Abstract
Partial agonism is primarily dependent upon receptor density and coupling efficiency. As these parameters are tissue/model dependent, intrinsic activity in different tissues can vary. We have utilised the ecdysone-inducible expression system containing the human nociceptin/orphanin FQ (N/OFQ) peptide receptor (hNOP) expressed in Chinese hamster ovary cells (CHOINDhNOP) to examine the activity of a range of partial agonists in receptor binding, GTPγ35S binding and inhibition of adenylyl cyclase studies.
Incubation of CHOINDhNOP cells with ponasterone A (PON) induced hNOP expression ([leucyl-3H]N/OFQ binding) of 24, 68, 191 and 1101 fmol mg−1 protein at 1, 2, 5 and 10 μM PON, respectively. At 191 fmol mg−1, protein hNOP pharmacology was identical to that reported for other traditional expression systems.
pEC50 values for GTPγ35S binding ranged from 7.23 to 7.72 (2–10 μM PON) for the partial agonist [Phe1ψ(CH2–NH)Gly2]N/OFQ(1–13)–NH2 ([F/G]N/OFQ(1–13)–NH2) and 8.12–8.60 (1–10 μM PON) for N/OFQ(1–13)–NH2 and Emax values (stimulation factor relative to basal) ranged from 1.51 to 3.21 (2–10 μM PON) for [F/G]N/OFQ(1–13)–NH2 and 1.28–6.95 (1–10 μM) for N/OFQ(1–13)–NH2. Intrinsic activity of [F/G]N/OFQ(1–13)–NH2 relative to N/OFQ(1–13)–NH2 was 0.3–0.5. [F/G]N/OFQ(1–13)–NH2 did not stimulate GTPγ35S binding at 1 μM PON, but competitively antagonised the effects of N/OFQ(1–13)–NH2 with a pKB=7.62.
pEC50 values for cAMP inhibition ranged from 8.26 to 8.32 (2–10 μM PON) for [F/G]N/OFQ(1–13)–NH2 and 9.42–10.35 for N/OFQ(1–13)–NH2 and Emax values (% inhibition) ranged from 19.6 to 83.2 for [F/G]N/OFQ(1–13)–NH2 and 40.9–86.0 for N/OFQ(1–13)–NH2. The intrinsic activity of [F/G]N/OFQ(1–13)–NH2 relative to N/OFQ(1–13)–NH2 was 0.48–0.97.
In the same cellular environment with receptor density as the only variable, we show that the profile of [F/G]N/OFQ(1–13)–NH2 can be manipulated to encompass full and partial agonism along with antagonism.
Keywords: Nociceptin/orphanin FQ, nociceptin receptor, ecdysone-inducible expression, partial agonists, GTPγ35S binding, cAMP
Introduction
Nociceptin/orphanin FQ (N/OFQ) is the endogenous peptide ligand for the Gi-coupled N/OFQ peptide receptor (NOP). The terminology used in this paper with respect to nomenclature is in line with recent IUPHAR guidelines (Cox et al., 2000). At a cellular level, N/OFQ causes a reduction in cAMP formation, activation of potassium channels and inhibition of voltage-gated calcium channels, thereby reducing neuronal excitability and inhibiting transmitter release (Meunier et al., 1995; Reinscheid et al., 1995; Knoflach et al., 1996; Vaughan & Christie, 1996; Hawes et al., 2000; Schlicker & Morari, 2000; Jennings, 2001; New & Wong, 2002). Central administration of N/OFQ has been shown to cause analgesia, hyperalgesia and allodynia, hypotension and bradycardia, diuresis and antinatriuresis and have anxiolytic properties (Calo et al., 2000b; Meunier, 2000; Mogil & Pasternak, 2001).
As a result of structure–activity relationship studies and combinatorial library screens, several selective, potent agonists and antagonists have been described and these molecules have greatly enhanced our understanding of the physiological role(s) of the N/OFQ-NOP system. These molecules include the peptides [(pF)Phe4]N/OFQ(1–13)–NH2, [Phe1ψ(CH2–NH)Gly2]N/OFQ(1–13)–NH2 ([F/G]N/OFQ(1–13)–NH2), [Nphe1]N/OFQ(1–13)–NH2, UFP-101 and nonpeptides Ro65-6570/Ro64-6198 (agonists) and J-113397/JTC-801 (antagonists) (Guerrini et al., 1998; 2000; Jenck et al., 2000; Ozaki et al., 2000; Bigoni et al., 2002b; Calo et al., 2002; McDonald et al., 2002; Yamada et al., 2002). From a combinatorial library of 52 million, Dooley et al. (1997) identified five hexapeptides with high affinity for NOP (Berger et al., 2000a). Functionally, these peptides are all partial agonists with varying degrees of efficacy. The opioid antagonist naloxone benzoylhydrazone (NalBzOH) has been shown to possess low partial agonist activity at NOP, but activity at classical opioid receptors limits the use of this synthetic compound (Nicholson et al., 1998; Bigoni et al., 2002a).
Initial studies with [F/G]N/OFQ(1–13)–NH2 in the mouse vas deferens and guinea-pig ileum indicated that the peptide behaved as an NOP-selective antagonist (Guerrini et al., 1998). However, subsequent studies reported variable intrinsic activity of this molecule from zero (pure antagonism) to one (full agonism) (Calo et al., 1998; Grisel et al., 1998; Meis & Pape, 1998; Menzies et al., 1999; Okawa et al., 1999; Mason et al., 2001; Calo et al., 2000a). Consensus is that [F/G]N/OFQ(1–13)–NH2 is in fact a low-efficacy partial agonist (Calo et al., 2000b). However, there are no detailed studies on NOP from one species at differing levels of expression using an identical cell background that specifically addresses this question.
To date, NOP has been expressed in a variety of mammalian cell lines including CHO and HEK-293 (Guerrini et al., 2000; Dautzenberg et al., 2001), which either utilise a transient expression strategy (hence relatively uncontrolled expression) or are used to generate stable clones (usually with high levels of expression). It would be desirable, with particular reference to the evaluation of agonist intrinsic activity, to have a range of lines available with differing levels of receptor expression. The ecdysone-inducible expression system represents a simple method allowing the production of cultures with differing expression levels of a receptor of interest. In this system, addition of ponasterone A (an ecdysone analogue) will produce a concentration-dependent increase in transcription and hence receptor expression. This has been demonstrated for sst2 (Cole et al., 2001), 5-HT (h5-HT 1B, 1F, 4B) (Van Craenenbroeck et al., 2001) and DOP receptors (Law et al., 2000).
In this study, we have utilised the ecdysone-inducible expression system containing the hNOP receptor to examine the activity of a range of partial agonist molecules. Initially, we have characterised the system with reference to ponasterone A induction concentrations in (a) receptor binding, (b) GTPγ35S binding and (c) cAMP assays. A pharmacological characterisation of the receptor expressed at levels similar to those typically obtained in saturation studies of rat cerebrocortical membranes (∼180 fmol mg−1 protein (Okawa et al., 1998)) is presented. Finally, we have examined the effects of a range of partial agonists including [F/G]N/OFQ(1–13)–NH2, Ac-RYYRIK–NH2 and Ac-RYYRWK–NH2, and NalBzOH at differing levels of NOP receptor expression.
Methods
Sources of materials
N/OFQ, N/OFQ–NH2, N/OFQ(1–13)–NH2, [F/G]N/OFQ(1–13)–NH2, J-113397, Ac-RYYRIK–NH2 and Ac-RYYRWK–NH2 were synthesised at the Department of Pharmaceutical Sciences at the University of Ferrara as described previously (Guerrini et al., 1997; De Risi et al., 2001; Rizzi et al., 2002). NalBzOH was purchased from Sigma (Poole, U.K.). Radioactivity, [leucyl-3H]N/OFQ (149–152 Ci mmol−1) was obtained from Amersham Pharmacia Biotech (Buckinghamshire, U.K.), GTPγ35S (1250 Ci mmol−1) and [2,8-3H]cAMP (28 Ci mmol−1) were obtained from NEN DuPont (Boston, MA, U.S.A.). Pertussis toxin was obtained from Sigma. Ponasterone A, zeocin, all tissue culture media and supplements were obtained from Invitrogen (Paisley, U.K.). Chinese hamster ovary (CHO) cells containing the ecdysone-inducible expression system with the hNOP and CHO cells stably expressing the hNOP were kindly provided by Dr F. Marshall and Mrs N. Bevan of GSK (Stevenage, Herts, U.K.).
The ecdysone-inducible mammalian expression system
The system is based on the induction, molting and metamorphosis process utilised by Drosophila, which activates gene expression through the ecdysone receptor. This system has been modified for use in mammalian cell lines so that a chosen gene can be expressed by the application of a steroid promoter. The ecdysone system makes use of a heterodimeric nuclear receptor consisting of the ecdysone receptor (VgEcR) and the retinoid X receptor (RXR, modified from mammalian cells), which bind a hybrid response element (E/GRE) in the presence of the synthetic analogue of ecdysone, ponasterone A. The hybrid response element lies upstream of a minimal heat shock promoter, activation of which leads to transcription of the gene of interest (in this study, human NOP).
Cell culture and induction
CHO cells stably expressing the ecdysone-inducible mammalian expression system containing the hNOP (CHOINDhNOP) were cultured in HAMS F12 supplemented with 10% foetal calf serum, penicillin (100 IU ml−1), streptomycin (100 μg ml−1) and fungizone (2.5 μg ml−1). Stock cultures were further supplemented with geneticin (1 mg ml−1) and zeocin (250 μg ml−1). CHO cells stably expressing the hNOP receptor (CHOhNOP) were routinely cultured as described (Hashiba et al., 2001). All cultures were maintained at 37°C with 5% carbon dioxide humidified air and subcultured as required using trypsin/EDTA. Cells were induced as they approached confluence for 20 h with the steroid ponasterone A, at concentrations of 1, 2, 5, 10 and 20 μM. Non-induced CHOINDhNOP cultures were used as negative controls in which the medium was replaced 20 h prior to use. In studies using pertussis toxin (PTx), 100 ng ml−1 was added to media at the time of induction.
Membrane preparation
Membranes were prepared from freshly harvested cells, CHOhNOP at confluence and CHOINDhNOP 20 h postmedia change/induction. Cells were suspended in a homogenising buffer of either Tris-HCl (50 mM), MgSO4 (5 mM) pH 7.4 with KOH (saturation and displacement) or Tris-HCl (50 mM), EGTA (0.2 mM) pH 7.4 with NaOH (GTPγ35S). Suspensions were homogenised followed by centrifugation at 13,500 rpm, for 10 min at 4°C. This was repeated three times in total. The membrane pellet was finally resuspended as appropriate for each experiment, the protein concentration was determined (Lowry et al., 1951) and finally adjusted as required for the experimental procedure.
Saturation binding
The membrane protein (15–350 μg) (depending on induction level) was incubated in 0.5 ml of homogenisation buffer containing 0.5% BSA, 10 μM peptidase inhibitors (amastatin, bestatin, captopril, phosphoramidon) and various concentrations of [leucyl-3H]N/OFQ (∼2 nM–0.002 pM) for 1 h at room temperature. Nonspecific binding (NSB) was defined in the presence of 1 μM unlabelled N/OFQ. Reactions were terminated by vacuum filtration through polyethylenimine (PEI) (0.5%)-soaked Whatman GF/B filters using a Brandel harvester.
Displacement binding
The membrane protein (35–70 μg) was incubated in the buffer used in saturation assays, but containing a fixed concentration of [leucyl-3H]N/OFQ (∼200 pM) and varying concentrations of a range of displacers. NSB was determined in the presence of 1 μM unlabelled N/OFQ. Assays were incubated at room temperature for 1 h and reactions terminated via filtration through PEI (0.5%)-soaked Whatman GF/B filters using a Brandel harvester.
GTPγ35S assays
CHOINDhNOP (40 μg) or 20 μg CHOhNOP membranes were incubated in 0.5 ml buffer containing Tris (50 mM), EGTA (0.2 mM), MgCl2 (1 mM), NaCl (100 mM) BSA (1 mg ml−1) pH 7.4 with NaOH to which bacitracin (0.15 mM), amastatin, bestatin, captopril and phosphoramidon (10 μM); GDP (5 μM/100 μM) and ∼150 pM GTPγ35S were added. NOP ligands were included in various combinations and at various concentrations. NSB was determined in the presence of 10 μM GTPγS. All receptor ligands were omitted when defining basal and NSB binding of GTPγ35S. Reactions were incubated for 1 h at 30°C with gentle shaking and terminated by filtration through Whatman GF/B filters using a Brandel harvester.
In all cases, radioactivity was determined following filter extraction (8 h, Optiphase Safe, Wallac) using liquid scintillation spectroscopy.
Inhibition of forskolin-stimulated cAMP formation
Inhibition of forskolin-stimulated cAMP formation was measured using whole CHO cells induced at 1, 2, 5 and 10 μM ponasterone A. Confluent adherent cell cultures (grown in 24-well tissue culture trays) were incubated in the presence of 1 mM isobutylmethylxanthine (IBMX) and forskolin (1 μM) for 15 min. NOP ligands were included in various combinations and at different concentrations. Reactions were terminated using 10 M HCl and neutralised with 10 M NaOH/1 mM Tris, pH 7.4. The concentration of cAMP was measured using the protein-binding method set out by Brown et al. (1971).
Analysis of data
All data are expressed as mean±s.e.m., from a minimum of three experiments performed as single points or in duplicate. Concentration–response curves and statistical analyses (paired/unpaired Students' t-test and ANOVA with Bonferroni correction for multiple comparison where appropriate) were performed using PRISM V3.0 (GraphPad, San Diego, U.S.A). pKi values were calculated using the Cheng & Prusoff equation (log{IC50/(1+[Radiolabel]/KD)}) (Cheng & Prusoff, 1973). A KD of 60.3 pM for [leucyl-3H]N/OFQ, measured from saturation binding using 5 μM-induced membranes was used. pKB values were calculated using the formula pKB=−log{(CR−1)/[antagonist]}, where CR is the ratio of the EC50 of the agonist in the presence and absence of antagonist, assuming a slope value of unity. In GTPγ35S binding studies, data are either presented as DPM 35S bound (in studies where the GDP concentration is varied, as ‘stimulation factor' is GDP dependent) or stimulation factor (i.e. the ratio between specific agonist-stimulated GTPγ35S binding and basal specific binding). cAMP data are presented as percentage inhibition of the forskolin-stimulated response.
Results
Saturation binding assays
Incubation of CHOINDhNOP cells with ponasterone A induced the expression of NOP, as measured by the binding of [leucyl-3H]N/OFQ. The total specific binding of [leucyl-3H]N/OFQ increased from 24 to 1101 fmol mg−1 protein as the concentration of ponasterone A was increased (1–10 μM) (Table 1). In non-induced cultures, there was no significant specific binding despite the use of large quantities of membrane protein. Interestingly, the induction–expression relationship appeared to be bell-shaped, such that an apparent maximum was obtained at 10 μM ponasterone A, above which (i.e. 20 μM ponasterone A) binding decreased. In a simple series of trypan blue exclusion experiments (n=6, data not shown), 20 μM ponasterone A did not cause any significant cytotoxicity.
Table 1.
The binding of [leucyl-3H]N/OFQ to CHOINDhNOP was ponasterone A dependent
| Induction (ponasterone A) (μM) | pKD | KD (pM) | Bmax (fmol mg protein−1) |
|---|---|---|---|
| 0 (noninduced) | — | — | — |
| 1 | 9.91±0.04 | 123 | 23.5±4.4 |
| 2 | 9.83±0.09 | 148 | 68.3±9.7 |
| 5 | 10.22±0.15 | 60 | 190.6±25.5 |
| 10 | 9.89±0.14 | 129 | 1101.0±145.3 |
| 20 | 9.89±0.13 | 129 | 191.2±33.9 |
Saturation analysis of log-transformed specific data was used to estimate Bmax and pKD. Data are mean±s.e.m. for n≥3 experiments.
The expression of NOP at 5 μM ponasterone A induction (∼200 fmol mg−1 protein) is similar to that measured in brain tissues, for example, in rat cerebral cortex membranes (179.7 fmol [125I]Tyr14-N/OFQ mg−1 protein) (Okawa et al., 1998; Hashiba et al., 2001) and so this induction level has been used to perform a series of displacements and GTPγ35S /cAMP studies in order to detail the pharmacology of the induced hNOP receptor.
Displacement binding assays
The binding of a fixed concentration of [leucyl-3H]N/OFQ was displaced in a concentration-dependent and saturable manner by a range of NOP peptide and non-peptide ligands in membranes prepared from CHOINDhNOP cells induced with 5 μM ponasterone A. pKi values for these data are summarised in Table 2. The rank order pKi is N/OFQ–NH2=N/OFQ(1–13)–NH2>N/OFQ=Ac-RYYRWK–NH2>[F/G]N/OFQ(1–13)–NH2>Ac-RYYRIK–NH2=J-113397>NalBzOH.
Table 2.
pKi values for a range of NOP ligands measured in CHOINDhNOP membranes induced with 5 μM ponasterone A
| Ligand Agonist | Class Peptide (P)/non-peptide (NP) | pKi |
|---|---|---|
| N/OFQ | P | 9.93±0.08 |
| N/OFQ–NH2 | P | 10.37±0.04 |
| N/OFQ(1-13)–NH2 | P | 10.35±0.04 |
| Presumed partial agonists NalBzOH | NP | 7.1±0.02 |
| [F/G]N/OFQ(1-13)–NH2 | P | 9.6±0.1 |
| Ac-RYYRIK–NH2 | P | 9.12±0.02 |
| Ac-RYYRWK–NH2 | P | 9.99±0.03 |
| Antagonist | ||
| J-113397 | NP | 9.09±0.11 |
pKi values were calculated using the Cheng and Prusoff equation using a KD of 60.3 pM for [leucyl-3H]N/OFQ, measured in saturation experiments for the same induction (Table 1). Data are mean±s.e.m. (n=4).
GTPγ35S and cAMP functional data
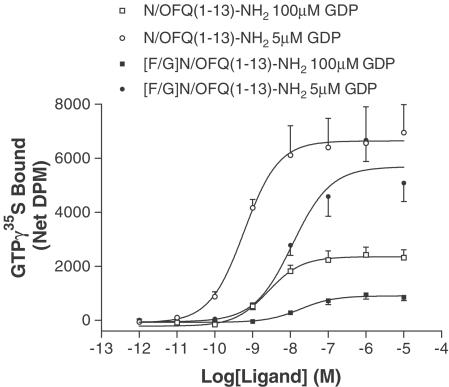
Initially, we made a comparison of GTPγ35S binding stimulated by N/OFQ(1–13)–NH2 and [F/G]N/OFQ(1–13)–NH2 in CHOhNOP and CHOINDhNOP cells (5 μM ponasterone A induction) at high (100 μM) and low (5 μM) concentrations of GDP. It has been hypothesised that low-efficacy (partial) agonists activate G proteins with vacant guanine nucleotide-binding sites more effectively. Hence, reducing the GDP concentration should lead to fewer occupied guanine nucleotide-binding sites and result in an increased intrinsic activity (Breivogel et al., 1998; Berger et al., 2000b; Bigoni et al., 2002a).
The stable CHOhNOP cell line used here expresses ∼1.9 pmol mg−1 protein NOP and has been used extensively by us in the past (Okawa et al., 1998; Guerrini et al., 2000; 2001; Hashiba et al., 2001). At 100 μM GDP, both N/OFQ(1–13)–NH2 and [F/G]N/OFQ(1–13)–NH2 stimulated GTPγ35S binding to CHOhNOP membranes in a concentration-dependent and saturable manner with pEC50 values of 9.11 and 8.28, respectively. N/OFQ(1–13)–NH2 was a full agonist, Emax 10,117 net DPM, while [F/G]N/OFQ(1–13)–NH2 displayed partial agonist activity in this preparation with an Emax of 6221 net DPM (Figure 1, Table 3). At 5 μM GDP, the potency of both [F/G]N/OFQ(1–13)–NH2 (pEC50 8.68) and N/OFQ(1–13)–NH2 (pEC50 9.57) increased as did the net stimulated binding of GTPγ35S, 24,255 and 16,999 DPM, respectively. More importantly, the relative intrinsic activity of [F/G]N/OFQ(1–13)–NH2 compared to N/OFQ(1–13)–NH2 increased from 0.61 to 0.70. The same is true for N/OFQ(1–13)–NH2 and [F/G]N/OFQ(1–13)–NH2 using membranes from 5 μM ponasterone A-induced cells (Table 3) with relative intrinsic activity increasing from 0.38 to 0.81. A similar increase in relative intrinsic activity can also be observed using Ac-RYYRWK–NH2 and NalBzOH, the latter having been previously published (Bigoni et al., 2002a).
Figure 1.
Net GTPγ35S binding by N/OFQ(1–13)–NH2 and [F/G]N/OFQ(1–13)–NH2 in membranes from CHOINDhNOP (5 μM ponasterone A induction) with 100 and 5 μM GDP. Data are mean±s.e.m. for n=4.
Table 3.
Effects of [F/G]N/OFQ(1–13)–NH2 Ac-RYYRWK–NH2 and NalBzOH on GTPγ35S binding in CHOhNOP and CHOINDhNOP (5 μM ponasterone A) membranes in the presence of either 100 or 5 μM GDP
| CHOhNOP | CHOINDhNOP | CHOhNOP | CHOhNOP (Bigoni et al., 2002a) | |||||
|---|---|---|---|---|---|---|---|---|
| N/OFQ(1–13) | F/G(1–13) | N/OFQ(1–13) | F/G(1–13) | N/OFQ | RYYRWK | N/OFQ | NalBzOH | |
| 100 μm GDP | ||||||||
| pEC50 | 9.11±0.03 | 8.28±0.01 | 8.65±0.07 | 7.72±0.05 | 8.30±0.04 | 8.97±0.03 | 8.53±0.12 | Inactive |
| Emax (DPM) | 10117±134 | 6221±374 | 2368±301 | 908±104 | 9548±86 | 5291±78 | 4140±40a | Inactive |
| α | 1.00 | 0.61 | 1.00 | 0.38 | 1.00 | 0.55 | 1.00 | 0 |
| 5 μm GDP | ||||||||
| pEC50 | 9.57±0.02* | 8.68±0.12* | 9.27±0.12* | 8.02±0.03* | 8.70±0.01* | 9.34±0.09* | 9.29±0.02* | 7.00±0.10 |
| Emax (DPM) | 24255±964* | 16999±242* | 6635±1080* | 5388±834* | 14894±317* | 11775±130* | 18190±65* | 2278±238 |
| α | 1.00 | 0.70 | 1.00 | 0.81 | 1.00 | 0.79 | 1.00 | 0.13 |
N/OFQ(1–13)–NH2 and N/OFQ were used as reference full agonists. Data derived from PRISM-Fits as in Figure 1 and are mean±s.e.m. (n≥3).
P<0.05 (paired t-test) increased pEC50 or Emax compared with 100 μM.
While it appears that there is some variation in the Emax (DPM) for N/OFQ and N/OFQ(1–13)NH2 for CHOhNOP cells reported in Bigoni et al. (2002a) relative to this study, it should be borne in mind that the study of Bigoni et al. (2002a) was performed with different batches of cells and GTPγ35S producing lower net DPM for basal, NSB and stimulated binding. However, the stimulation factors for N/OFQ(1–13)NH2 in this study (100 μM GDP; 10.26±0.72) are comparable to those for N/OFQ (100 μM GDP; 8.12±0.29) in Bigoni et al. (2002a).
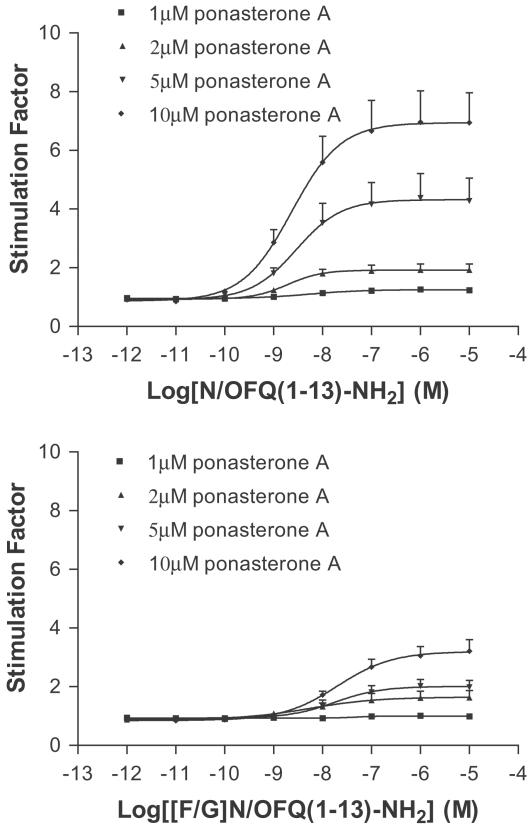
In membranes prepared from CHOINDhNOP cells incubated with 1, 2, 5 and 10 μM ponasterone A, both N/OFQ(1–13)–NH2 and [F/G]N/OFQ(1–13)–NH2 stimulated the binding of GTPγ35S in a concentration-dependent and saturable manner (Figure 2). As the induction concentration of ponasterone A was increased, the Emax (stimulation factor) of N/OFQ(1–13)–NH2 increased from 1.28 (1 μM) to 6.95 (10 μM; see Table 4). The Emax of [F/G]N/OFQ(1–13)–NH2 also increased as a function of the induction concentration, from 0.98, that is, basal (1 μM) to 3.21 (10 μM). However, the relative intrinsic activity of [F/G]N/OFQ(1–13)–NH2 (relative to N/OFQ(1–13)–NH2) remained similar at 0.37–0.55 for all induction levels.
Figure 2.
N/OFQ(1–13)–NH2 (upper panel) and [F/G]N/OFQ(1–13)–NH2 (lower panel)-stimulated GTPγ35S binding to membranes prepared from CHOINDhNOP cells induced with 1, 2, 5 and 10 μM ponasterone A. Data are mean±s.e.m. for n≥4.
Table 4.
N/OFQ(1–13)–NH2 and [F/G]N/OFQ(1–13)–NH2 stimulation of GTPγ35S binding and inhibition of cAMP formation in CHOINDhNOP membranes and cells respectively, induced for 20 h with 1, 2, 5, 10 μM ponasterone A
| GTPγ35S | cAMP | |||||
|---|---|---|---|---|---|---|
| Induction (Ponasterone A) | pEC50/Emax N/OFQ(1–13)NH2 | pEC50/Emax [F/G](1–13)NH2 | N/OFQ(1–13)NH2/[F/G](1–13)NH2 | pEC50/Emax N/OFQ(1–13)NH2 | pEC50/Emax [F/G](1–13)NH2 | N/OFQ(1–13)NH2/[F/G](1–13)NH2 |
| 1 μM | 8.12±0.32 | Not analysable | — | Not analysable | Not analysable | — |
| 1.28±0.03 | ||||||
| 2 μM | 8.68±0.11 | 7.23±0.38 | 9.42±0.49 | 8.26±0.87 | ||
| 1.93±0.20 | 1.51±0.15 | 0.55 | 40.9±2.2 | 19.6±4.8 | 0.48 | |
| 5 μM | 8.52±0.06 | 7.68±0.10 | 9.72±0.40 | 8.99±0.18 | ||
| 4.33±0.80 | 2.01±0.23 | 0.30 | 79.5±4.1 | 59.37±5.8 | 0.75 | |
| 10 μM | 8.60±0.07 | 7.72±0.06 | 10.35±0.22 | 8.32±0.13 | ||
| 6.95±1.05 | 3.21±0.38 | 0.37 | 86.0±3.7 | 83.23±4.0 | 0.97 | |
Data are mean±s.e.m. for n⩾3 experiments. pEC50 values for N/OFQ(1–13)–NH2 and [F/G](1–13)–NH2 did not differ (P>0.05, ANOVA). There was a ponasterone concentration-dependent increase in Emax for N/OFQ(1–13)–NH2 and [F/G](1–13)–NH2 (P<0.05, ANOVA).
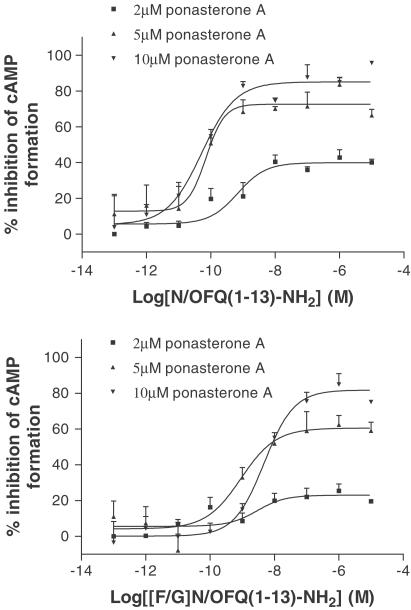
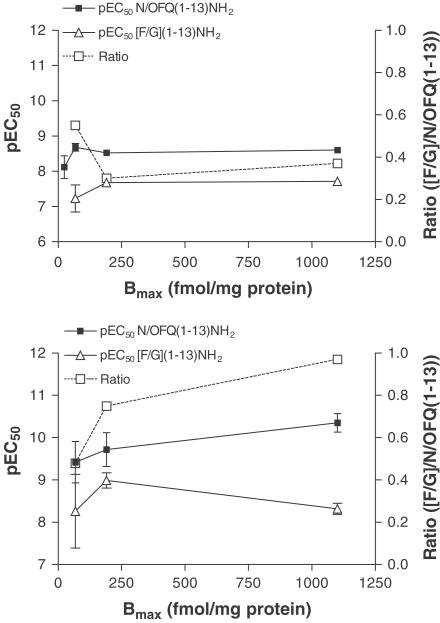
In cAMP inhibition studies, the Emax of both N/OFQ(1–13)–NH2 and [F/G]N/OFQ(1–13)–NH2 also varied as a function of the induction concentration, from 41 to 86% and from 20 to 83% at low and high ponasterone A induction, respectively (Figure 3). The relative intrinsic activity of [F/G]N/OFQ(1–13)–NH2 changed from 0.48 to 0.97 (Table 4), indicating that at 10 μM ponasterone A this molecule, in this assay, behaved as a full agonist. At the lower 1 μM ponasterone A induction, due to low expression of hNOP and sensitivity of this assay, data for cAMP studies could not be reliably analysed. These and GTPγ35S binding data are shown as a function of receptor density in Figure 4.
Figure 3.
N/OFQ(1–13)–NH2 (top panel) and [F/G]N/OFQ(1–13)–NH2 (bottom panel) inhibition of forskolin-stimulated cAMP formation in whole CHOINDhNOP cells induced with 2, 5 and 10 μM ponasterone A. Data are mean±s.e.m. for n≥3.
Figure 4.
Summary of pEC50 values for N/OFQ(1–13)–NH2 (top-GTPγ35S) and [F/G]N/OFQ(1–13)–NH2 in CHOINDhNOP (bottom-cAMP) as a function of receptor density (Bmax). Also shown is the relative intrinsic activity (N/OFQ(1–13)–NH2/[F/G]N/OFQ(1–13)–NH2 ratio).
Pertussis toxin (PTx) sensitivity
CHOINDhNOP cells were induced for 20 h at 5 μM ponasterone A in the absence and presence of PTx (100 ng ml−1). Membrane fragments or whole cells were then tested for their ability to stimulate the binding of GTPγ35S or inhibit cAMP formation by a range of NOP-selective agonists (Table 5). While in this series of experiments the degree of GTPγ35S stimulation and inhibition of cAMP was slightly reduced, PTx treatment clearly prevented agonist-stimulated GTPγ35S binding and CAMP inhibition by N/OFQ, N/OFQ(1–13)–NH2, [F/G]N/OFQ(1–13)–NH2 and confirms NOP action through either a Gi and/or Go in CHOINDhNOP cells.
Table 5.
PTx sensitivity of agonist-stimulated GTPγ35S binding and cAMP inhibition for CHOINDhNOP membranes and cells (5 μM ponasterone A), respectively
| Ligand | GTPγ35S binding (stimulation factor) | cAMP inhibition (%) | ||
|---|---|---|---|---|
| Control | +PTx | Control | +PTx | |
| N/OFQ | 2.45±0.34 | 1.01±0.16* | 43.1±8.9 | 0.0±9.6* |
| N/OFQ(1–13)–NH2 | 2.49±0.38 | 1.17±0.13* | 50.3±8.8 | 1.7±7.0* |
| [F/G]N/OFQ(1–13)–NH2 | 1.48±0.13 | 0.74±0.20* | 45.5±16.7 | 3.4±15.3* |
Agonists were included at 10 μM for GTPγ35S experiments and 100 nM for cAMP measurements. Data are mean±s.e.m.; n⩾3.
Values are significantly reduced compared with control, P<0.05 (unpaired t-test).
In CHOINDhNOP induced at 5 μM ponasterone A, we examined the behaviour of a range of other NOP ligands including Ac-RYYRIK–NH2, Ac-RYYRWK–NH2 and NalBzOH. Both N/OFQ and N/OFQ–NH2 produced concentration-dependent and saturable increases in the binding of GTPγ35S (Table 6). Both were full agonists since Emax values did not vary significantly from one another or N/OFQ(1–13)–NH2. In the GTPγ35S assay at the same induction level, both Ac-RYYRIK–NH2 and Ac-RYYWK–NH2 were clear partial agonists (Emax 1.66±0.02 and 2.16±0.08, respectively) with relative intrinsic activity values not significantly different from that of [F/G]N/OFQ(1–13)–NH2 (Table 6). NalBzOH produced no measurable stimulation of GTPγ35S binding up to 100 μM in membranes from cells induced at 1–10 μM ponasterone A and is therefore classed as an antagonist in this assay system (Okawa et al., 1999).
Table 6.
Effects of NalBzOH, Ac-RYYRIK–NH2 and Ac-RYYRWK–NH2 at different induction concentrations on GTPγ35S binding and comparison with N/OFQ and N/OFQ–NH2
| Ligand | [Induction] (ponasterone A) (μM) | pEC50 | Emax |
|---|---|---|---|
| N/OFQ | 5 | 8.26±0.01 | 3.72±1.01 |
| N/OFQ–NH2 | 5 | 8.92±0.05 | 4.13±0.62 |
| NalBzOH | 1 | No response at 100 μM | |
| 5 | No response at 100 μM | ||
| 10 | No response at 100 μM | ||
| Ac-RYYRIK–NH2 | 1 | 7.76±0.26 | 1.10±0.04 |
| 5 | 8.27±0.27 | 1.66±0.02* | |
| Ac-RYYRWK–NH2 | 1 | 8.39±0.27 | 1.22±0.07 |
| 5 | 8.69±0.11 | 2.16±0.08* | |
Data are mean±s.e.m. for n≥3 experiments.
P<0.05 (unpaired t-test) significantly different compared with 1 μM ponasterone A-induced cells.
Antagonism studies
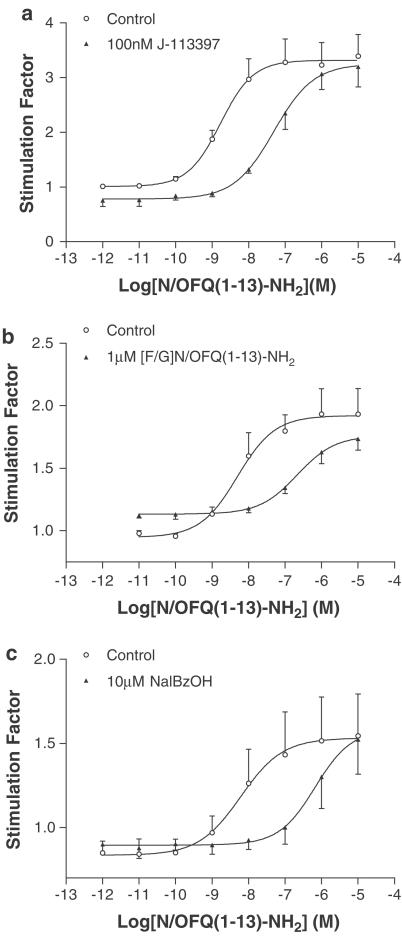
The NOP-selective, nonpeptide antagonist J-113397 was evaluated. J-113397 (100 nM) antagonised GTPγ35S binding stimulated by N/OFQ(1–13)–NH2 with an apparent pKB of 8.45 (Figure 5a). This value is essentially identical to the pA2 for J-113397 of 8.53 measured previously in CHOhNOP membranes (McDonald et al., 2002).
Figure 5.
N/OFQ(1–13)–NH2-stimulated GTPγ35S binding is reversed by 100 nM J-113397 at 5 μM ponasterone A induction (a), 1 μM [F/G]N/OFQ(1–13)–NH2 at 1 μM ponasterone A induction (b) and 10 μM NalBzOH at 1 μM ponasterone A induction (c). Data are mean±s.e.m., n=3.
At a 1 μM ponasterone A induction, [F/G]N/OFQ(1–13)–NH2 produced little or no measurable binding of GTPγ35S (Table 4), and hence this expression level was used to examine antagonist activity. At 1 μM ponasterone A, the binding of GTPγ35S stimulated by N/OFQ(1–13)–NH2 was competitively antagonised by 1 μM [F/G]N/OFQ(1–13)–NH2 with an apparent pKB of 7.62±0.08 (Figure 5b). Using the same induction concentration in the GTPγ35S assay, the nonselective partial agonist NalBzOH (which was devoid of any agonist activity in this system, Table 6) was used to antagonise the actions of N/OFQ(1–13)–NH2. NalBzOH (10 μM) competitively antagonised GTPγ35S binding stimulated by N/OFQ(1–13)–NH2 with an apparent pKB of 7.02±0.13 (Figure 5c).
Discussions
We show that the estimated intrinsic activity of a range of NOP partial agonists is dependent upon receptor density. In the ecdysone-inducible expression system, NOP not only displays the same pharmacology observed in different cell lines and tissues, but also allows reliable titration of receptor density.
To discriminate between antagonists and partial agonists, Berger et al. (2000b) described a method based upon decreasing the GDP concentration in GTPγ35S-binding studies. High GDP (⩾100 μM) concentration can mask the low activity of partial agonists. Previously, we have shown that stimulation of GTPγ35S binding by the partial agonist NalBzOH depended on the GDP concentration (Bigoni et al., 2002a). Here, we further describe this effect for [F/G]N/OFQ(1–13)–NH2 and Ac-RYYWK–NH2. Decreasing the GDP concentration to 5 μM increased the net stimulated GTPγ35S binding. Moreover, the intrinsic activity of the partial agonists [F/G]N/OFQ(1–13)–NH2 and Ac-RYYRWK–NH2 relative to N/OFQ and N/OFQ(1–13)–NH2 in both CHOhNOP and CHOINDhNOP (5 μM ponasterone A) systems increased. This greater increase in intrinsic activity for [F/G]N/OFQ(1–13)–NH2 and Ac-RYYRWK–NH2 may suggest that partial agonists and full agonists differ in their dependency for GDP.
In order to carry out a more detailed study of the effects that differential expression of hNOP has on the intrinsic activity of different ligands in one system, the ecdysone expression system has been used (Van Craenenbroeck et al., 2001). The higher concentration (10 μM ponasterone A) produced receptor densities (∼1 pmol mg−1) similar to many commonly used transfected cell systems, for example, CHOhNOP here used 1.9 pmol mg−1 (Hashiba et al., 2002), HEK 293 1.2 pmol mg−1 (Dautzenberg et al., 2001) and CHOhNOP 0.9 pmol mg−1 (Mason et al., 2001). The 5 μM induced receptor density (∼200 fmol mg−1) was similar to that reported in rat central tissue, for example, rat cortex 236 fmol mg−1 (Berger et al., 2000a), rat frontal cortex 246 fmol mg−1 (Mason et al., 2001) and rat cerebral cortex 180 fmol mg−1 (Okawa et al., 1998) and represents a pseudo-physiological level of receptor expression. Competition binding assays at this expression density indicated a pharmacology consistent with that reported in the literature. In GTPγ35S and cAMP assays at 5 μM induction, N/OFQ and N/OFQ–NH2 were both full agonists with pEC50 values of 8.26 and 8.92 (GTPγ35S), 9.38 and 9.66 (cAMP), respectively. Furthermore, in GTPγ35S-binding assays at this induction concentration, Ac-RYYRIK–NH2 and Ac-RYYRWK–NH2 (Dooley et al., 1997) were partial agonists and the effects of N/OFQ(1–13)–NH2 were antagonised by J-113397 (pKB∼8.45). In all assays, agonist effects were PTx sensitive, confirming the expected Gi/Go coupling in this system.
In GTPγ35S binding, N/OFQ(1–13)–NH2 was always a full agonist, while [F/G]N/OFQ(1–13)–NH2 produced submaximal stimulation, possessing little or no response at 1 μM induction. In cAMP measurements, N/OFQ(1–13)–NH2 was again a full agonist in all preparations. However, [F/G]N/OFQ(1–13)–NH2 displayed full agonism at the 10 μM ponasterone A concentration, with the percentage inhibition being similar to that reported by us in our higher stable expressing CHOhNOP transfects (see Okawa et al., 1999), and partial agonism at all lower expressions of hNOP. This is typical of the amplification seen when measuring a downstream effector such as cAMP, that is, saturation of stimulus–response mechanisms becomes more evident the further down the stimulus–response chain the response is measured (Kenakin, 1997). In GTPγ35S assays at the lowest induction concentration (1 μM ponasterone A), [F/G]N/OFQ(1–13)–NH2 and NalBzOH acted as competitive antagonists with pKB values of 7.62 and 7.02, similar to their pEC50 values of 7.68 and 7.00 (Bigoni et al., 2002a), respectively.
Conflicting data from different groups using similar and differing preparations reported agonism, partial agonism and antagonism for [F/G]N/OFQ(1–13)–NH2 and also for Ac-RYYRIK–NH2, Ac-RYYRWK–NH2 and NalBzOH (Okawa et al., 1999; Berger et al., 2000a; Calo et al., 2000a; Mason et al., 2001). In vitro [F/G]N/OFQ(1–13)–NH2 was a full agonist for inhibition of cAMP formation in CHOhNOP cells and inhibition of glutamate release from synaptasomes (Okawa et al., 1999). Following i.c.v. injection in rats, [F/G]N/OFQ(1–13)–NH2 caused a decrease in heart rate, mean arterial pressure, urinary sodium excretion and a marked increase in urine flow, similar to N/OFQ but of longer duration (Kapusta et al., 1999). Partial agonism was also reported for the stimulation of GTPγ35S binding in mouse N1E-115 cells (Olianas et al., 1999). For a detailed review of the actions of [F/G]N/OFQ(1–13)–NH2, see Calo et al. (2000a). This difference in signalling between central and peripheral NOP was explained by [F/G]N/OFQ(1–13)–NH2 being a partial agonist with strong coupling in central tissue and high-expression transfected systems and weak coupling in peripheral tissue and low-expression systems (Okawa et al., 1999). To date, the variable pharmacology of these partial agonists has not been carefully examined in the same expression system.
This problem has been addressed by only a few groups, using either cells transfected with different levels of NOP or using peripheral and central tissue (Mason et al., 2001). A recent paper by Mason et al. (2001) showed differences in the relative intrinsic activities of [F/G]N/OFQ(1–13)–NH2, Ac-RYYRIK–NH2 and Ac-RYYRWK–NH2 using transfected cells, central preparations and peripheral tissue. Differences in coupling efficiency are just one variable seen between different tissue preparations and native and recombinant NOP, which can affect values of relative intrinsic activity. Hence, differences in relative intrinsic activity may not be the result of changes in receptor number, but due to changes in coupling efficiency or other local cellular factors such as GDP concentration. Recent suggestions and data have shown that agonists differ in their efficacy for different cellular responses or subtypes of downstream effector (Berg et al., 1998; Cordeaux et al., 2000). Indeed, N/OFQ can stimulate PLC activity, with differential potency via a Gα14-mediated PTx-insensitive pathway (in Gα14-transfected cells, EC50 5 nM; Yung et al., 1999) and via a Gαi PTx-sensitive pathway (EC50 0.4 nM; Reinscheid et al., 1995). Therefore, different subtypes of effector or cellular pathways leading to a given response between different cell types or tissue preparations could give rise to differential efficacy/potency making conclusions about relative activities of ligands awkward, i.e., differences in relative efficacy of a ligand between tissues may not be due to receptor density alone.
As noted, it has been suggested that the variable activity reported for the actions of [F/G]N/OFQ(1–13)–NH2, that is, agonist, partial agonist and antagonist, was the result of different expressions of NOP at those sites assayed. Since we were able to control the expression of hNOP by changing the induction concentration of ponasterone A, we could measure the effect this had on the intrinsic activity of both N/OFQ(1–13)–NH2 and [F/G]N/OFQ(1–13)–NH2 in two functional assays. The efficacy of N/OFQ(1–13)–NH2 with respect to its ability to stimulate GTPγ35S binding and inhibit adenylyl cyclase was full agonist in nature for all expression levels. However, the relative intrinsic activity of [F/G]N/OFQ(1–13)–NH2 varied at different expression levels and between assays. In cAMP measurements, [F/G]N/OFQ(1–13)–NH2 was a full agonist at 10 μM ponasterone A and partial agonist at all other induction concentrations. An increased receptor reserve at 10 μM ponasterone A induction and amplification steps in the pathway leading to the inhibition of adenylyl cyclase may explain this finding. However, it may be more fitting to suggest a coupling reserve (i.e. only a small proportion of the activated G protein is required to generate a full response), since no change in relative intrinsic activity is seen in GTPγ35S binding, suggesting that there is no receptor reserve. At expression levels greater than that induced by 1 μM ponasterone A, that is, ∼30 fmol mg−1, [F/G]N/OFQ(1–13)–NH2 was a partial agonist in GTPγ35S measurements. Below this expression, [F/G]N/OFQ(1–13)–NH2 produced no response. This is due to the very low density of hNOP and [F/G]N/OFQ(1–13)–NH2 reduced efficacy for the receptor. Indeed, it is tempting to suggest that in previous studies where [F/G]N/OFQ(1–13)–NH2 behaved as an antagonist, this is due to similar low expression as that shown here, although as mentioned previously other factors can also play a role.
It can be seen from our data that intrinsic activity is a property of both the ligand and the tissue; hence relative values change with hNOP expression. Intrinsic activity is useful as a comparison of ligand efficacy in a sense of rank order (i.e. it can be said that N/OFQ(1–13)–NH2 is more efficacious than [F/G]N/OFQ(1–13)–NH2), but it is not possible to infer the molecular properties of agonism, such as intrinsic efficacy (i.e. the response per unit pharmacon receptor), from comparison of tissue maxima (Kenakin, 1997). However, in cases where fractional occupancy–response curves are of a more linear nature, in the absence of a receptor reserve or when the maximal tissue response has not been saturated, comparison of intrinsic activity (maximal tissue response) may represent a good measure of intrinsic efficacy, although this will need rigorous experimental validation (Kenakin, 2002). Since with GTPγ35S binding there appears to be no excess of receptor or saturation in response (suggested by the static nature of the relative intrinsic activity), for any receptor density, [F/G]N/OFQ(1–13)–NH2 has between 0.37–0.55 relative intrinsic activity. Given that little or no change is seen in relative intrinsic activity between [F/G]N/OFQ(1–13)–NH2 and N/OFQ(1–13)–NH2, it could be suggested that the latter ligand is not returning the system maximum response with regard to GTPγ35S binding. Given a high density of available guanine nucleotide-binding sites (Albrecht et al., 1998), it is not surprising that in this tissue and under these assay conditions (high GDP), GTPγ35S binding is apparently not saturating; hence no clear receptor reserve and static relative intrinsic activity. However, in cAMP measurements at 2 μM ponasterone A induction where the maximal tissue response has not been reached even by the full agonist N/OFQ(1–13)–NH2, the relative intrinsic activity is 0.48. Therefore, it can be suggested that the intrinsic efficacy of [F/G]N/OFQ(1–13)–NH2 is ∼0.4–0.5.
Finally, the issue that for a partial agonist the pEC50 should predict its pA2 or pKB has been addressed. Using the lowest induction concentration of 1 μM ponasterone A, the ability of N/OFQ(1–13)–NH2 to stimulate GTPγ35S binding was competitively antagonised by [F/G]N/OFQ(1–13)–NH2 with a pKB of 7.62, which is essentially identical to its pEC50 of 7.68 (5 μM ponasterone A). This was also true for NalBzOH that antagonised N/OFQ(1–13)–NH2-stimulated GTPγ35S binding with a pKB of 7.02 (pEC50 7.00; Bigoni et al., 2002a). In this assay NalBzOH was devoid of agonist activity and is therefore a very-low-efficacy partial agonist. Ac-RYYRIK–NH2 and Ac-RYYRWK–NH2 displayed similar intrinsic activity to [F/G]N/OFQ(1–13)–NH2 for GTPγ35S binding. Overall, (at 5 μM ponasterone A) these partial agonists display a rank order intrinsic activity of Ac-RYYRWK–NH2>[F/G]N/OFQ(1–13)–NH2>Ac-RYYRIK–NH2>NalBzOH.
Acknowledgments
This work was funded in part by a small project grant from The British Journal of Anaesthesia and The Royal College of Anaesthetists (DGL and DJR). We would like to thank the International Association for the Study of Pain for provision of a collaborative travel grant between Leicester (UK) and Ferrara (Italy). This work was presented in part at the Brighton meeting of The British Pharmacological Society, January 2003.
Abbreviations
- CHOhNOP
Chinese hamster ovary cells expressing human NOP
- CHOINDhNOP
Chinese hamster ovary cells expressing the ecdysone-inducible mammalian expression system containing the human NOP
- [F/G]N/OFQ(1–13)–NH2
[Phe1ψ(CH2–NH)Gly2]N/OFQ(1–13)–NH2)
- NalBzOH
naloxone benzoylhydrazone
- N/OFQ
nociceptin/orphanin FQ
- NOP
N/OFQ peptide receptor.
References
- ALBRECHT E., SAMOVILOVA N.N., OSWALD S., BAEGER I., BERGER H. Nociceptin (orphanin FQ): high-affinity and high-capacity binding site coupled to low-potency stimulation of guanylyl-5′-O-(gamma-thio)-triphosphate binding in rat brain membranes. J. Pharmacol. Exp. Ther. 1998;286:896–902. [PubMed] [Google Scholar]
- BERG K.A., MAAYANI S., GOLDFARB J., SCARAMELLINI C., LEFF P., CLARKE W.P. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol. Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- BERGER H., BIGONI R., ALBRECHT E., RICHTER R.M., KRAUSE E., BIENERT M., CALO G. The nociceptin/orphanin FQ receptor ligand acetyl-RYYRIK-amide exhibits antagonistic and agonistic properties. Peptides. 2000a;21:1131–1139. doi: 10.1016/s0196-9781(00)00251-5. [DOI] [PubMed] [Google Scholar]
- BERGER H., CALO G., ALBRECHT E., GUERRINI R., BIENERT M. [Nphe(1)]NC(1–13)NH(2) selectively antagonizes nociceptin/orphanin FQ-stimulated G-protein activation in rat brain. J. Pharmacol. Exp. Ther. 2000b;294:428–433. [PubMed] [Google Scholar]
- BIGONI R., CALO G., RIZZI A., OKAWA H., REGOLI D., SMART D., LAMBERT D.G. Effects of naloxone benzoylhydrazone on native and recombinant nociceptin/orphanin FQ receptors. Can. J. Physiol. Pharmacol. 2002a;80:407–412. doi: 10.1139/y02-040. [DOI] [PubMed] [Google Scholar]
- BIGONI R., RIZZI D., RIZZI A., CAMARDA V., GUERRINI R., LAMBERT D.G., HASHIBA E., BERGER H., SALVADORI S., REGOLI D., CALO G. Pharmacological characterization of [(pX)Phe4]nociceptin(1–13)amide analogs: I) in vitro studies. Naunyn –Schmiedeberg's Arch. Pharmacol. 2002b;365:442–449. doi: 10.1007/s00210-002-0548-8. [DOI] [PubMed] [Google Scholar]
- BREIVOGEL C.S., SELLEY D.E., CHILDERS S.R. Cannabinoid receptor agonist efficacy for stimulating [35S]GTPgammaS binding to rat cerebellar membranes correlates with agonist-induced decreases in GDP affinity. J. Biol. Chem. 1998;273:16865–16873. doi: 10.1074/jbc.273.27.16865. [DOI] [PubMed] [Google Scholar]
- BROWN B.L., ALBANO J.D., EKINS R.P., SGHERZI A.M. A simple and sensitive saturation assay method for the measurement of adenosine 3′ : 5′-cyclic monophosphate. Biochem. J. 1971;121:561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALO G., BIGONI R., RIZZI A., GUERRINI R., SALVADORI S., REGOLI D. Nociceptin/orphanin FQ receptor ligands. Peptides. 2000a;21:935–947. doi: 10.1016/s0196-9781(00)00230-8. [DOI] [PubMed] [Google Scholar]
- CALO G., GUERRINI R., RIZZI A., SALVADORI S., REGOLI D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br. J. Pharmacol. 2000b;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALO G., RIZZI A., MARZOLA G., GUERRINI R., SALVADORI S., BEANI L., REGOLI D., BIANCHI C. Pharmacological characterization of the nociceptin receptor mediating hyperalgesia in the mouse tail withdrawal assay. Br. J. Pharmacol. 1998;125:373–378. doi: 10.1038/sj.bjp.0702087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALO G., RIZZI A., RIZZI D., BIGONI R., GUERRINI R., MARZOLA G., MARTI M., MCDONALD J., MORARI M., LAMBERT D.G., SALVADORI S., REGOLI D. [Nphe(1),Arg(14),Lys(15)]Nociceptin–NH(2), a novel potent and selective antagonist of the nociceptin/orphanin FQ receptor. Br. J. Pharmacol. 2002;136:303–311. doi: 10.1038/sj.bjp.0704706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG Y., PRUSOFF W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- COLE S.L., SCHINDLER M., SELLERS L.A., HUMPHREY P.P. Titrating the expression of a Gi protein-coupled receptor using an ecdysone-inducible system in CHO-K1 cells. Recept. Channels. 2001;7:289–302. [PubMed] [Google Scholar]
- CORDEAUX Y., BRIDDON S.J., MEGSON A.E., MCDONNELL J., DICKENSON J.M., HILL S.J. Influence of receptor number on functional responses elicited by agonists acting at the human adenosine A(1) receptor: evidence for signaling pathway-dependent changes in agonist potency and relative intrinsic activity. Mol. Pharmacol. 2000;58:1075–1084. doi: 10.1124/mol.58.5.1075. [DOI] [PubMed] [Google Scholar]
- COX B.M., CHAVKIN C., CHRISTIE M.J., CIVELLI O., EVANS C., HAMON M.D., HOELLT V., KIEFFER B., KITCHEN I., Mcknight A.T., MEUNIER J.C., PORTOGHESE P.S.Opioid receptors The IUPHAR Compendium of Receptor Characterization and Classification 2000London: IUPHAR Media Ltd; 321–333.ed. Girdlestone, D. pp [Google Scholar]
- DAUTZENBERG F.M., WICHMANN J., HIGELIN J., PY-LANG G., KRATZEISEN C., MALHERBE P., KILPATRICK G.J., JENCK F. Pharmacological characterization of the novel nonpeptide orphanin FQ/nociceptin receptor agonist Ro 64-6198: rapid and reversible desensitization of the ORL1 receptor in vitro and lack of tolerance in vivo. J. Pharmacol. Exp. Ther. 2001;298:812–819. [PubMed] [Google Scholar]
- DE RISI C., PIERO POLLINI G., TRAPELLA C., PERETTO I., RONZONI S., GIARDINA G.A. A new synthetic approach to 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-benzimidazol-2-one(J-113397), the first non-peptide ORL-1 receptor antagonist. Bioorg. Med. Chem. 2001;9:1871–1877. doi: 10.1016/s0968-0896(01)00085-2. [DOI] [PubMed] [Google Scholar]
- DOOLEY C.T., SPAETH C.G., BERZETEI-GURSKE I.P., CRAYMER K., ADAPA I.D., BRANDT S.R., HOUGHTEN R.A., TOLL L. Binding and in vitro activities of peptides with high affinity for the nociceptin/orphanin FQ receptor, ORL1. J. Pharmacol. Exp. Ther. 1997;283:735–741. [PubMed] [Google Scholar]
- GRISEL J.E., FARRIER D.E., WILSON S.G., MOGIL J.S. [Phe1psi(CH2–NH)Gly2]nociceptin-(1–13)–NH2 acts as an agonist of the orphanin FQ/nociceptin receptor in vivo. Eur. J. Pharmacol. 1998;357:R1–R3. doi: 10.1016/s0014-2999(98)00567-6. [DOI] [PubMed] [Google Scholar]
- GUERRINI R., CALO G., BIGONI R., RIZZI A., VARANI K., TOTH G., GESSI S., HASHIBA E., HASHIMOTO Y., LAMBERT D.G., BOREA P.A., TOMATIS R., SALVADORI S., REGOLI D. Further studies on nociceptin-related peptides: discovery of a new chemical template with antagonist activity on the nociceptin receptor. J. Med. Chem. 2000;43:2805–2813. doi: 10.1021/jm990075h. [DOI] [PubMed] [Google Scholar]
- GUERRINI R., CALO G., BIGONI R., RIZZI D., RIZZI A., ZUCCHINI M., VARANI K., HASHIBA E., LAMBERT D.G., TOTH G., BOREA P.A., SALVADORI S., REGOLI D. Structure–activity studies of the Phe(4) residue of nociceptin (1–13)–NH(2): identification of highly potent agonists of the nociceptin/orphanin FQ receptor. J. Med. Chem. 2001;44:3956–3964. doi: 10.1021/jm010221v. [DOI] [PubMed] [Google Scholar]
- GUERRINI R., CALO G., RIZZI A., BIANCHI C., LAZARUS L.H., SALVADORI S., TEMUSSI P.A., REGOLI D. Address and message sequences for the nociceptin receptor: a structure–activity study of nociceptin-(1–13)-peptide amide. J. Med. Chem. 1997;40:1789–1793. doi: 10.1021/jm970011b. [DOI] [PubMed] [Google Scholar]
- GUERRINI R., CALO G., RIZZI A., BIGONI R., BIANCHI C., SALVADORI S., REGOLI D. A new selective antagonist of the nociceptin receptor. Br. J. Pharmacol. 1998;123:163–165. doi: 10.1038/sj.bjp.0701640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIBA E., HARRISON C., GALO G., GUERRINI R., ROWBOTHAM D.J., SMITH G., LAMBERT D.G. Characterisation and comparison of novel ligands for the nociceptin/orphanin FQ receptor. Naunyn Schmiedeberg's Arch. Pharmacol. 2001;363:28–33. doi: 10.1007/s002100000327. [DOI] [PubMed] [Google Scholar]
- HASHIBA E., LAMBERT D.G., JENCK F., WICHMANN J., SMITH G. Characterisation of the non-peptide nociceptin receptor agonist, Ro64-6198 in Chinese hamster ovary cells expressing recombinant human nociceptin receptors. Life Sci. 2002;70:1719–1725. doi: 10.1016/s0024-3205(02)01477-7. [DOI] [PubMed] [Google Scholar]
- HAWES B.E., GRAZIANO M.P., LAMBERT D.G. Cellular actions of nociceptin: transduction mechanisms. Peptides. 2000;21:961–967. doi: 10.1016/s0196-9781(00)00232-1. [DOI] [PubMed] [Google Scholar]
- JENCK F., WICHMANN J., DAUTZENBERG F.M., MOREAU J.L., OUAGAZZAL A.M., MARTIN J.R., LUNDSTROM K., CESURA A.M., POLI S.M., ROEVER S., KOLCZEWSKI S., ADAM G., KILPATRICK G. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4938–4943. doi: 10.1073/pnas.090514397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENNINGS E.A. Postsynaptic K+ current induced by nociceptin in medullary dorsal horn neurons. Neuroreport. 2001;12:645–648. doi: 10.1097/00001756-200103050-00043. [DOI] [PubMed] [Google Scholar]
- KAPUSTA D.R., CHANG J.K., KENIGS V.A. Central administration of [Phe1psi(CH2–NH)Gly2]nociceptin(1–13)–NH2 and orphanin FQ/nociceptin (OFQ/N) produce similar cardiovascular and renal responses in conscious rats. J. Pharmacol. Exp. Ther. 1999;289:173–180. [PubMed] [Google Scholar]
- KENAKIN T. Pharmacologic Analysis of Drug-Receptor Interaction. Philadelphia, PA: Lippincott-Raven, U.S.A; 1997. [Google Scholar]
- KENAKIN T. Drug efficacy at G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- KNOFLACH F., REINSCHEID R.K., CIVELLI O., KEMP J.A. Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. J. Neurosci. 1996;16:6657–6664. doi: 10.1523/JNEUROSCI.16-21-06657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW P.Y., KOUHEN O.M., SOLBERG J., WANG W., ERICKSON L.J., LOH H.H. Deltorphin II-induced rapid desensitization of delta-opioid receptor requires both phosphorylation and internalization of the receptor. J. Biol. Chem. 2000;275:32057–32065. doi: 10.1074/jbc.M002395200. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., NIRA J., ROSENBROUGH A., FARR L., RANDALL R.J. Protein measurements with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MASON S.L., HO M., NICHOLSON J., MCKNIGHT A.T. In vitro characterization of Ac-RYYRWK–NH(2), Ac-RYYRIK–NH(2) and [Phe(1)CPsi(CH(2)–NH)Gly(2)] nociceptin(1–13)NH(2) at rat native and recombinant ORL(1) receptors. Neuropeptides. 2001;35:244–256. doi: 10.1054/npep.2001.0882. [DOI] [PubMed] [Google Scholar]
- MCDONALD J., BARNES T.A., CALO G., GUERRINI R., ROWBOTHAM D.J., LAMBERT D.G. Effects of [(pF)Phe(4)]nociceptin/orphanin FQ-(1–13)NH(2) on GTPgamma35S binding and cAMP formation in Chinese hamster ovary cells expressing the human nociceptin/orphanin FQ receptor. Eur. J. Pharmacol. 2002;443:7–12. doi: 10.1016/s0014-2999(02)01577-7. [DOI] [PubMed] [Google Scholar]
- MEIS S., PAPE H.C. Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to nociceptin/orphanin FQ. J. Neurosci. 1998;18:8133–8144. doi: 10.1523/JNEUROSCI.18-20-08133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENZIES J.R., GLEN T., DAVIES M.R., PATERSON S.J., CORBETT A.D. In vitro agonist effects of nociceptin and [Phe(1)psi(CH(2)–NH)Gly(2)]nociceptin(1–13)NH(2) in the mouse and rat colon and the mouse vas deferens. Eur. J. Pharmacol. 1999;385:217–223. doi: 10.1016/s0014-2999(99)00700-1. [DOI] [PubMed] [Google Scholar]
- MEUNIER J. The potential therapeutic value of nociceptin receptor agonists and antagonists. Exp. Opin. Ther. Patents. 2000;10:371–388. [Google Scholar]
- MEUNIER J.C., MOLLEREAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.C., FERRARA P., MONSARRAT B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MOGIL J.S., PASTERNAK G.W. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol. Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- NEW D.C., WONG Y.H. The ORL1 receptor: molecular pharmacology and signalling mechanisms. Neurosignals. 2002;11:197–212. doi: 10.1159/000065432. [DOI] [PubMed] [Google Scholar]
- NICHOLSON J.R., PATERSON S.J., MENZIES J.R., CORBETT A.D., MCKNIGHT A.T. Pharmacological studies on the ‘orphan' opioid receptor in central and peripheral sites. Can. J. Physiol. Pharmacol. 1998;76:304–313. [PubMed] [Google Scholar]
- OKAWA H., HIRST R.A., SMART D., MCKNIGHT A.T., LAMBERT D.G. Rat central ORL-1 receptor uncouples from adenylyl cyclase during membrane preparation. Neurosci. Lett. 1998;246:49–52. doi: 10.1016/s0304-3940(98)00228-6. [DOI] [PubMed] [Google Scholar]
- OKAWA H., NICOL B., BIGONI R., HIRST R.A., CALO G., GUERRINI R., ROWBOTHAM D.J., SMART D., MCKNIGHT A.T., LAMBERT D.G. Comparison of the effects of [Phe1psi(CH2–NH)Gly2]nociceptin(1–13)NH2 in rat brain, rat vas deferens and CHO cells expressing recombinant human nociceptin receptors. Br. J. Pharmacol. 1999;127:123–130. doi: 10.1038/sj.bjp.0702539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIANAS M.C., MAULLU C., INGIANNI A., ONALI P. [Phe1phi(CH2–NH)Gly2]nociceptin-(1–13)–NH2 acts as a partial agonist at ORL1 receptor endogenously expressed in mouse N1E-115 neuroblastoma cells. Neuroreport. 1999;10:1127–1131. doi: 10.1097/00001756-199904060-00041. [DOI] [PubMed] [Google Scholar]
- OZAKI S., KAWAMOTO H., ITOH Y., MIYAJI M., IWASAWA Y., OHTA H. A potent and highly selective nonpeptidyl nociceptin/orphanin FQ receptor (ORL1) antagonist: J-113397. Eur. J. Pharmacol. 2000;387:R17–R18. doi: 10.1016/s0014-2999(99)00822-5. [DOI] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J., JR, CIVELLI O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- RIZZI D., RIZZI A., BIGONI R., CAMARDA V., MARZOLA G., GUERRINI R., DE RISI C., REGOLI D., CALO G. [Arg(14),Lys(15)]nociceptin, a highly potent agonist of the nociceptin/orphanin FQ receptor: in vitro and in vivo studies. J. Pharmacol. Exp. Ther. 2002;300:57–63. doi: 10.1124/jpet.300.1.57. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., MORARI M. Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides. 2000;21:1023–1029. doi: 10.1016/s0196-9781(00)00233-3. [DOI] [PubMed] [Google Scholar]
- VAN CRAENENBROECK K., VANHOENACKER P., LEYSEN J.E., HAEGEMAN G. Evaluation of the tetracycline- and ecdysone-inducible systems for expression of neurotransmitter receptors in mammalian cells. Eur. J. Neurosci. 2001;14:968–976. doi: 10.1046/j.0953-816x.2001.01719.x. [DOI] [PubMed] [Google Scholar]
- VAUGHAN C.W., CHRISTIE M.J. Increase by the ORL1 receptor (opioid receptor-like1) ligand, nociceptin, of inwardly rectifying K conductance in dorsal raphe nucleus neurones. Br. J. Pharmacol. 1996;117:1609–1611. doi: 10.1111/j.1476-5381.1996.tb15329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMADA H., NAKAMOTO H., SUZUKI Y., ITO T., AISAKA K. Pharmacological profiles of a novel opioid receptor-like1 (ORL(1)) receptor antagonist, JTC-801. Br. J. Pharmacol. 2002;135:323–332. doi: 10.1038/sj.bjp.0704478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUNG L.Y., JOSHI S.A., CHAN R.Y., CHAN J.S., PEI G., WONG Y.H. GalphaL1 (Galpha14) couples the opioid receptor-like1 receptor to stimulation of phospholipase C. J. Pharmacol. Exp. Ther. 1999;288:232–238. [PubMed] [Google Scholar]