Abstract
The neuronal system plays a fundamental role in the maturation of primitive embryonic vascular network by providing a paracrine template for blood vessel branching and arterial differentiation. Furthermore, postnatal vascular and neural regeneration cooperate in the healing of damaged tissue.
Neurogenesis continues in adulthood although confined to specific brain regions. Following ischaemic insult, neural staminal cells contribute towards the healing process through the stimulation of neurogenesis and vasculogenesis.
Evidence indicates that nerves and blood vessels exert a reciprocal control of their own growth by paracrine mechanisms. For instance, guidance factors, including vascular endothelial growth factor A (VEGF-A) and semaphorins, which share the ability of binding neuropilin receptors, play a pivotal role in the tridimensional growth pattern of arterial vessels and nerves.
Animal models and clinical studies have demonstrated a role of VEGF-A in the pathogenesis of ischaemic and diabetic neuropathies. Further, supplementation with VEGF-A ameliorates neuronal recovery by exerting protective effects on nerves and stimulating reparative neovascularization. Human tissue kallikrein, a recently discovered angiogenic and arteriogenic factor, accelerates neuronal recovery by stimulating the growth of vasa nervorum.
Conversely, the neurotrophin nerve growth factor, known to regulate neuronal survival and differentiation, is now regarded as a stimulator of angiogenesis and arteriogenesis.
These results indicate that angiogenesis and neurogenesis are paracrinally regulated by growth factors released by endothelial cells and neurons. Supplementation of these growth factors, alone or in combination, could benefit the treatment of ischaemic diseases and neuropathies.
Keywords: Angiogenesis, neurogenesis, neurotrophins, diabetes, ischaemia, endothelium
Vascular regeneration is a fundamental survival response that helps in the healing of damaged tissue. Postnatal neovascularization occurs through (1) capillary sprouting of resident endothelial cells (EC), a process named angiogenesis, (2) proliferation of pre-existing arteriolar connections, a process called arteriogenesis and (3) de novo vascularization from EC precursors, that is, vasculogenesis, a process thought to be peculiar to the embryo, but now recognized to be operative also in the adult (Takahashi et al., 1999; Carmeliet, 2000).
Reciprocal interaction between nerves and arteries during development
The neuronal system plays a fundamental role in the maturation of primitive embryonic vasculature. This is supported by the finding that mutations that disrupt peripheral sensory nerves or Schwann cells prevent proper arteriogenesis, while those that disorganize the nerves maintain the alignment of arteries with misrouted axons (Mukouyama et al., 2002). The same authors documented that sensory neurons modulate the expression of arterial markers on ECs via the secretion of vascular endothelial growth factor (VEGF) 164/120. These data suggest that during prenatal development, peripheral nerves provide a paracrine template that determines the organotypic pattern of blood vessel branching and arterial differentiation (Miller, 2002).
Neuronal plasticity facilitates vascular regeneration during adulthood
Neural stem cells (NSCs) have been identified in diverse areas of mouse and human central nervous system (CNS) (Gritti et al., 1999; Pagano et al., 2000), indicating that neurogenesis continues in adulthood, although confined to specific brain regions and decreasing with age. Interestingly, brain insults, including focal ischaemia, may stimulate NSC proliferation, migration and differentiation thereby promoting neurogenesis in the damaged regions. Further, the hypothesis was elaborated that NSCs may improve ischaemic brain recovery not only through trophic support but also by trans-differentiation into glial and vascular cells. Accordingly, NSCs were found to be positive for endothelial stem cell markers and responsive to different environmental signals guiding stem cell-fate conversion (Parati et al., 2002).
Paracrine control of neural regeneration and angiogenesis
This review will principally focus on recent evidence documenting that nerves and blood vessels, which share the characteristic of being branched structures, may control each other in a paracrine manner that is, angiogenic growth factors released by ECs modulate nerve regeneration, while neurotrophins, most often associated with the promotion of neuronal growth and survival (Chao, 2003), stimulate neovascularization. In the following sections, we will provide examples regarding the involvement of VEGF, a prototypical angiogenic molecule, in the maintenance and restoration of peripheral nerve integrity (Carmeliet & Storkebaum, 2002). Reciprocally, we will provide documentation that vascular growth may be modulated by neural factors during postnatal development (Emanueli et al., 2002a).
Spatial guidance of neural and vascular regeneration: neuropilins and VEGF interaction
Angiogenesis is a complex process that occurs in steps. During the progression phase, ECs migrate and proliferate. During the subsequent maturation phase, angiopoietin-1 promotes arteriogenesis by recruiting mural cells and assembling a more stable vascular network. Further, guidance factors play a pivotal role in the establishment of a tridimensional pattern. In concordance with this model, spatial guidance of neural growth is controlled by the binding of semaphorins to neuropilin-1 (np1) and neuropilin-2 (np2) receptors (Neufeld et al., 2002). In addition, both np1 and np2 are able to bind to certain heparin-binding splice forms of VEGF, indicating that neuropilins play a role in the control of the cardiovascular function as well. Gene-targeting experiments indicate that np1 does indeed function as an important modulator of VEGF during vasculogenesis and angiogenesis, but the role of np2 in the cardiovascular system remains unknown. The other way round, spatial guidance of neuronal outgrowth may be controlled by the interaction of VEGF with neuropilins.
VEGF neurotrophic actions
Until recently, VEGF was considered to be specific in its actions for EC survival, proliferation and migration. The molecular basis for this isolated action of VEGF on ECs is the fact that the VEGF receptor-2 (also called KDR) was thought to be restricted to ECs.
In the past few years, however, basic research and clinical observations have disclosed the existence of a strategic interconnection between neural and vascular actions of VEGF. These interconnections are related to the requirement for oxygen supply of nervous tissues and the limited regenerative capacity of neuronal cells after hypoxic damage. In the nervous system, VEGF mRNA has been found in neurons in the capillary-rich areas of the brain, for instance in the pars distalis cells of the pituitary gland and also in glial cells in the retina after hypoxia (Ferrara & Davis-Smith, 1997; Stone et al., 1995). Furthermore, VEGF expression is induced in astrocytes at the site of a spinal cord injury (Bartholdi et al., 1997). The location of VEGF and pattern of expression after injury in the nervous system thus appears consistent with the role of VEGF as an angiogenic factor. The other way round, VEGF was documented to exert trophic functions on neuronal cells, as shown by induction of neurite outgrowth and by demonstration of proliferative and antiapoptotic effects of VEGF on neuronal cells of the dorsal root ganglia and superior cervical ganglia through the action of VEGF receptor-2 (Sondell et al., 1999). VEGF protects cultured neural cells from death induced by serum starvation or hypoxia via VEGF-2-mediated activation of phosphatidylinositol 3′-kinase (PI3 K), Akt and nuclear factor-kappa B pathways. Further, in mouse cortical neuron cultures subjected to hypoxia, the neuroprotective effect of VEGF involves the suppression of cell-death pathways mediated by caspase-3 (Jin et al., 2001). Interestingly enough, another recently published paper showed that the binding site for the hypoxia-inducible factor in the VEGF gene has an obligatory role in spinal motor neuron survival (Oosthuyse et al., 2001). Deletion of this binding site causes adult-onset progressive motor neuron degeneration, reminiscent of amyotrophic lateral sclerosis, probably due to chronic vascular insufficiency of the spinal cord (Oosthuyse et al., 2001). These results indicate that chronic vascular insufficiency and, possibly, insufficient VEGF-dependent neuroprotection lead to the selective degeneration of motor neurons.
VEGF supplementation as a remedy for diabetic and ischaemic neuropathies
Peripheral nerves are supplied by a system of longitudinal and segmental arteries, known as the ‘vasa nervorum'. These are intimately connected to each other: interfascicular vessels within the epineurium and perineurium, and infrafascicular vessels within the endoneurium. Compared to the brain, the endoneurial vascular network in peripheral nerves has a rather limited surface area of metabolic exchange and lacks autoregulation. Owing to the lack of autoregulation and absence of precapillary sphincters, the peripheral nerve must be sufficiently vascularized to avoid ischaemia. These efficient intercommunicating anastomoses between both vascular networks are essential to ensure an uninterrupted blood supply in the event of the flow through one or several nutrient vessels being interrupted.
The peripheral nervous system is commonly affected in patients with diabetes. Remarkably, the pathogenesis of diabetic neuropathy has remained enigmatic. Microangiopathic abnormalities with impaired blood flow and ischaemia in diabetic nerves have been implicated (Yagihashi, 1997; Boulton & Malik, 1998). Similarly, in diabetic skeletal muscles, accelerated EC apoptotic death and capillary occlusion lead to progressive microvascular rarefaction (Emanueli et al., 2002b). In the epineurial vessels, structural abnormalities include arteriolar attenuation, venous distension, arteriovenous shunting, new vessel formation along with intimal hyperplasia and hypertrophy, and denervation. Transperineurial vessels also demonstrate denervation with luminal narrowing. Epi- and peri-neurial arterioles are supplied by abundant sympathetic nerve endings in their walls and the degenerative changes of these nerve endings of vasa nervorum in patients with diabetes may impair endoneurial blood flow.
Diabetic microvascular alterations are associated with inappropriate production of angiogenic inducers. The deficit, however, is not irreversible and indeed supplementation with angiogenic factors is emerging as a remedy for both microvascular insufficiency and diabetic neuropathy. Recently, Schratzberger et al. (2001) demonstrated that the destruction of the vasa nervorum in two different animal models of experimental diabetic neuropathy induced by streptozotocin or alloxan can be reversed by administration of VEGF. Intramuscular gene transfer of plasmid DNA encoding VEGF preserved the nerve blood flow and function in diabetic animals. However, the clinical implications of these important discoveries still remain uncovered (Veves & King, 2001).
Arterial insufficiency is often complicated by peripheral neuropathy (Ugalde & Rosen, 2001). This pathology affects nerve fibres of all sizes, predominantly of the sensory type (Weinberg, et al., 2001). Schratzberger et al. (2000) reported that ischaemic peripheral neuropathy could be prevented and/or reversed by VEGF, Intriguingly, improved nerve function was attributable to enhanced hindlimb perfusion and possibly, also to a direct neurotrophic effect of VEGF on Schwann cells, which express the VEGF receptors np-1, Flt-1 and Flk-1.
A beneficial effect of VEGF gene therapy on ischaemic neuropathy in patients with critical limb ischaemia has been reported (Simovic et al., 2001). Notably, improvement in the vascular ankle-brachial index in treated legs corres-ponded to improvement in neuropathy in the same limb. Collectively, the above reports support the hypothesis that VEGF, besides its action on the vascular system, also exerts neuroprotection via the activation of specific receptors on neurons and Schwann cells. The growth factor could represent a promising tool for the treatment of diabetic or ischaemic neuropathy.
Local angiogenesis gene therapy with human tissue kallikrein improves peri-neural circulation and accelerates postischaemic neural recovery
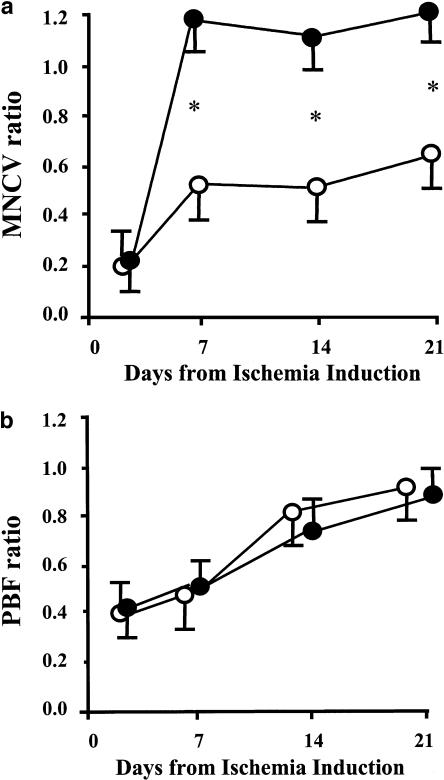
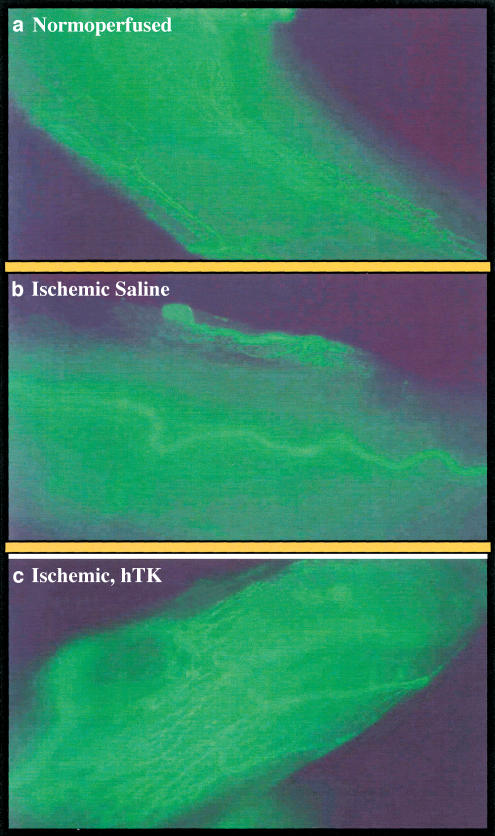
The potentiality of local human tissue kallikrein (hTK) gene transfer for the treatment of limb ischaemia has been documented in rodent models with or without associated endothelial dysfunction (Emanueli et al., 2001a, 2001b). Con-sequently, we challenged the therapeutic potential of the hTK gene transfer for the rescue of ischaemic neuropathy. As shown in Figure 1, plantar blood flow recovery was not altered by peri-neural injection of hTK gene, possibly due to lack of diffusion of the transgene to the ischaemic muscle. At variance, motor nerve conduction velocity of ischaemic limb was drastically accelerated by hTK transfer. Furthermore, as shown in Figure 2, hTK contrasted the rarefaction of the peri-neural vascular network. These studies clearly document the efficacy of hTK in rescuing ischaemic neuronal damage through potentiation of neoangiogenesis.
Figure 1.
Line graph showing the postischaemic recovery in terms of motor nerve conduction velocity (MNCV, Letica Electromiography System, panel a) and plantar blood flow (PBF, Transonic Laser Doppler Flowmetry, panel b). Limb ischaemia was induced in Wistar rats by surgical resection of left femoral artery according to established methodology (Emanueli et al., 2001b). After 3 days later, 50 μl sterile saline or 5 × l09 p.f.u. (in 50 μl saline) adenovirus containing the hTK gene or luciferase gene was injected around the ischaemic sciatic nerve (six rats per group). MNCV and PBF were recorded sequentially up to the 21st day following induction of ischaemia. Values are means±s.e.m. and represent the ratio of ischaemic to contralateral side. Solid circles represent hTK-treated and open circles represent vehicle and luciferase-treated rats. *P<0.05.
Figure 2.
Microphotographs showing the perineural microvasculature of sciatic nerves 21 days after induction of limb ischaemia produced in anaesthetized rats by surgical excision of left femoral artery. Contralateral normoperfused nerve microvasculature was counted for control. Animals (six per group) were injected with 50 μl sterile saline or 5 × l09 p.f.u. (in 50 μl saline) adenovirus containing the hTK gene or luciferase gene around the ischaemic sciatic nerve. At 21 days after induction of ischaemia, the animals were killed after sciatic nerve harvesting. Perineural microvasculature was visualized by in situ fluorescent staining using the endothelial cell-specific marker BS-1 lectin conjugated to FITC (Vector Laboratories, Burlingame, CA, U.S.A.). Images were captured using a Nikon Diaphot fluorescence microscope and an Optronics digital camera. Panel a shows the normal vascularization (in green) of normo-perfused nerve. Panel b shows rarefaction of vasa -nevorum network 21 days after ischaemia in saline-treated rat. Panel c shows the increased vascularity of sciatic nerve that was submitted to ischaemic insult and 3 days after receiving hTK gene transfer.
Control of neural integrity and vascular repair by nerve growth factor
Nerve growth factor (NGF) is a neurotrophin (NTR) discovered in the early 1950 s and represents the first isolated and best-characterized member of a growing family of neurotrophins, which includes brain-derived neurotrophic factor (BDNF) and NTRs 3–5. NGF is known to regulate the survival and differentiation of neurons through the tyrosine kinase activity of its constitutive receptor, p140trkA. The functions of the p75NTR receptor are still unknown, although the signalling cascade seems to culminate in the activation of caspases (Hempstead, 2002; Chao, 2003). In animal models of diabetes, NGF is depleted in peripheral nerves and this deficit produces hypoalgesia. Deficiency of NGF has been related to degeneration or impaired regeneration of peripheral nerves and to dysfunction of sensory small-diameter fibres that release substance P (Anand et al., 1996). A significant correlation exists between NGF depletion and decreased skin axon-reflex vasodilatation mediated by substance P release from sensory fibres. Supplementation with exogenous NGF reverses the pathological changes typical of diabetic neuropathy (Unger et al., 1998) and, for this property, the neurotrophic factor is currently under investigation in clinical trials. The beneficial effect exerted by NGF may be related to its ability in restoring neurotransmitter content and reverse distinct diabetes-related alterations in myelinated nerve fibre morphology. These changes occur in the entire myelinated population of sensory nerves and are not restricted to nociceptive nerve fibres (Unger et al., 1998).
Lambiase et al. (2000) detected NGF trkA and p75 receptors on cultured ECs and vascular smooth muscle cells (VSMCs). Additional studies documented that activation of NGF receptors influences vascular cell biology and fate in vitro (Kraemer et al., 1999; Wang et al., 2000; Raychaudhuri et al., 2001). NGF treatment causes a rapid phosphorylation of trkA(NGFR) in cultured human ECs, determining a parallel increase of phosphorylated ERK1/2. By this mechanism, NGF induces direct proliferative effects on human ECs (abolished by the trkA inhibitor K252a) and promotes neoangiogenesis when delivered onto the chorioallantoic membrane of the chicken embryo (Cantarella et al., 2002). NGF may also act as an indirect activator of EC growth by stimulating the release of other vascular growth factors. For instance, in superior cervical ganglia of newborn rats, capillary sprouting is promoted by NGF via the release of VEGF (Calzà, et al., 2001). In addition, NGF-induced capillarization is seemingly mediated by the neurokinin substance P that stimulates the release of downstream angiogenic activator nitric oxide (NO) (Ziche et al., 1994). Finally, a subsidiary clue for NGF acting as a proangiogenic agent emerges from preclinical and clinical studies documenting the wound-healing properties of the growth factor (Bernabei et al., 1999).
Recent studies from our group firmly established the implication of endogenous NGF in reparative angiogenesis (Emanueli et al., 2002a). Ischaemia enhances NGF content and trkA expression in skeletal muscles. Native angiogenic response is profoundly impaired by administration of NGF antibody and not by control IgG. Furthermore, ischaemia-induced apoptosis is augmented by NGF antibody at the level of myofibres. Therefore, ischaemia induces the expression of NGF system components and this native response is instrumental to reparative vascular growth.
We also documented that potentiation of the above mechanism by supplementation with exogenous NGF improves the native microvascular response to ischaemia and inhibits endothelial and muscular cell apoptosis, thereby accelerating the rate of perfusion recovery. Interestingly enough, NGF modulates the expression of its own receptors and by this action it restores the balance between EC growth and death, which is essential for mounting an efficient neovascularization. The pathways implicated in NGF-induced vascular effects are becoming more clear. The neuropeptide stimulates the phosphorylation of Akt, a protein kinase acting downstream of VEGF and angiopoietin. Akt also induces NO production through its ability to phosphorylate endothelial NO synthase at serine 1179 or 1177. Using inhibitors of the above pathway in vivo, we documented that NGF acts as a pro-survival factor for ECs and improves neovascularization via a VEGF-Akt-NO-dependent mechanism.
Control of vascular repair by sympathetic nervous system
Substance P and CGRP, reportedly implicated in neoangiogenesis, have originated from nonadrenergic, noncholinergic sensory neurons. In addition, sympathetic nerves have long been suspected to produce trophic influences on the innervated tissues, but a possible angiogenic action has been neglected. Implication in ischaemia-related angiogenesis is seemingly negated by the observation that total sympathectomy early during postnatal life (Madeddu, unpublished observations) or unilateral sympathectomy in adult animals (Lee et al., 2003) do not affect the spontaneous capillarization response to ischaemia. However, the sympathetic vasoconstrictor neuropeptide Y and its receptors have been reported to be positively modulated in ischaemic tissues and ECs seemingly start producing and releasing neuropeptide Y into the circulation after arterial occlusion (Lee et al., 2003). Therefore, increase in neuropeptide Y levels derive mainly from extraneuronal sources. Furthermore, administration of exogenous neuro-peptide Y at physiological, nonvasoconstrictor concentrations induces the Y2/Y5 receptors, stimulates neovascularization and restores ischaemic muscle blood flow and performance, with these effects being reduced in knock outs for the Y2 receptor or eNOS.
Conclusions
Angiogenesis and neurogenesis are strictly interconnected during prenatal development and continue to play a physiological role during postnatal life. Whenever vessels and neurons are damaged, the concerted action of nerve and vascular growth factors contribute to the healing process. Interestingly, ischaemia induces important modifications in the expression of nerve and vascular growth factors and their receptors. Furthermore, ECs are normally quiescent cells, but when submitted to hypoxia or starvation, they lose their mutual contact, proliferate and acquire the ability to produce angiogenic or neurotrophic factors. The latter phenomenon acts as a reverberating intracrine mechanism instrumental to proper capillarization. It is now becoming clear that treatment of various diseases can take advantage of the potentiation of this endogenous mechanism. Preclinical studies have clearly documented that postischaemic recovery is accelerated by supplementation of trophic factors that target both neuronal and vascular tissues. Therefore, this supply-side strategy with complementary approaches based on stem-cell transfer could hopefully open new avenues for the treatment of ischaemic and neurodegenerative pathologies.
Acknowledgments
This study was funded by grants from the Juvenile Diabetes Research Foundation (JDRF, U.S.A. No. 1-2001-877), Telethon (GP0300Y01) and National Council CNR grant to Dr Madeddu.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- CNS
central nervous system
- EC
endothelial cells
- hTK
human tissue kallikrein
- KDR
VEGF receptor-2
- MNCV
motor nerve conduction velocity
- NGF
nerve growth factor
- NSC
neural stem cells
- NTR
neurotrophin
- np1
neuropilin-1
- np2
neuropilin-2
- PI3K
phosphatidylinositol 3′-kinase
- PBF
plantar blood flow
- VEGF-A
vascular endothelial growth factor A
- VSMC
vascular smooth muscle cells
References
- ANAND P., TERENGHI G., WARNER G., KOPELMAN P., WILLIAMS-CHESTNUT R.E., SINICROPI D.V. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat. Med. 1996;2:703–707. doi: 10.1038/nm0696-703. [DOI] [PubMed] [Google Scholar]
- BARTHOLDI D., RUBIN B.P., SOHWB M.E. VEGFmRNA induction correlates with changes in the vascular architecture upon spinal cord damage in the rat. Eur. J. Neurosci. 1997;9:2549–2560. doi: 10.1111/j.1460-9568.1997.tb01684.x. [DOI] [PubMed] [Google Scholar]
- BERNABEI R., LANDI F., BONINI S., ONDER G., LAMBIASE A., POLA R., ALOE L. Effect of topical application of nerve-growth factor on pressure ulcers. Lancet. 1999;354:307. doi: 10.1016/S0140-6736(99)02784-1. [DOI] [PubMed] [Google Scholar]
- BOULTON A.J., MALIK R.A. Diabetic neuropathy. Med. Clin. N. Am. 1998;82:909–929. doi: 10.1016/s0025-7125(05)70029-8. [DOI] [PubMed] [Google Scholar]
- CALZA L., GIARDINO L., GIULIANI A., ALOE L., LEVI-MONTALCINI R. Nerve growth factor control of neuronal expression of angiogenic and vasoactive factors. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4160–4165. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANTARELLA G., LEMPEREUR L., PRESTA M., RIBATTI D., LOMBARDO G., LAZAROVICI P., ZAPPALA G., PAFUMI C., BERNARDINI R.Nerve growth factor–endothelial cell interaction leads to angiogenesis in vitro and in vivo FASEB J. 2002Published online [DOI] [PubMed]
- CARMELIET P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- CARMELIET P., STORKEBAUM E. Vascular and neuronal effects of VEGF in the nervous system: implications for neurological disorders. Semin. Cell Dev. Biol. 2002;13:39–53. doi: 10.1006/scdb.2001.0290. [DOI] [PubMed] [Google Scholar]
- CHAO M.V. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- EMANUELI C., MINASI A., ZACHEO A., CHAO J., CHAO L., SALIS M.B., STRAINO S., TOZZI M.G., SMITH R., GASPA L., BIANCHINI G., STILLO F., CAPOGROSSI M.C., MADEDDU P. Local delivery of human tissue kallikrein gene accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia. Circulation. 2001a;103:125–132. doi: 10.1161/01.cir.103.1.125. [DOI] [PubMed] [Google Scholar]
- EMANUELI C., SALIS M.B., STACCA T., GASPA L., CHAO J., CHAO L., PIANA A., MADEDDU P. Rescue of impaired angiogenesis in spontaneously hypertensive rats by intramuscular human tissue kallikrein gene transfer. Hypertension. 2001b;38:136–141. doi: 10.1161/01.hyp.38.1.136. [DOI] [PubMed] [Google Scholar]
- EMANUELI C., SALIS M.B., PINNA A., GRAIANI G., MANNI L., MADEDDU P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002a;106:2257–2262. doi: 10.1161/01.cir.0000033971.56802.c5. [DOI] [PubMed] [Google Scholar]
- EMANUELI C., SALIS M.B., PINNA A., STACCA T., MILIA A.F., SPANO A., CHAO J., CHAO L., SCIOLA L., MADEDDU P. Prevention of diabetes-induced microangiopathy by human tissue kallikrein gene transfer. Circulation. 2002b;106:993–999. doi: 10.1161/01.cir.0000027104.33206.c8. [DOI] [PubMed] [Google Scholar]
- FERRARA N., DAVIS-SMYTH T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- GRITTI A., FROLICHSTHAL-SCHOELLER P., GALLI R., PARATI E.A., COVA L., PAGANO S.F., BJORNSON C.R., VESCOVI A.L. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J. Neurosci. 1999;19:3287–3297. doi: 10.1523/JNEUROSCI.19-09-03287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEMPSTEAD B.L. The many faces of p75NTR. Curr. Opin. Neurobiol. 2002;12:260–267. doi: 10.1016/s0959-4388(02)00321-5. [DOI] [PubMed] [Google Scholar]
- JIN K., MAO X.O., BATTEUR S.P., MCEACHRON E., LEAHY A., GREENBERG D.A. Caspase-3 and the regulation of hypoxic neuronal death by vascular endothelial growth factor. Neuroscience. 2001;108:351–358. doi: 10.1016/s0306-4522(01)00154-3. [DOI] [PubMed] [Google Scholar]
- KRAEMER R., NGUYEN H., MARCH K.L., HEMPSTEAD B. NGF activates similar intracellular signaling pathways in vascular smooth muscle cells as PDGF-BB but elicits different biological responses. Artherioscler. Thromb. Vasc. Biol. 1999;19:1041–1050. doi: 10.1161/01.atv.19.4.1041. [DOI] [PubMed] [Google Scholar]
- LAMBIASE A., MANNI L., BONINI S., RAMA R., MICERA A., ALOE L. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest. Ophthalmol. Visl. Sci. 2000;41:1063–1069. [PubMed] [Google Scholar]
- LEE E.W., MICHALKIEWICZ M., KITLINSKA J., KALEZIC I., SWITALSKA H., YOO P., SANGKHARAT A., JI H., LI L., MICHALKIEWICZ T., LJUBISAVLJEVIC M., JOHANSSON H., GRANT D.S., ZUKOWSKA Z. Neuropeptide Y induces ischemic angiogenesis and restores function of ischemic skeletal muscles. J. Clin. Invest. 2003;111:1853–1862. doi: 10.1172/JCI16929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER G. Developmental biology. Nerves tell arteries to make like a tree. Science. 2002;296:2121–2123. doi: 10.1126/science.296.5576.2121a. [DOI] [PubMed] [Google Scholar]
- MUKOUYAMA Y.S., SHIN D., BRITSCH S., TANIGUCHI M., ANDERSON D.J. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- NEUFELD G., COHEN T., SHRAGA N., LANGE T., KESSLER O., HERZOG Y. The neuropillins: multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovasc. Med. 2002;12:13–19. doi: 10.1016/s1050-1738(01)00140-2. [DOI] [PubMed] [Google Scholar]
- OOSTHUYSE B., MOONS L., STORKEBAUM E., BECK H., NUYENS D., BRUSSELMANS K., VAN DORPE J., HELLINGS P., GORSELINK M., HEYMANS S., THEILMEIER G., DEWERCHIN M., LAUDENBACH V., VERMYLEN P., RAAT H., ACKER T., VLEMINCKX V., VAN DEN BOSCH L., CASHMAN N., FUJISAWA H., DROST M.R., SCIOT R., BRUYNINCKX F., HICKLIN D.J., INCE C., GRESSENS P., LUPU F., PLATE K.H., ROBBERECHT W., HERBERT J.M., COLLEN D., CARMELIET P. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 2001;28:118–131. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- PAGANO S.F., IMPAGNATIELLO F., GIRELLI M., COVA L., GRIONI E., ONOFRI M., CAVALLARO M., ETTERI S., VITELLO F., GIOMBINI S., SOLERO C.L., PARATI E.A. Isolation and characterization of neural stem cells from the adult human olfactory bulb. Stem Cells. 2000;18:295–300. doi: 10.1634/stemcells.18-4-295. [DOI] [PubMed] [Google Scholar]
- PARATI E.A., BEZ A., PONTI D., DE GRAZIA U., CORSINI E., COVA L., SALA S., COLOMBO A., ALESSANDRI G., PAGANO S.F. Human neural stem cells express extra-neural markers. Brain Res. 2002;925:213–221. doi: 10.1016/s0006-8993(01)03291-7. [DOI] [PubMed] [Google Scholar]
- RAYCHAUDHURI S.K., RAYCHAUDHURI S.P., WELTMAN H., WELTMAN H., FARBER E.M. Effect of nerve growth factor on endothelial cell biology: proliferation and adherence molecule expression on human dermal microvascular endothelial cells. Arch. Dermatol. Res. 2001;293:291–295. doi: 10.1007/s004030100224. [DOI] [PubMed] [Google Scholar]
- SCHRATZBERGER P., SCHRATZBERGER G., SILVER M., CURRY C., KEARNEY M., MAGNER M., ALROY J., ADELMAN L.S., WEINBERG D.H., ROPPER A.H., ISNER J.M. Favorable effect of VEGF gene transfer on ischemic peripheral neuropathy. Nat. Med. 2000;6:405–413. doi: 10.1038/74664. [DOI] [PubMed] [Google Scholar]
- SCHRATZBERGER P., WALTER D.H., RITTIG K., BAHLMANN F.H., POLA R., CURRY C., SILVER M., KRAININ J.G., WEINBERG D.H., ROPPER A.H., ISNER J.M. Reversal of experimental diabetic neuropathy by VEGF gene transfer. J. Clin. Invest. 2001;107:1083–1092. doi: 10.1172/JCI12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMOVIC D., ISNER J.M., ROPPER A.H., PIECZEK A., WEINBERG D.H. Improvement in chronic ischemic neuropathy after intramuscular phVEGF 165 gene transfer in patients with critical limb ischemia. Arch. Neurol. 2001;58:761–768. doi: 10.1001/archneur.58.5.761. [DOI] [PubMed] [Google Scholar]
- SONDELL M., LUNDBORG G., KANJE M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J. Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STONE J., ITIN A., ALON T., PE'ER J., GNESSIN H., CHAN-LING T., KESHET E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI T., KALKA C., MASUDA H., CHEN D., SILVER M., KEARNEY M., MAGNER M., ISNER J.M., ASAHARA T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- UGALDE V., ROSEN B.S. Ischemic peripheral neuropathy. Phys. Med. Rehabil. Clin. N. Am. 2001;12:365–380. [PubMed] [Google Scholar]
- UNGER J.W., KLITZSCH T., PERA S., REITER R. Nerve growth factor (NGF) and diabetic neuropathy in the rat: morphological investigations of the sural nerve, dorsal root ganglion, and spinal cord. Exp. Neurol. 1998;153:23–34. doi: 10.1006/exnr.1998.6856. [DOI] [PubMed] [Google Scholar]
- WANG S., BRAY P., MCCAFFREY T., MARCH K., HEMPSTEAD B.L., KRAEMER R. p75(NTR) mediates neurotrophin-induced apoptosis of vascular smooth muscle cells. Am. J. Pathol. 2000;157:1125–1247. doi: 10.1016/S0002-9440(10)64640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEVES A., KING G.L. Can VEGF reverse diabetic neuropathy in human subjects. J. Clin. Invest. 2001;107:1215–1218. doi: 10.1172/JCI13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINBERG D.H., SIMOVIC D., ISNER J., ROPPER A.H. Chronic ischemic monomelic neuropathy from critical limb ischemia. Neurology. 2001;57:1008–1012. doi: 10.1212/wnl.57.6.1008. [DOI] [PubMed] [Google Scholar]
- YAGIHASHI S. Pathogenetic mechanisms of diabetic neuropathy: lessons from animal models. J. Peripher. Nerv. Syst. 1997;2:113–132. [PubMed] [Google Scholar]
- ZICHE M., MORBIDELLI L., MASINI E., AMERINI S., GRANGER H.J., MAGGI C.A., GEPPETTI P., LEDDA F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]