC antagonists on cytochrome c levels in response to TNFα treatment. Representative autoradiogram of Western blot is displayed above the figure. Values represent the mean (±s.e.) of three experiments each performed in triplicate. *P<0.05 compared to control groups. +P<0.05 compared to TNF-treated groups.
Discussion
This is the first report that TNF-α challenge to a nontumour-derived human intestinal epithelial cell line will result in an activation of specific PKC isoforms and an alteration in cellular integrity, as assessed by changes in cellular metabolism and apoptotic index. The human epithelial cells used in these studies were the SCBN cell line. We chose this cell line because it is nontransformed and is derived from humans. These cells do not form tumours when inoculated into nude mice and show morphological and functional characteristics typical of small intestinal crypt cells (Pang et al., 1993; Buresi et al., 2001). In previous studies, it has been demonstrated that these cells respond in culture in a similar manner to animal-derived intestinal cell lines and will respond appropriately to proinflammatory challenges (Buresi et al., 2001). Furthermore, the increase in cellular injury and apoptosis observed here using these human cells is similar to our previously published report using TNFα treatment of rat ileal epithelial IEC-18 cells (Chang & Tepperman, 2001).
The present data also indicate that, by itself, TNF-α induced a small degree of cell injury but that the level of damage could be enhanced by coincubation with the transcription inhibitor AMD. Previous studies have similarly indicated that cytokines, including TNF, by themselves, were relatively ineffective in inducing cytotoxicity in intestinal epithelial cell lines (Wyatt et al., 1997). This confirms finding in other cell types treated with the cytokine and AMD (Leist Gantner et al., 1994) and suggests that the complete intracellular machinery needed for TNF to mediate apoptosis pre-exists in these cells and that new protein synthesis may play a role in determining susceptibility of some cell types to the cytotoxic effects of TNFα. The identity of the protein(s) that may play a role in this regard is unknown, although a number of candidates have been associated with TNF-induced challenge to hepatocytes and fibroblasts (Rath & Aggarwal, 1999).
In the present study, the isoenzymes α, δ, ɛ and ζ of PKC were detected in the human SCBN cells. This array of isoforms is similar to what has been previously detected in rodent intestinal cells (O'Connell et al., 1997; Weller et al., 1999; Chang & Tepperman, 2001). Furthermore, TNF-α treatment of SCBN cells induced an increase in translocation of some PKC isoforms from cytosol to membrane fractions of these cells. Such translocation of PKC is commonly used as an index of PKC activation (Miyawaki et al., 1996; Wang & Ashraf, 1999). This result in SCBN cells confirms previous findings in a variety of cell types including our previous work using rodent IEC-18 colonic cell line (Wyatt et al., 1997; Li et al., 1999; Chang & Tepperman, 2001). The present and our previous work suggest that this activation in response to TNF is cytotoxic to human as well as rodent intestinal epithelial cells.
TNF-α-mediated challenge to various cell types has been associated with changes in the activities of different PKC isoforms (Mayne & Murray, 1998; Laouar et al., 1999). In previous studies using intestinal epithelial cells, the translocation of PKCδ and ɛ has been most closely associated with cytolysis and apoptosis (Tepperman et al., 1999,2000; Chang & Tepperman, 2001). The precise contribution of these isoforms to these processes has been difficult to determine in the past since most studies have used relatively nonselective PKC isozyme tools. In the present study, we have used newly developed isozyme-selective peptide inhibitors and activators of PKCα, δ and ɛ (Dorn & Mochly-Rosen, 2002). The results of the present study demonstrate that activation and inhibition of these isoforms were associated with the regulation of the integrity of human SCBN cells to TNF-α challenge with changes in PKCɛ most consistently mirroring these responses. This isoform has been shown to play a similar role in the regulation of the integrity of other cell types including hepatocytes, fibroblasts and cardiac myocytes (Jones et al., 1997; O'Connell et al., 1997; Dorn & Mochly-Rosen, 2002).
In the present study, we found that TNF treatment would trigger degradation of the cytoplasmic family of inhibitory proteins termed I-κB. Furthermore, TNF challenge was also associated with activation of the transcription factor complex NF-κB. Increased NF-κB activity has been found in inflamed intestinal mucosa, and factors that are implicated in inflammatory bowel disease such as TNF and LPS are potent activators of NF-κB (Rogler et al., 1998). Previous studies have demonstrated that stimulation of cells by cytokines, free radicals and oxidants would induce rapid degradation of I-κB (Henkel et al., 1993; Palombella et al., 1994). I-κB is bound to NF-κB in a stable cytoplasmic ternary complex. Degradation of I-κB allows NF-κB to translocate to the nucleus, where it can activate a pleitropic group of proinflammatory genes. While NF-κB has been demonstrated to be involved in the rapid induction of a number of cytokine genes implicated in the inflammatory process (Baeuerle & Henkel, 1994), NF-κB activation has also been demonstrated to be a very strong antiapoptotic signal by inhibition of caspase-8 in the phenotype of RelA−/− (NF-κB-deficient) mice (Wang et al., 1998). TRAF1 and inhibitor-of-apoptosis proteins (IAP), which are products of NF-κB-responsive genes, can directly bind or block caspase-8 activation and prevent TNF-induced cell death. Our current data show that TNF alone does not kill SCBN cells. However, TNF caused a significant increase in cell death and apoptosis in the presence of AMD, which may inhibit the synthesis or function of proteins such as IAP in TNFR1 pathway. Previous studies have also demonstrated that NF-κB is activated at early stages of adenoviral-induced hepatitis (Kühnel et al., 2000). Other proinflammatory cytokines such as IL-1β cause degradation of I-κB and activation of NF-κB in a variety of intestinal epithelial cell lines including HT-29, Caco-2 and T84 (Jobin et al., 1997).
The present studies also indicate that degradation of I-κB and activation of NF-κB in response to TNF-α were reversed by PKC inhibition. Similarly, PKC selective activators would mimic the effects of TNF treatment. This result confirms and extends previous findings that PKC activation via PMA would induce NF-κB activation and that this effect was inhibited by treatment with the nonselective PKC inhibitor, staurosporine (Wilson et al., 1999). These data suggest the involvement of IκB-NF-κB in the PKC mediation of cell damage in intestinal epithelial cells. However, the data also suggest that not all PKC isozymes are equally involved since specific PKCδ antagonism did not inhibit the degradation of I-κB induced by TNF-α, but caused some increase in NF-κB binding. In contrast, PKCɛ antagonism consistently reduced the I-κB degradation in response to TNF, but was ineffective in reducing NF-κB activation. It is possible that some of the PKC isoforms induce NF-κB activation independent of I-κB degradation. This result confirms previous contentions of a PKC-dependent activation of this transcription factor in enterocytes (Wilson et al., 1999).
Our data also demonstrate that TNF treatment resulted in mitochondrial cytochrome c release. Cytochrome c is released into the cytosol as a result of mitochondrial impairment (Kowaltowski et al., 2001) and is an activator of caspases, especially caspase-3 which participates in the initiation, amplification and execution of apoptosis (Bossy-Wetzel & Green, 1999; Renz et al., 2001). Activation of caspase-3 in mammalian cells has been linked with the proteolytic cleavage of cellular substrates including poly-ADP-ribose-polymerase (Tewardi et al., 1995). Cytochrome c-mediated caspase release has been demonstrated as an important mechanism in cardiomyocyte apoptosis (de Moissac et al., 2000). Our present results extend these findings to include human intestinal epithelial cells.
In summary, we have demonstrated that TNF-α can induce activation of selective PKC isoforms in the human intestinal epithelial cell line, SCBN. Furthermore, selective PKCɛ activation was associated with an increase in cellular damage and apoptosis, while selective PKCɛ inhibition resulted in a reduction in these parameters. These data suggest that PKCɛ is most closely linked causally with the regulation of integrity of intestinal epithelial cells in response to a TNF-α challenge. Furthermore, our data also demonstrate that selective isoforms of PKC mediate this cellular injury and apoptosis via NF-κB activation, although the degradation of I-κB is not an essential requirement for this to occur.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research MOP-6426. We thank Dr Andre Buret, Department of Biology, University of Calgary, for the kind donation of the SCBN cells.
Abbreviations
- AMD
actinomycin D
- DMEM
Dulbecco's modified Eagle's medium
- EMSA
electrophoretic mobility shift assay
- PKC
protein kinase C
- PMA
phorbol myristate acetate
- TNF
tumour necrosis factor
References
- ALBRIGHT C.D., BORGMAN C., CRACIUNESCU C.N. Activation of a caspase-dependent oxidative damage response mediates TGFbeta 1 apoptosis in rat hepatocytes. Exp. Mol. Pathol. 2003;74:256–261. doi: 10.1016/s0014-4800(03)00002-9. [DOI] [PubMed] [Google Scholar]
- BAEUERLE P.A., HENKEL T. Function and activation of NF-κB in the immune system. Ann. Rev. Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- BEIL W.J., WELLER P.F., PEPPERCORN M.A., GALLI S.J., DVORAK A.M. Ultrastructural immunogold localization of subcellular sites of TNF-α in colonic Crohn's disease. J. Leukoc. Biol. 1995;58:284–298. doi: 10.1002/jlb.58.3.284. [DOI] [PubMed] [Google Scholar]
- BOSSY-WETZEL E., GREEN D.R. Apoptosis: checkpoint at the mitochondrial frontier. Mutat. Res. 1999;434:243–251. doi: 10.1016/s0921-8777(99)00032-4. [DOI] [PubMed] [Google Scholar]
- BRADFORD M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1994;72:248–285. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BROWN J.F., CHANG Q., SOPER B.D., TEPPERMAN B.L. Protein kinase C mediates experimental colitis in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 1999;276:G583–G590. doi: 10.1152/ajpgi.1999.276.3.G583. [DOI] [PubMed] [Google Scholar]
- BURESI M.C., SCHLEIHAUF E., VERGNOLLE N., BURET A., WALLACE J.L., HOLLENBERG M.D., MACNAUGHTON W.K. Protease-activated receptor-1 stimulates Ca2+-dependent Cl− secretion in human intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G323–G332. doi: 10.1152/ajpgi.2001.281.2.G323. [DOI] [PubMed] [Google Scholar]
- CESPINSKAS C., LUSH C.W., KVIETYS P.R. Anoxia/reoxygenation-induced tolerance with respect to polymorphonuclear leukocyte adhesion to cultured endothelial cells. A nuclear factor-κB mediated phenomenon. Circ. Res. 1999;84:103–112. doi: 10.1161/01.res.84.1.103. [DOI] [PubMed] [Google Scholar]
- CHANG Q., TEPPERMAN B.L. The role of protein kinase C isozymes in TNF-α-induced cytotoxicity to a rat intestinal epithelial cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G572–G583. doi: 10.1152/ajpgi.2001.280.4.G572. [DOI] [PubMed] [Google Scholar]
- CSUKAI M., MOCHLY-ROSEN D. Pharmacologic modulation of protein kinase C isozymes: the role of RACKs and subcellular localization. Pharmacol. Res. 1999;39:253–259. doi: 10.1006/phrs.1998.0418. [DOI] [PubMed] [Google Scholar]
- DORN G.W., MOCHLY-ROSEN D. Intracellular transport mechanisms of signal transducers. Annu. Rev. Physiol. 2002;64:407–429. doi: 10.1146/annurev.physiol.64.081501.155903. [DOI] [PubMed] [Google Scholar]
- GARSIDE P., BUNCE C., TOMLINSON C., NICHOLS B.L., MOWAT A.M. Analysis of enteropathy-induced by tumor necrosis factor α. Cytokine. 1993;5:24–30. doi: 10.1016/1043-4666(93)90020-6. [DOI] [PubMed] [Google Scholar]
- GHAYUAR T., HUGUNIN M., TALANIAN R.V., RATNOFSKY S., QINLAN C., EMOTO Y., PARDEY P., DATTA R., HUANG Y., KHARBANDA L., ALLEN H., KAMEN R., WONG W., KUFE D. Proteolytic activation of protein kinase C-δ by an IEC/CED 3-like protease induces characteristics of apoptosis. J. Exp. Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENKEL T., MACHLEIDT T., ALKALAY I., KRONKE M., BEN-NERIAH Y., BAEUERLE P. Rapid proteolysis of I-kappa B alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993;365:182–184. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- HU K., MOCHLY-ROSEN D., BOUTJDIR M. Evidence for functional role of epsilon PKC isoenzyme in the regulation of cardiac Ca2+ channels. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2658–H2664. doi: 10.1152/ajpheart.2000.279.6.H2658. [DOI] [PubMed] [Google Scholar]
- JOBIN C., HASKILL S., MAYER L., PANJA A., BALFOUR SARTOR R. Evidence for altered regulation of IκBα degradation in human colonic epithelial cells. J. Immunol. 1997;158:226–234. [PubMed] [Google Scholar]
- JONES B.A., RAO Y.P., STRAVITZ R.T., GORES G.J. Bile salt-induced apoptosis of hepatocytes involves activation of protein kinase C. Am. J. Physiol. Gastrointest. Liver Physiol. 1997;272:G1109–G1115. doi: 10.1152/ajpgi.1997.272.5.G1109. [DOI] [PubMed] [Google Scholar]
- KANG P.M., HAUNSTETTER A., AOKI H., USHEVA A., IZUMO S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxigenation. Circ. Res. 2000;87:118–125. doi: 10.1161/01.res.87.2.118. [DOI] [PubMed] [Google Scholar]
- KIM J.M., ECKMAN L., SAVIDGE T.C., LOWE D.C., WITTHOFT T., KAGNOFF M.F. Apoptosis of human intestinal epithelial cells after bacterial invasion. J. Clin. Invest. 1998;102:1815–1823. doi: 10.1172/JCI2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOJOUHAROFF G., HANS W., OBREMEIRER F., MANNEL D.N., ANDUS T., SCHOLMERICH J., GROSS V., FALK W. Neutralization of tumor necrosis factor (TNF) but not IL-1 reduces inflammation in chronic dextran sulphate-sodium-induced colitis in mice. Clin. Exp. Immunol. 1997;107:353–358. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOWALTOWSKI A., CASTILHO R.F., VERCESI A.E. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495 doi: 10.1016/s0014-5793(01)02316-x. [DOI] [PubMed] [Google Scholar]
- KÜHNEL F., SENDER L., PAUL Y., TIETZE M.K., TRAUTWEIN C., MANNS M., KUBICKA S. NFκB mediates apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. J. Biol. Chem. 2000;275:6421–6427. doi: 10.1074/jbc.275.9.6421. [DOI] [PubMed] [Google Scholar]
- LAOUAR A., GLESNE D., HUBERMAN E. Involvement of protein kinase C-β and ceramide in tumor necrosis factor-α-induced but not Fas-induced apoptosis of human myeloid cells. J. Biol. Chem. 1999;274:23526–23534. doi: 10.1074/jbc.274.33.23526. [DOI] [PubMed] [Google Scholar]
- LEIST GANTNER M.F., BOHLINGER I., GERMANN P.G., TIEGS G., WENDEL A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-α requires transcriptional arrest. J. Immunol. 1994;153:1778–1788. [PubMed] [Google Scholar]
- LI D., YANG B., MEHTA J.L. Tumor necrosis factor α enhances hypoxia-reoxygenation-mediated apoptosis in cultured human coronary artery endothelial cells: critical role of protein kinase C. Cardiovasc. Res. 1999;42:805–813. doi: 10.1016/s0008-6363(98)00342-3. [DOI] [PubMed] [Google Scholar]
- MAYNE G.L., MURRAY A.W. Evidence that protein kinase Cɛ mediates phorbol ester inhibition of calphostin C and tumor necrosis factor α induced apoptosis in U937 histiocytic lymphoma cells. J. Biol. Chem. 1998;273:24115–24121. doi: 10.1074/jbc.273.37.24115. [DOI] [PubMed] [Google Scholar]
- MIYAWAKI H., ZHOU X., ASHRAF M. Calcium preconditioning elicits strong protection against ischemic injury via protein kinase C signaling pathway. Circ. Res. 1996;79:137–146. doi: 10.1161/01.res.79.1.137. [DOI] [PubMed] [Google Scholar]
- MOCHLY-ROSEN D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- MOCHLY-ROSEN D., FAGIN J.A., KNAUF J.A., NIKIFOROV Y., LIRON T., SCHECHTMAN D.Spontaneous occurrence of an inhibitor of protein kinase C epsilon localization in a thyroid cancer cell line: role in thyroid tumorigenesis Advances in Enzyme Regulation 2001New York: Elsevier Pergamon Press; 87–97.ed. Weber, G. pp [DOI] [PubMed] [Google Scholar]
- NEURATH M.E., FASS I., PASPARAKIS M., ALEXOPOULOU L., HARALAMBOUS S., BUSCHENFELDE K.H., STROBER W., KOLLIAS G. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur. J. Immunol. 1997;27:1741–1750. doi: 10.1002/eji.1830270722. [DOI] [PubMed] [Google Scholar]
- NISHIZUKA Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- O'CONNELL M.A., KELLEHER D., LISKAMP R.M., HALL N., O'NEILL L.A., LONG M. TNF-mediated cytotoxicity of L929 cells: role of staurosporine in enhancement of cytotoxicity and translocation of protein kinase C isozymes. Cytokine. 1997;9:83–92. doi: 10.1006/cyto.1996.0140. [DOI] [PubMed] [Google Scholar]
- PALOMBELLA V., RANDO O., GOLDBERG A., MANIATIS T. The ubiquitin–proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- PANG G., BURET A., LOUGHLIN E.O., SMITH A., BATEY R., CLANCY R. Immunologic, functional and morphological characterization of three new human small intestinal epithelial cell lines. Gastroenterology. 1993;111:8–18. doi: 10.1053/gast.1996.v111.pm8698229. [DOI] [PubMed] [Google Scholar]
- RATH P.C., AGGARWAL B.B. TNF-induced signaling in apoptosis. J. Clin. Immunol. 1999;19:350–364. doi: 10.1023/a:1020546615229. [DOI] [PubMed] [Google Scholar]
- RENZ A., BERDEL W.E., KREUTER M., BELKA C., SCHULZE-OSTHOFF K., LOS M. Rapid extracellular release of cytochrome c is specific for apoptosis and marks cell death in vivo. Blood. 2001;98:1542–1548. doi: 10.1182/blood.v98.5.1542. [DOI] [PubMed] [Google Scholar]
- ROGLER G., BRAND K., VOGL D., PAGE S., HOFMEISTER R., ANDUS T., KNUECHEL R., BAEUERLE P.A., SCHÖLMERICH J., GROSS V. Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- SCHMITZ H., FROMM M., BENTZEL C.J., SCHOLZ P., DETJEN K., MANKERTZ J., BODE H., EPPLE H.T., RIECKEN E.O., SCHULZKE T.O. Tumor necrosis factor-alpha (TNF-alpha) regulates the epithelial barrier in human intestinal epithelial cell line, HT-29/BGF. J. Cell. Sci. 1999;112:137–146. doi: 10.1242/jcs.112.1.137. [DOI] [PubMed] [Google Scholar]
- SOUROUJON M.C., MOCHLY-ROSEN D. Peptide modulators of protein–protein interactions in intracellular signaling. Nat. Biotechnol. 1998;16:919–924. doi: 10.1038/nbt1098-919. [DOI] [PubMed] [Google Scholar]
- TEPPERMAN B.L., CHANG Q., SOPER B.D. The involvement of protein kinase C in nitric oxide-induced damage to rat isolated colonic mucosal cells. Br. J. Pharmacol. 1999;128:1268–1274. doi: 10.1038/sj.bjp.0702891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEPPERMAN B.L., SOPER B.D., CHANG Q., BROWN J.F., WAKULICH C.A. The effect of protein kinase C activation on colonic epithelial cellular integrity. Eur. J. Pharmacol. 2000;389:131–140. doi: 10.1016/s0014-2999(99)00892-4. [DOI] [PubMed] [Google Scholar]
- TWENTYMAN P.R., LUSCOMBE M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer. 1994;56:279–285. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y., ASHRAF M. Role of protein kinase C in mitochondrial KATP channel-mediated protection against Ca2+ overload injury in rat myocardium. Circ. Res. 1999;84:1156–1165. doi: 10.1161/01.res.84.10.1156. [DOI] [PubMed] [Google Scholar]
- WANG C.-Y., MAYO M.W., KORNELUK R.G., GOEDDEL D.V., BALDWIN A.S., Jr NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- WELLER S.G., KLEIN I.K., PENNINGTON R.C., KARNES W.E., Jr Distinct protein kinase C isozymes signal mitogenesis and apoptosis in human colon cancer cells. Gastroenterology. 1999;117:848–857. doi: 10.1016/s0016-5085(99)70343-4. [DOI] [PubMed] [Google Scholar]
- WILSON L., SZABO C., SALZMAN A.L. Protein kinase C-dependent activation of NF-κB in enterocytes is independent of IκB degradation. Gastroenterology. 1999;117:106–114. doi: 10.1016/s0016-5085(99)70556-1. [DOI] [PubMed] [Google Scholar]
- WRIGHT K., KOLIOS G., WESTWICK J., WARD S.G. Cytokine-induced apoptosis in epithelial HT-29 cells is independent of nitric oxide formation. J. Biol. Chem. 1999;274:17193–17201. doi: 10.1074/jbc.274.24.17193. [DOI] [PubMed] [Google Scholar]
- WYATT T.A., ITO H., VEYS T.J., SPURZEM J.R. Stimulation of protein kinase C activity by tumor necrosis factor-α in bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 1997;273:L1007–L1012. doi: 10.1152/ajplung.1997.273.5.L1007. [DOI] [PubMed] [Google Scholar]