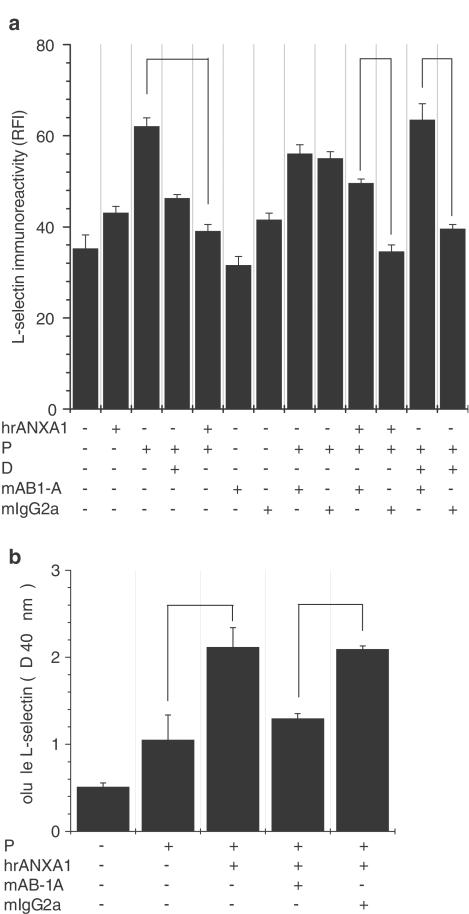
Figure 2.
Exogenous human recombinant ANXA1 mimicked the endogenous protein by inducing L-selectin shedding. Both effects were reversed by a neutralizing antibody. Nonstimulated U-937 cells (C) were treated with 1 nM PMA for 24 h (P) or with 1 μM dexamethasone for 16 h (D) or with the combination PMA (24 h)+dexamethasone (additional 16 h) (P+D). Control samples were incubated in RPMI+5% serum medium for the same period (24 h+16 h), (a) The contribution of ANXA1 to the downregulation of L-selectin expression was confirmed by PMA treatment of U-937 cells for 24 h, followed by 30 min incubation at 37°C with 5 μg ml−1 of human recombinant ANXA1 (PMA+hrANXA1). Cells were treated with PMA for 24 h, then incubated with a specific anti-ANXA1 mAb at a concentration of 20 μg ml−1 (+mAB1-A) for 10 min, and finally incubated with either hrANXA1 for 30 min or dexamethasone for 16 h. A control antibody (+mIgG2a) was used at the same concentration and incubated for the same period of time. Data represent mean±s.e.m. of three independent experiments done in duplicate, *P⩽0.05. (b) ELISA analysis for soluble L-selectin in U-937 cell-free supernatants. Neutralizing anti-ANXA1 mAb (20 μg ml−1) or its isotype-matched control antibody (+mIgG2a) was incubated with PMA-treated cells 10 min before adding hrANXA1 for 30 min. Soluble L-selectin was trapped with a polyclonal L-selectin antibody and detected with a monoclonal L-selectin antibody (Dreg-56), as described in Methods. Data represent mean±s.e.m. of three independent experiments done in triplicate, P≤0.05.