Chronic inflammatory diseases such as bronchial asthma, allergic gastrointestinal disease, and atopic dermatitis are characterized by the selective recruitment and activation of distinct subtypes of leukocytes, particularly eosinophils, into the tissue from peripheral blood (Rothenberg, 1998). Eosinophils, along with mast cells, are postulated to play a key role in the pathophysiology of these chronic diseases. The striking accumulation of eosinophils in allergic disease has stimulated intense scientific examination leading to the discovery of numerous molecular mechanisms responsible for this process. Eosinophils, like all leukocytes, migrate from the vascular lumen, across the endothelial cell surface into the appropriate tissue sites. This elaborate mechanism, known as transendothelial migration, has been shown to be a highly orchestrated process (Springer, 1994). The migratory pathway, or chemotaxis, of the leukocyte is directly influenced by soluble molecules known as chemoattractants. These molecules reside along a tissue gradient, and bind to and activate G-protein coupled receptors (GPCRs) on the leukocyte cell surface. Several eosinophil chemoattractants have been extensively studied. Some of these molecules, the classical chemoattractants, include the complement cleavage fragments, leukotrienes and platelet-activating factor (PAF). These chemoattractants have been shown to stimulate the recruitment of a wide variety of leukocyte subtypes, and are therefore nonselective. In contrast, chemotactic cytokines (or chemokines) such as eotaxin-1, -2, -3, and monocyte chemoattractant protein (MCP)-4 are more selective for the recruitment of leukocytes associated with allergic inflammation, such as eosinophils, mast cells, and basophils.
Histamine is one of the most intensely studied biomolecules in medicine and is the single most potent mediator of immediate hypersensitivity reactions (MacGlashan, 2003). While the effects of histamine on smooth muscle contraction, vascular permeability, and regulation of stomach acid are well known, its biological effects on blood leukocytes are not. Histamine was first described as a selective chemoattractant for eosinophils almost 3 decades ago (Clark et al., 1975). Subsequent studies revealed that this effect was unlikely to be mediated through the known histamine receptor subtypes (Clark et al., 1977; Raible et al., 1994). Since these studies were published, further information regarding the histamine receptor responsible for eosinophil chemotaxis has remained sparse. Now, two reports in the British Journal of Pharmacology (Buckland et al., 2003; Ling et al., 2004, this issue) show unequivocally that histamine possesses all the properties of a classical leukocyte chemoattractant (i.e., agonist-induced actin polymerization, mobilization of intracellular calcium, alteration in cell shape, and upregulation of adhesion molecule expression) and that the histamine receptor responsible for the selective recruitment of eosinophils, is of the H4 subtype; a Gαi-linked pertussis toxin-sensitive GPCR. These important studies followed the recent identification of the histamine H4 receptor, the fourth receptor family member cloned and characterized, by seven independent research groups through orphan GPCR/homology cloning methods (Morse et al., 2001; O'Reilly et al., 2002; MacGlashan, 2003).
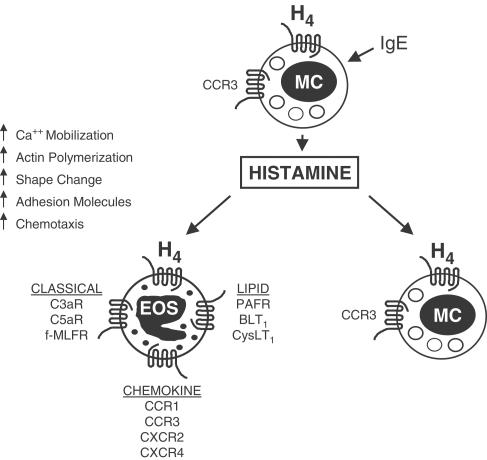
Synthetic organic chemistry targeting the histamine receptors over the past several decades has resulted in the discovery of a diverse array of pharmacological tools, some with very interesting properties with respect to their histamine receptor specificity and agonism/antagonism activity. For example, in the paper by Buckland et al. (2003), the authors used the compounds clobenpropit and clozapine, which act as antagonists at the H3 receptor and agonists at the H4 receptor. These compounds induced an eosinophil shape change, an effect that was blocked by thioperamide, an H3/H4 receptor dual antagonist. Further investigation showed that thioperamide inhibited histamine-induced actin polymerization, calcium mobilization, and upregulation of the adhesion molecule CD11b. In this issue, Ling et al. (2004) describe the use of the first selective H4 receptor antagonist, JNJ7777120, whose selectivity for the H4 receptor was recently reported to be greater than 1000-fold over the H1, H2, and H3 receptors (Jablonowski et al., 2003). This molecule was able to block eosinophil shape change via histamine, while diphenhydramine, ranitidine, and JNJ6379490 (H1, H2, and H3 selective receptor antagonists, respectively) were not effective. In addition, JNJ7777120 blocked chemotaxis as well as the histamine-induced upregulation of the adhesion molecules CD11b and CD54. The reports by Buckland et al. (2003), and Ling et al. (2004, this issue) follow a recent study which showed that H4 was responsible for chemotaxis of mast cells (Hofstra et al., 2003). To illustrate this, these authors performed elegant chemotaxis experiments on mast cells isolated from H3- and H4- receptor gene-deficient mice. Mast cells from wild-type and H3- receptor-deficient mice migrated in response to histamine (effect blocked by thioperamide), while mast cells from the H4 receptor knockout failed to respond at all. Taken together, these results provide direct proof that chemotaxis of eosinophils and mast cells via histamine is triggered though the H4 receptor. Consequently, it is postulated that H4 serves as a mechanism for tissue-derived mast cells upon allergen challenge, to amplify histamine-mediated allergic reactions. This results in the selective recruitment of these major effector cells into tissue sites leading to chronic allergic inflammation (Figure 1).
Figure 1.
Mechanism of histamine-induced recruitment of eosinophils and mast cells in chronic allergic inflammation. Histamine H4 GPCRs are expressed on the surface of both eosinophils and mast cells. Antigen-IgE complex-dependent crosslinking of FcɛRI on the surface of resident mast cells stimulates the secretion of histamine, which binds to and activates the H4 receptor on eosinophils. Signalling through the H4 receptor triggers a series of signal transduction events (calcium mobilization, actin polymerization, shape change, upregulation of adhesion molecule expression) leading to directional migration (chemotaxis) and accumulation of eosinophils into sites of inflammation. Upon release, histamine can also stimulate the recruitment of additional mast cells into the inflammatory site, by activation of the mast cell expressed H4 receptor. As shown in the figure, the H4 receptor is one of a number of G-protein coupled chemottractant receptors expressed on eosinophils. These include members of the chemokine receptor family, receptors for classical chemoattractants (complement cleavage fragments, formyl peptides and lipid mediators). Abbreviations: EOS, eosinophil; MC, mast cell; IgE, immunoglobulin E; H4, histamine receptor type 4; PAFR, platelet activating factor receptor; BLT1, leukotriene B4 receptor type 1; CysLT1, leukotriene D4 receptor type 1; CCR1, C-C chemokine receptor type 1; CCR3, C-C chemokine receptor type 3; CXCR2, C-X-C chemokine receptor type 2; CXCR4, C-X-C chemokine receptor type 4; C3aR, complement 3a receptor; C5aR, complement 5a receptor; f-MLFR, N-formyl-methionyl-leucyl-phenylalanine receptor.
The ability of histamine to mediate an eosinophil shape change and act as a chemoattractant in vitro is weak, at best, in both potency and maximal effect, when compared to the potent CCR3-active β-chemokines, eotaxin and eotaxin-2 (Clark et al., 1975; O'Reilly et al., 2002; Buckland et al., 2003; Ling et al., 2004, this issue). This lack of functional efficacy raises questions regarding the role of histamine acting through H4 on eosinophils in vivo. In spite of this, it is postulated that high concentrations of histamine, at sites of mast cell degranulation in vivo, can trigger H4, conceivably functioning as a chemoattractant for eosinophils to that site. Additionally, the H4 receptor is expressed in conjunction with many other chemoattractant receptors on the eosinophil cell surface (Figure 1), and these receptors are activated by the molecular nature of the local inflammatory milieu. The activation of H4 sequentially with these other receptors, in a coordinated fashion (via a distinct temporal and spatial pattern of ligand exposure), allows emigration of eosinophils on the path from the vasculature, through the various compartments, to the involved site. Furthermore, perhaps a key biological function of histamine in vivo is to prime eosinophils for chemotaxis in response to other chemoattractants and, in fact, both research groups present compelling evidence for this hypothesis. Preincubation of eosinophils with histamine resulted in enhanced migration towards eotaxin and eotaxin-2 (Buckland et al., 2003; Ling et al., 2004, this issue). On the other hand, the potential of histamine alone to act as an eosinophil chemoattractant in vivo, might be augmented by other factors, such as growth factors or cytokines. Indeed, O'Reilly et al. (2002) demonstrated that the number of eosinophils migrating in response to histamine was greatly increased by interleukin-5, the cytokine specific for the differentiation, activation, and survival of eosinophils. It remains to be determined what effects histamine, acting via H4, has on the actions of other chemoattractants, such as lipid mediators and/or complement cleavage fragments, upon ligation of their cognate GPCRs on eosinophils, and vice versa.
The development of potent, highly selective H4 receptor antagonists presents considerable therapeutic potential and will launch new avenues for antiallergy therapy. Clearly, novel small molecule H4 receptor antagonists such as JNJ7777120 (Jablonowski et al., 2003; Ling et al., 2004, this issue) will be evaluated in animals, and these studies, along with those with the available H4- receptor-deficient mouse (Hofstra et al., 2003), will elucidate the role of H4 in animal models of allergic inflammation. It is highly likely that JNJ7777120 and/or its analogs, given appropriate pharmacokinetic/pharmacodynamic profiles and bioavailability, will soon be tested in the clinic, to treat chronic inflammatory diseases in humans, such as allergic respiratory, gastrointestinal, and skin diseases, where eosinophils and mast cells are thought to play prominent roles in disease pathogenesis (Rothenberg, 1998).
It is tempting to speculate that combination therapies would be considered, whereby these new histamine H4 receptor antagonist drugs would be paired with other drugs currently on the market and/or in development. Such combinations would include administration of an H4 antagonist with the widely prescribed H1 antagonists, thus antagonizing both the classical anti-inflammatory acute effects of histamine (H1-mediated immediate hypersensitivity reactions) and the chronic effects of histamine (H4-mediated recruitment of effector cells into inflammatory sites leading to late phase reactions and chronic inflammation). Furthermore, the development of selective H1/H4 dual receptor antagonists would be relatively straightforward, since it was recently reported that the H1 antagonists, doxepin, cinnarizine, and promethazine exhibit high affinity binding to the H4 receptor (Nguyen et al., 2001), and thus define the initial structure activity relationships for medicinal chemists to design rapidly such dual antagonist molecules. Notably, the fact that histamine, upon activation of the H4 receptor, augmented the chemotactic effect of CCR3-active β-chemokines on eosinophils, has implications for combination therapy beyond that with just H1 receptor antagonists. These combinations might include pairing an H4 antagonist with a leukotriene receptor antagonist, a PAF receptor antagonist, and/or a chemokine receptor antagonist. Importantly, these novel treatment paradigms have the potential to be glucocorticoid sparing beyond that with currently available therapy. At best, the histamine H4 receptor antagonists will fulfill their potential in clinical use and so open up new therapeutic modalities for the treatment of allergic disease.
Abbreviations
- GPCR
G-protein coupled receptor
- MCP
monocyte chemoattractant protein
- PAF
platelet-activating factor
References
- BUCKLAND K.F., WILLIAMS T.J., CONROY D.M. Histamine induces cytoskeletal changes in human eosinophils via the H4 receptor. Br. J. Pharmacol. 2003;140:1117–1127. doi: 10.1038/sj.bjp.0705530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK R.A.F., GALLIN J.I., KAPLAN A.P. The selective eosinophil chemotactic activity of histamine. J. Exp. Med. 1975;142:1462–1476. doi: 10.1084/jem.142.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK R.A.F., SANDLER J.A., GALLIN J.I., KAPLAN A.P. Histamine modulation of eosinophil migration. J. Immunol. 1977;118:137–145. [PubMed] [Google Scholar]
- HOFSTRA C.L., DESAI P.J., THURMOND R.L., FUNG-LEUNG W.-P. Histamine H4 receptor mediated chemotaxis and calcium mobilization of mast cells. J. Pharmacol. Exp. Ther. 2003;305:1212–1221. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- JABLONOWSKI J.A., GRICE C.A., CHAI W., DVORAK C.A., VENABLE J.D., KWOK A.K., LY K.S., WEI J., BAKER S.M., DESAI P.J., JIANG W., WILSON S.J., THURMOND R.L., KARLSSON L., EDWARDS J.P., LOVENBERG T.W., CARRUTHERS N.I. The first potent and selective non-imidazole human histamine H4 receptor antagonists. J. Med. Chem. 2003;46:3957–3960. doi: 10.1021/jm0341047. [DOI] [PubMed] [Google Scholar]
- LING P., NGO K., NGUYEN S., THURMOND R.L., EDWARDS J.P., KARLSSON L., FUNG-LEUNG W.-P.Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule up-regulation Br. J. Pharmacol. 2004(this issue) [DOI] [PMC free article] [PubMed]
- MACGLASHAN D. Histamine: a mediator of inflammation. J. Allergy Clin. Immunol. 2003;112:S53–S59. doi: 10.1016/s0091-6749(03)01877-3. [DOI] [PubMed] [Google Scholar]
- MORSE K.L., BEHAN J., LAZ T.M., WEST R.E., GREENFEDER S.A., ANTHES J.C., UMLAND S., WAN Y., HIPKIN R.W., GONSIOREK W., SHIN N., GUSTAFSON E.L., QIAO X., WANG S., HEDRICK J.A., GREENE J., BAYNE M., MONSMA F.J. Cloning and characterization of a novel human histamine receptor. J. Phamacol. Exp. Ther. 2001;296:1058–1066. [PubMed] [Google Scholar]
- NGUYEN T., SHAPIRO D.A., GEORGE S.R., SETOLA V., LEE D.K., CHENG R., RAUSER L., LEE S.P., LYNCH K.R., ROTH B.L., O'DOWD B.F. Discovery of a novel member of the histamine receptor family. Mol. Pharmacol. 2001;59:427–433. doi: 10.1124/mol.59.3.427. [DOI] [PubMed] [Google Scholar]
- O'REILLY M., ALPERT R., JENKINSON S., GLADUE R.P., FOO S., TRIM S., PETER B., TREVETHICK M., FIDOCK M. Identification of a histamine H4 receptor on human eosinophils-role in eosinophil chemotaxis. J. Recept. Signal Transduc. 2002;22:431–448. doi: 10.1081/rrs-120014612. [DOI] [PubMed] [Google Scholar]
- RAIBLE D.G., LENAHAN T FAYVILEVICH Y., KOSINSKI R., SCHULMAN E.S. Pharmacologic characterization of a novel histamine receptor on human eosinophils. Am. J. Respir. Crit. Care Med. 1994;149:1506–1511. doi: 10.1164/ajrccm.149.6.8004306. [DOI] [PubMed] [Google Scholar]
- ROTHENBERG M.E. Eosinophilia. N. Engl. J. Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- SPRINGER T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]