Abstract
Various putative drug targets for suppression of ischaemia-induced ventricular fibrillation (VF) have been proposed, but therapeutic success in the suppression of sudden cardiac death (SCD) has been disappointing. Platelet-activating factor (PAF) is a known component of the ischaemic milieu. We examined its arrhythmogenic activity, and its interaction with two other putative mediators, norepinephrine and K+, using an ischaemia-free in vitro heart bioassay, and a specific PAF antagonist (BN-50739).
PAF (0.1–100 nmol) was administered selectively to the left coronary bed of rat isolated hearts using a specially designed catheter. In some hearts, PAF was administered to the left coronary bed during concomitant regional perfusion with norepinephrine and/or K+. In separate studies, PAF accumulation in the perfused cardiac tissue was evaluated using 3H-PAF.
PAF evoked ventricular arrhythmias concentration-dependently (P<0.05). It also widened QT interval and reduced coronary flow selectively in the PAF-exposed left coronary bed (both P<0.05). Two exposures of hearts to PAF were necessary to evoke the QT and rhythm effects.
The PAF-induced arrhythmias and coronary vasoconstriction were partially suppressed by the PAF antagonist BN-50739 (10 μM), although BN-50739 itself widened QT interval.
K+ (8 and 15 mM) unexpectedly antagonised the arrhythmogenic effects of PAF without itself eliciting arrhythmias (P<0.05). Norepinephrine (0.1 μM) had little or no effect on the actions of PAF, while failing to evoke arrhythmias itself. Nevertheless, the combination of 15 mM K+ and 0.1 μM norepinephrine evoked arrhythmias of a severity similar to arrhythmias evoked by PAF alone, without adding to or diminishing the arrhythmogenic effects of PAF.
3H-PAF accumulated in the cardiac tissue, with 43±5% still present 5 min after bolus administration, accounting for the need for two exposures of the heart to PAF for evocation of arrhythmias.
Thus, PAF, by activating specific receptors in the ventricle, can be expected to contribute to arrhythmogenesis during ischaemia. However, its interaction with other components of the ischaemic milieu is complex, and selective block of its actions (or its accumulation) in the ischaemic milieu is alone unlikely to reduce VF/SCD.
Keywords: Arrhythmia, BN-50739, ischaemia, endogenous mediator, PAF, regional blood flow, ventricular arrhythmias
Introduction
Although the frequency of death from ischaemic heart disease is slowly falling year on year (British Heart Foundation, 2003), presumably owing to improvements in diet and lifestyle, there are no effective antiarrhythmic drug treatments to prevent sudden cardiac death (SCD) resulting from ventricular fibrillation (VF), and successive clinical trials have had unanticipated undesired outcomes (CAST, 1989; Waldo et al., 1996). Most antiarrhythmic drugs tested clinically are targeted towards cardiac ion channels (CAST, 1989; Waldo et al., 1996; Janse, 2003).
There is a large body of preclinical data that has explored the alternative approach of targeting the synthesis or receptor-mediated actions of individual biochemical components of the ischaemic milieu with putative arrhythmogenic properties (reviewed by Curtis et al., 1993, and Clements-Jewery & Curtis, 2003). Unfortunately, this approach has yet to yield clinically effective VF-suppressing drugs. This implies that either the key mediator has not yet been identified, or that the relative importance of individual components of the milieu and their possible interactions have not yet been accurately characterised. With regard to the latter, if several components interact in a complex way to influence susceptibility to arrhythmias, this may make the targeting of an individual component unlikely to achieve effective protection against SCD. Thus, it is essential that the complexity of mediator interactions be better characterised.
Platelet-activating factor (PAF) has been proposed as an arrhythmogenic mediator during ischaemia (Flores & Sheridan, 1990). Several separate pieces of evidence support this. For example, PAF accumulates in the ischaemic myocardium (Berti et al., 1990), exogenously applied PAF worsens the severity of low-flow global ischaemia-induced VF (Flores & Sheridan, 1990), and the PAF antagonist BN-50739 protects against ischaemia-induced VF when delivered to the left (and subsequently ischaemic) coronary bed (Baker & Curtis, 1999). However, these data all have their limitations. For example, superimposition of PAF perfusion and low flow global ischaemia (e.g., Flores & Sheridan, 1990) is of questionable pathophysiological relevance, since effects outside the relevant region of myocardium (i.e., the sinoatrial and atrioventricular nodes) will occur. These effects may disturb the rhythm and mask any specific effects in the ventricle itself. Moreover, global (i.e., whole-heart) ischaemia does not elicit arrhythmias to the same extent or by the same mechanism as regional (local) ischaemia (Ridley et al., 1992) such as occurs in ischaemic heart disease. Furthermore, PAF antagonists (BN-50739 being the most extensively characterised) may have PAF-independent effects on cardiac electrophysiology which, until it can be shown that the drugs antagonise the arrhythmogenic actions of PAF itself, may mean that PAF antagonist drug effects on ischaemia-induced VF are unrelated to PAF antagonism (Baker & Curtis, 1999).
To resolve these problems, in the present study we have made use of a preparation that replaces the normal Langendorff aortic cannula with dual lumen catheter to perfuse independently the left and right coronary beds of an isolated heart (Avkiran & Curtis, 1991). When coronary flow to the left coronary bed is selectively shut off in this preparation, this results in ischaemia and arrhythmias that are almost identical to those evoked by coronary ligation in the standard rat Langendorff arrhythmia bioassay (Rees & Curtis, 1995a; Baker & Curtis, 1999), making the preparation ideal for the study of putative mediators of ischaemia-induced VF and thus preferable to global perfusion/ischaemia models. The preparation additionally allows the monitoring of effects on rhythm and antecedent variables such as heart rate and coronary flow, without the confounding influence of nerves, blood and hormones (Avkiran & Curtis, 1991). The preparation was applied to determine whether PAF, in the absence of ischaemia, would elicit arrhythmias when delivered to the region that would be made ischaemic were the left coronary artery to be subjected to ligation, and whether the arrhythmias were sensitive to specific PAF receptor antagonism.
PAF is only one of many candidate mediators of arrhythmias present in the ischaemic milieu (Curtis et al., 1993). Therefore, PAF's effects may interact with those of other mediators. To explore this possibility, norepinephrine and K+, two additional candidate mediators of longstanding interest (Curtis et al., 1993), were incorporated into the study to determine whether they facilitate or antagonise the actions of PAF and of one another.
Methods
All experiments were performed according to the United Kingdom Home Office ‘Guide on the operation of the Animals (Scientific Procedures) Act 1986'.
Basis for choice of species
The rat was the species of choice, in part because the rat heart has a low level of collateral flow (approximately 5% of flow in the uninvolved region; Maxwell et al., 1987). This minimises the scope for cross-flow between the independently perfused left and right coronary beds (Avkiran & Curtis, 1991). In addition, the rat is the species used in our previous studies on ischaemia-induced VF and effects of PAF antagonists (Baker & Curtis, 1999).
Heart perfusion
Male Wistar rats (Bantin and Kingman, UK; 250–300 g) were anaesthetised with pentobarbitone (60 mg kg−1 i.p.) and heparinised (sodium heparin 250 i.u. i.p.). Once reflex responses to the pinching of a hind paw and touching of the cornea had ceased, hearts were excised and arrested in ice-cold perfusion solution containing (in mM): NaCl 118.5, NaHCO3 25.0, MgSO4 1.2, NaH2PO4 1.2, CaCl2 1.4, KCl 3.0 and glucose 11.1. All solutions were filtered (5 μM pore size) before use to remove particulate matter.
Excised hearts were perfused under conditions of constant temperature (37°C), pH (7.4) and pressure (80 mmHg). Each heart was attached, via the aorta, to a specially made cannula (3.3 mm external diameter), the interior of which is divided into two equal hemicylinders by a central septum (Avkiran & Curtis, 1991). The cannula was aligned in the aorta such that each hemicylinder apposed the left or right coronary ostium (alignment verified by in-line flowmeter – see below). Each hemicylinder received an independent supply of perfusion solution, thus allowing the left and right coronary beds to be perfused independently via their ostia. To minimise cardiac cooling, the right atrium and sinoatrial node were continuously superfused with drug-free oxygenated perfusion solution at a constant flow rate of 5 ml min−1, which is sufficient to maintain a normal sinus rate (Curtis & Hearse, 1989a).
Arrhythmia diagnosis and electrocardiogram (ECG) analysis
A unipolar ECG was recorded by implanting one stainless steel electrode into the central anterior region of the left ventricle, with a second connected to the aortic cannula. MacLab™ software for the Apple Macintosh™ computer was used in the identification and analysis of the ECG.
From the ECG, the incidence of arrhythmias, the heart rate, PR interval and QT interval were obtained. In rat hearts the T wave is superimposed upon the end of the QRS complex and the signal returns to isoelectric asymptotically. This makes it difficult to ascertain when the T wave ends. For these reasons, the QT interval was measured at the point of 90% repolarisation (QT90) by measuring the amplitude of the ventricular complex, taking the point of 90% repolarisation, and measuring the interval between this and the start of the ventricular complex, as described previously (Ridley et al., 1992). No attempt was made to ‘correct' QT for heart rate since previous studies have shown that QT is not rate-dependent in the rat heart over the rate range encountered in the present study (Rees & Curtis, 1993c; Farkas & Curtis, 2003).
The diagnostic criteria for discriminating between different ventricular arrhythmias were those described by the Lambeth Conventions (Walker et al., 1988) with the exception of VF, for which revised criteria were used (Tsuchihashi & Curtis, 1991) (see below). Thus, ventricular premature beats (VPBs) were defined as discrete and identifiable premature QRS complexes. Bigeminy was defined by the minimum sequence P, QRS, VPB, P, QRS, VPB. A salvo was defined as a run of two or three consecutive VPBs. A run of four or more consecutive VPBs was defined as ventricular tachycardia (VT). VF was defined as a signal for which individual QRS deflections varied in amplitude and coupling interval on a cycle-to-cycle basis and could no longer be distinguished from one another.
Measurement of the size of the left and right coronary beds and regional coronary flow
The coronary flow through each hemicylinder of the dual perfusion cannula was continuously monitored using a pair of in-line, ultrasonic flow meters (Transonic T206 Flowmeter, Transonic Systems Inc., U.S.A.).
The size of the left and right coronary beds was quantified at the end of each experiment using the disulphine blue dye exclusion method (Curtis & Hearse, 1989b). In all, 5 ml of disulphine blue dye (0.016% w v−1) was introduced into the left coronary bed via a syringe attached to a side arm of the aortic cannula, then the heart was removed from the perfusion apparatus with the dye trapped in the left coronary bed. The atria and any non-cardiac mediastinal tissue were removed. The blue (left coronary bed) and pink (right coronary bed) portions of the ventricles were dissected and weighed. The size of the left coronary bed was expressed as % total ventricular weight. Values of weight-corrected coronary flow (ml min−1 g−1) in the left and right coronary beds were calculated from the measured uncorrected coronary flow (ml min−1) and the weight of the two regions (Curtis & Hearse, 1989a).
Experimental protocols used to investigate the actions of exogenous PAF in the absence of ischaemia
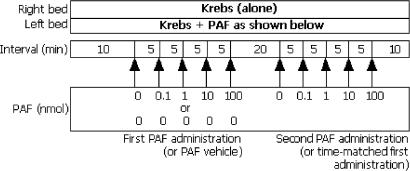
All groups for arrhythmia studies were n=12. The first series of experiments was designed to examine whether PAF delivered regionally in the absence of other components of ischaemia could evoke ventricular arrhythmias (protocol shown in Figure 1). Hearts were perfused for an initial 10 min with modified Krebs solution. Over the next 20 min, PAF vehicle (comprising 0.9% NaCl, 1% bovine serum albumin (BSA)), 0.1 nmol, 1 nmol, 10 nmol and 100 nmol PAF were administered in sequence at 5 min intervals by bolus injections (0.1 ml volume) into the left coronary perfusion line 2 cm proximal to the aortic cannula. The hearts were then perfused with Krebs for a further 20 min, whereupon the PAF administration protocol was repeated. Hearts were exposed twice to increasing amounts of PAF because we intended, in subsequent studies, to examine the effects of the PAF antagonist BN-50739, using each heart as its own control (in order to minimise animal usage). However, in order to exclude any influence of differences in the timing of the first and second exposures to PAF, a second group of hearts receiving identically timed bolus injections of PAF vehicle prior to exposure to PAF was incorporated into the protocol (Figure 1).
Figure 1.
First experimental protocol used to investigate the actions of exogenous PAF in the absence of ischaemia. The left coronary bed was perfused with Krebs, then exposed to PAF or vehicle by bolus injection into the perfusion line at the concentrations and intervals indicated.
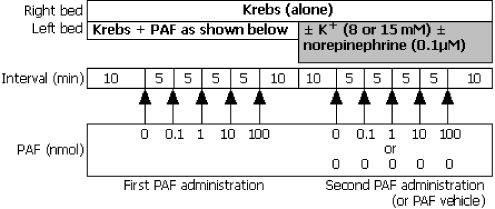
A second series of experiments was designed to investigate the ability of PAF to evoke arrhythmias in the presence of the putative arrhythmogenic mediators, norepinephrine and K+ (choice of concentrations explained below). Following the first exposure to PAF, the hearts were perfused with Krebs for a further 10 min, whereupon the left coronary perfusion solution was exchanged with identical Krebs solution, or Krebs modified to contain 8 mM K+, 15 mM K+, 0.1 μM norepinephrine, 8 mM K+ plus 0.1 μM norepinephrine, or 15 mM K+ plus 0.1 μM norepinephrine. These solutions were delivered continuously for the remainder of the experiment, during which time hearts were exposed again to bolus injections of PAF or PAF vehicle (protocol shown in Figure 2).
Figure 2.
Second protocol in which the left coronary bed was exposed to PAF, then PAF or vehicle during continuous perfusion with K+ and/or norepinephrine.
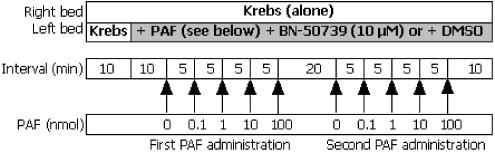
To test whether the postulated arrhythmogenic effects of PAF were receptor-mediated, a third series of experiments was performed with the PAF antagonist BN-50739. Hearts were perfused for an initial 10 min with drug-free solution, then the solution delivered to the left coronary bed was switched in a randomised, blinded fashion to one of two solutions containing 10 μM BN-50739, or BN-50739 vehicle (0.01% DMSO in Krebs). After a further 10 min perfusion, bolus injections of PAF vehicle, 0.1, 1, 10 and 100 nmol PAF were administered into the perfusion line in a sequence timed to match the sequence utilised in the first two experiments (above). Thus, a 5 min interval was allowed between each injection, during which time BN-50739 (or DMSO) was delivered continuously. In order to confirm suspicions that a prior exposure to PAF influenced the response to a subsequent exposure (see results), these hearts were subjected to a second exposure to bolus injections of PAF beginning 20 min later, during maintained perfusion with whichever solution (DMSO or BN-50739) that had been introduced earlier. The protocol is shown in Figure 3.
Figure 3.
Third protocol, in which the left coronary bed was perfused with BN-50739 or its vehicle (DMSO) during exposure to bolus injections of PAF.
Choice of concentration and means of administration of PAF and other substances
Bolus injections of PAF were utilised rather than continuous perfusion because of the requirement for the gassing of main perfusion solutions. It was found in preliminary experiments that gassing 100 nM solutions of PAF generated a foam of PAF at the top of the perfusion chamber. Thus, it was decided to inject small volumes of ungassed PAF-containing solution into the stream of gassed perfusion solution in close proximity to the heart.
The choice of PAF concentration (0.1–100 nmol per heart) and the decision to utilise bolus injection rather than continuous infusion were based primarily upon the observation that PAF can be administered by bolus injection in the nmol range to achieve pharmacological activity in the isolated rat heart (Piper & Stewart, 1986). Exposure times of 5 min were chosen between PAF injections because preliminary experiments carried out using bolus injections of PAF with a 20 min interval between injections revealed that the coronary vasoconstrictor actions of PAF occurred within the first 5 min of exposure (data not shown). The choice of BN-50739 concentration (10 μM) was based on the concentration used and found to suppress arrhythmias in previous coronary ligation studies from our laboratory (Baker & Curtis, 1999).
Norepinephrine and K+, on the other hand, were administered by continuous perfusion in order to examine their ability to facilitate the effects of PAF. This is because it is convenient to administer these substances as supplements to the Krebs perfusion solution (Curtis, 1991; Rees & Curtis, 1995a, 1995b; Clements-Jewery et al., 2002). The reason why these substances were chosen for study and the basis for the chosen concentrations are as follows.
The majority of studies involving norepinephrine and K+ as possible mediators of phase I (early ischaemia-induced) arrhythmias are supportive of an independent role (Hirche et al., 1980; Culling et al., 1984; Curtis & Hearse, 1989a; Curtis, 1991; Du et al., 1998).
The accumulation of norepinephrine within the ischaemic myocardium is believed to result from the local activation of sympathetic cardiac nerves (Mazenot et al., 1999), and the neuronal norepinephrine uptake mechanism, uptake one, operating in the reverse mode (Du et al., 1998). Sympathetic denervation using 6-hydroxy-dopamine has been shown to deplete myocardial catecholamine stores and significantly reduce the incidence of ischaemia-induced VT and VF in the isolated guinea-pig heart (Culling et al., 1984) and the anaesthetised cat (Sheridan et al., 1980). VF is also significantly reduced in the ischaemic rat heart by inhibitors of uptake one, such as desipramine and cocaine (Kurz et al., 1995; Du et al., 1998). In contrast, in non-ischaemic, normoxic rat hearts, desipramine increases the incidence of VF during perfusion with exogenous norepinephrine (0.01–1 μM), which itself evokes VF (Kurz et al., 1995). This, together with the observation that 0.1 μM norepinephrine is sufficient to produce significant pharmacological effects (e.g., in aortic rings from spontaneously hypertensive rats; Arribas et al. 1994), formed the basis for the choice of norepinephrine concentration (0.1 μM) used in this study. Ascorbate 5 μM was added to all catecholamine solutions to limit the extent of their oxidation, as in previous studies (Clements-Jewery et al., 2002). At this concentration, ascorbate has no effect on heart rate, PR and QT interval or coronary flow in isolated rat hearts, and does not evoke arrhythmias itself (Clements-Jewery et al., 2002).
K+ is a substance that satisfies most of the principal criteria for establishing a substance as an independent mediator of ischaemia-induced arrhythmias (Curtis et al., 1993). The accumulation of extracellular K+ within the ischaemic myocardium is known to occur in three stages. An initial 5–10 min phase, during which extracellular K+ rises rapidly, a second 10–15 min plateau phase, when extracellular K+ concentrations remain relatively constant, and a third phase, when a further rise in K+ occurs, are well characterised (Hirche et al., 1980). The onset and initial peak of extracellular K+ accumulation coincides with the appearance of phase I arrhythmias (Hirche et al., 1980). The choice of K+ concentration (8 and 15 mM) used in this study was based upon several observations. Firstly, during the initial phase of ischaemia-induced extracellular K+ accumulation, peak K+ concentrations as high as 15 mM have been detected within the myocardium at a time when ventricular arrhythmias occur (Cascio et al., 1995). Secondly, regional infusion of K+-containing solutions is arrhythmogenic in otherwise healthy, normally perfused hearts, albeit with an arrhythmogenic ‘efficacy' that is less than that of ischaemia itself. Thus, Morena et al. (1980) found that 15 mM K+ was capable of inducing VF in the isolated pig heart, and 9–18 mM K+ evoked arrhythmias in isolated rabbit heart in a concentration-dependent manner, with peak effects again occurring with 15 mM K+ (Curtis, 1991). Therefore, in order to investigate the ability of regional K+ to induce arrhythmias and interact with PAF and norepinephrine, maximal (15 mM) and submaximal (8 mM) effective K+ concentrations were chosen.
Methods for assessing the activity of 3H-PAF in isolated hearts
Verification of effective delivery and examination of the possible accumulation of PAF in cardiac tissue were achieved in parallel studies using radiolabelled PAF. In a separate group of hearts (n=6), after 10 min perfusion with modified Krebs solution the left coronary perfusion line received 0.1 ml of 100 nmol PAF containing 10 μl 3H-PAF by bolus injection. The deadspace between the injection site and the left coronary ostium was 0.09 ml. The coronary effluent was collected continuously for 5 min after the PAF injection, then the heart was removed from the cannula and the free left ventricular wall and right ventricle (plus septum) were dissected. Each section was weighed, homogenised and converted into a suspension using 20 ml NaOH (10 M). Aliquots of 0.5 ml were added to scintillation vials containing 4.5 ml of Liquescint scintillation fluid (Packard) and analysed for radioactive content using a liquid scintillation counter (Packard 1900 TR).
Measuring the recovery of 3H-PAF from both the coronary effluent and cardiac tissue allowed determination of (a) whether PAF delivered in this manner was able to access the heart, (b) the percentage of PAF likely to be ‘washed out' of the heart during a 5 min period and (c) the percentage of PAF likely to remain within the heart (at the end of consecutive 5 min periods of perfusion). An equivalent volume (0.1 ml) of PAF (100 nmol) containing 3H-PAF (10 μl) was prepared and immediately processed to calibrate the extraction procedure: by measuring the radioactive content of a known volume of PAF spiked with 3H-PAF, it was possible to ascertain whether any of the PAF injected was ‘lost' in the system. Typically, losses of PAF due to sticking to glass and plastic are common, making quantification of material from biological samples difficult (Alam et al., 1983).
The radioactive content of each sample was detected as β emission over 3 min. Radioactivity was analysed as disintegrations per min (DPMs), and corrected for volume using the equation:
Results were expressed as a percentage of the total radiation injected into the heart.
Exclusion criteria
In order to minimise the variation between baseline conditions of perfusion, and to ensure that any hearts damaged during cardiac excision and aortic cannulation were not included in the study, exclusion criteria were applied. These criteria have been developed from the lab database (e.g., Tsuchihashi & Curtis, 1991; Ridley et al., 1992; Rees & Curtis, 1993a, 1993b; Rees et al., 1993) according to the principle that data from a single population should be within 2 standard deviations of the population mean. Any outlier was excluded according to Le Chatlier's principle (Rees & Curtis, 1993c). Thus, during baseline perfusion with control solution, any heart with a sinus rate of less than 250 beats min−1 or more than 420 beats min−1, a global coronary flow of less than 8 ml min−1 (indicating coronary vasospasm or embolism), or more than 18 ml min−1 (indicating aortic rupture), 5 min before drug intervention was excluded. Likewise, any heart that was not in sinus rhythm 1 min before the introduction of PAF was also excluded. Arrhythmia incidences were evaluated within consecutive time bins (normally of 5 min duration). Any heart that was experiencing sustained ventricular tachyarrhythmias at the transition from one time bin to the next, and for which arrhythmias persisted for up to 1 min thereon, was excluded from analysis of arrhythmia incidence during the latter time bin. At worst, this resulted in temporary exclusion of no more than two hearts per group of n=12.
Statistics
Data were processed according to published guidelines (Walker et al., 1988; Kusuoka & Hoffman, 2002). Gaussian distributed variables were expressed as mean±s.e.m. and were subjected to unpaired Students t-test (if comparing one group with a control). If comparing more than two groups, variables were first subjected to one- or two-way ANOVA. If treatment constituted a significant source of variance, each group was compared with the control using Dunnett's test. For within-group comparisons, variables were subjected to paired t-tests. Mainland's contingency tables were used for nonparametric analysis (e.g., of arrhythmia incidence). P<0.05 was taken as indicative of a statistically significant difference between values.
Drugs and materials
BN-50739 (Beaufour Ipsen Industrie, France) was dissolved in 100% DMSO to achieve stock solutions equivalent to 10 μmol l−1 DMSO, and stored at 4°C. PAF powder (Sigma, U.K.) was stored at −20°C. Immediately before use, PAF was dissolved in 1% BSA (Sigma, U.K.) and 0.09% sodium chloride (BDH Chemicals, U.K.) to prepare a stock solution that contained 100 nmol PAF. Norepinephrine, ascorbate (Sigma, U.K.) and disulphine blue dye (patent blue V sodium salt) (BDH Chemicals, U.K.) were dissolved in distilled water (Milli-RO 10 and Milli-Q 50, Millipore Ltd, U.K.) and prepared on a daily basis. 3H-PAF was obtained from Amersham, U.K.
Results
Recovery of 3H-PAF
The method of PAF administration used in the present studies was effective in that, after 5 min of perfusion with Krebs following a bolus injection of PAF spiked with 3H-PAF, radioactivity was detected in both the coronary effluent and the cardiac tissue. Of the total 3H-PAF recovered, 43±5% was trapped within the heart, and 57±7% was detected in the coronary effluent. This implies that PAF will accumulate in the heart during successive injections. This has important implications (see later).
Effects of a first exposure of hearts to PAF
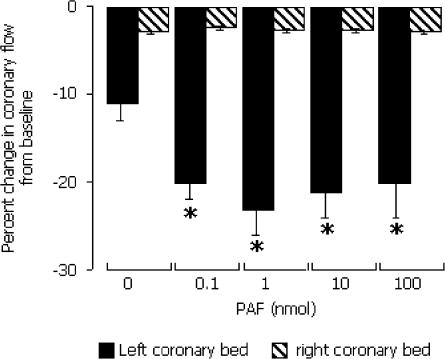
A first exposure of hearts to 0.1–100 nmol. PAF (initial exposure) evoked very few arrhythmias (Table 1). No heart developed VF. PAF did however produce coronary vasoconstriction, with a peak effect achieved by 1 nmol PAF. This was a highly selective effect since it was restricted to the left coronary bed (that which was exposed to PAF) with no changes in coronary flow in the right coronary bed (Figure 4). Initial exposure to PAF did not affect the QT interval, heart rate or PR interval (data not shown).
Table 1.
Arrhythmias during first exposure to PAF
| PAF (nmol) | |||||
|---|---|---|---|---|---|
| 0 | 0.1 | 1 | 10 | 100 | |
| VPBs | 8 | 17 | 8 | 17 | 33 |
| Bigeminy | 8 | 8 | 8 | 8 | 8 |
| Salovs | 0 | 0 | 0 | 8 | 8 |
| VT | 0 | 0 | 0 | 8 | 8 |
| VF | 0 | 0 | 0 | 0 | 0 |
The data represent arrhythmia incidence (%), during initial exposure to PAF. PAF (0–100 nmol) was delivered selectively to the left coronary bed (5 min exposure times). 0 nmol refers to perfusion with PAF vehicle; n=12 per group.
Figure 4.
Change in coronary flow in the left and right coronary beds during exposure to bolus injection of PAF (without prior exposure) into the left coronary perfusion line. *P<0.05 versus values for the right coronary bed.
Effects of a second exposure of hearts to PAF
By the time the second exposure to PAF was about to begin, coronary flow values were no longer different between hearts previously exposed to PAF (8.7±0. 5 ml min−1 g−1) versus hearts previously exposed to vehicle (7.2±0.9 ml min−1 g−1). A second exposure of hearts to 0.1–100 nmol PAF evoked VPBs, bigeminy, salvos and VT in the majority of hearts. The arrhythmogenic effects were concentration-dependent and, at >0.1 nmol, statistically significant (Table 2). VF also occurred, but the incidence was very low. Comparison of Tables 1 and 2 shows that the arrhythmogenic effect of the second exposure to PAF was more substantial than the effect of the first. The effect was not due to any time-dependent change in the heart associated with prolonged perfusion since a first exposure to 0.1–100 nmol PAF evoked few arrhythmias in a time-matched group of hearts initially exposed to vehicle rather than PAF (Table 2).
Table 2.
Arrhythmias during exposure to PAF following previous exposure to PAF or vehicle
| Previous solution | PAF (nmol) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 1 | 10 | 100 | ||
| VPBs | Vehicle | 17 | 25 | 25 | 8 | 33 |
| PAF | 33 | 58 | 75* | 83* | 91* | |
| Bigeminy | Vehicle | 17 | 25 | 25 | 17 | 42 |
| PAF | 42 | 58 | 67* | 73* | 82 | |
| Salvos | Vehicle | 8 | 8 | 0 | 0 | 8 |
| PAF | 42 | 25 | 42 | 45* | 55* | |
| VT | Vehicle | 0 | 0 | 0 | 0 | 0 |
| PAF | 33 | 42 | 42 | 45* | 45* | |
| VF | Vehicle | 0 | 0 | 0 | 0 | 0 |
| PAF | 8 | 8 | 17 | 9 | 9 | |
The data represent arrhythmia incidence (%), during identically timed exposure to PAF, following previous exposure to PAF or vehicle, according to the protocol in Figure 1. PAF (0–100 nmol) was delivered selectively to the left coronary bed. 0 nmol refers to PAF vehicle; n=12 per group.
P<0.05 versus the group previously exposed to vehicle.
A second exposure of hearts to PAF also led to a significant reduction in coronary flow in the left coronary bed (data not shown) of a magnitude similar to that evoked by the first exposure (Figure 4), again without change in flow in the Krebs-perfused right coronary bed.
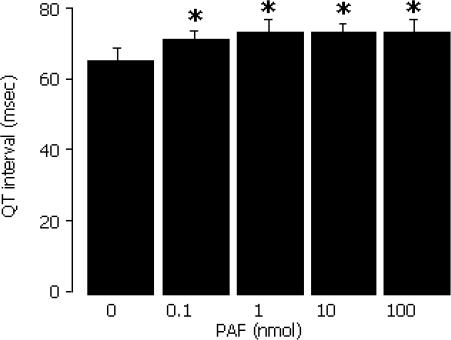
The QT interval was widened by PAF during a second exposure, compared with time-matched controls (Figure 5). This was significant with ≥0.1 nmol PAF, (P<0.05 versus vehicle), and contrasts with the absence of such an effect during the first exposure (see above). A second exposure of hearts to PAF did not affect the heart rate or PR interval (data not shown).
Figure 5.
QT interval in the left coronary bed during a second exposure to PAF by bolus injection into the left coronary perfusion line. *P<0.05 versus 0 nmol PAF.
Effects of a second exposure to PAF in combination with norepinephrine and K+ on rhythm
To examine the possibility of arrhythmogenic interactions between PAF, K+ and norepinephrine, a second study was performed. Hearts were exposed to PAF according to the protocol used in the first study (above), then exposed to PAF again during continuous perfusion with elevated levels of K+ and/or norepinephrine (administered as additives to the main perfusion solution; protocol shown in Figure 2). During the initial exposure to PAF (i.e. in the presence of ‘normal' K+, and no norepinephrine), changes in variables were similar to those seen in the first study under identical conditions (data not shown). The results described below refer exclusively to effects during the second exposure to PAF.
The incidences of VPBs, bigeminy, salvo and VT evoked by PAF were significantly reduced by co-perfusion with 8 mM K+, 15 mM K+ and 8 mM K+ plus 0.1 μM norepinephrine (Table 3). Perfusion with 0.1 μM norepinephrine alone did not suppress (or exacerbate) the arrhythmogenic effect of PAF.
Table 3.
Arrhythmias elicited by a second exposure to PAF during perfusion with elevated K+ for norepinephrine (NA)
| PAF (nmol) | |||||
|---|---|---|---|---|---|
| Perfusion solution modification | 0 | 0.1 | 1 | 10 | 100 |
| VPB (%) | |||||
| None | 33 | 58 | 75 | 82 | 91 |
| K elevated to 8 mM | 0 | 0* | 17* | 0* | 33* |
| K elevated to 15 mM K | 17 | 17 | 17* | 17* | 17* |
| +0.1 μM NA | 42 | 58 | 91 | 100 | 90 |
| K elevated to 8 mM+0.1 μM NA | 50 | 27 | 18* | 45 | 18* |
| K elevated to 15 mM+0.1 μM NA | 92* | 92 | 92 | 92 | 100 |
| Bigeminy(%) | |||||
| None | 33 | 58 | 67 | 73 | 82 |
| K elevated to 8 mM | 0 | 0* | 0* | 0* | 0* |
| K elevated to 15 mM K | 17 | 17 | 17* | 17* | 17* |
| +0.1 μM NA | 33 | 33 | 27 | 45 | 60 |
| K elevated to 8 mM+0.1 μM NA | 17 | 0* | 0* | 9* | 0* |
| K elevated to 15 mM+0.1 μM NA | 67 | 83 | 58 | 66 | 75 |
| Salvo(%) | |||||
| None | 33 | 25 | 42 | 45 | 55 |
| K elevated to 8 mM | 0 | 0 | 0 | 0* | 17* |
| K elevated to 15 mM K | 0 | 0 | 0 | 0* | 0* |
| +0.1 μM NA | 8 | 33 | 18 | 27 | 30 |
| K elevated to 8 mM+0.1 μM NA | 17 | 9 | 0 | 9 | 0* |
| K elevated to 15 mM+0.1 μM NA | 58 | 75 | 66 | 58 | 83 |
| VT(%) | |||||
| None | 33 | 42 | 42 | 45 | 45 |
| K elevated to 8 mM | 0 | 0 | 0 | 0* | 0* |
| K elevated to 15 mM K | 0 | 0 | 0 | 0* | 0* |
| +0.1 μM NA | 25 | 8 | 18 | 27 | 20 |
| K elevated to 8 mM+0.1 μM NA | 17 | 9 | 0 | 0* | 0* |
| K elevated to 15 mM+0.1 μM NA | 58 | 58 | 33 | 33 | 58 |
| VF(%) | |||||
| None | 8 | 8 | 17 | 9 | 9 |
| K elevated to 8 mM | 0 | 0 | 0 | 0 | 0 |
| K elevated to 15 mM K | 0 | 0 | 0 | 0 | 0 |
| +0.1 μM NA | 8 | 8 | 9 | 18 | 0 |
| K elevated to 8 mM+0.1 μM NA | 8 | 0 | 0 | 0 | 0 |
| K elevated to 15 mM+0.1 μM NA | 0 | 8 | 0 | 0 | 0 |
Left coronary beds were initially exposed to PAF. The data represent group incidence (%) of arrhythmias during a second exposure to PAF (0–100 nmol). During this second exposure, PAF was delivered to the left coronary bed in combination with the perfusion solutions indicated. 0 nmol refers to PAF vehicle; n=12 per group. Throughout, the right coronary bed was perfused with unadulterated Krebs solution
P<0.05 versus none (no addition to Krebs perfusion).
A parallel set of experiments was performed in which hearts received the standard first exposure to PAF, followed by perfusion with various concentrations of K+ and/or norepinephrine without a second exposure to PAF. To provide a data set that was time-matched (with the data shown in Table 3), hearts were exposed five times to PAF vehicle during perfusion with norepinephrine and/or K+ (protocol shown in Figure 2). This allowed examination of whether norepinephrine and/or K+ possessed arrhythmogenic activity in the absence of concurrent administration of PAF. The data (Table 4) show that, in the absence of PAF, neither K+ (8 or 15 mM) nor norepinephrine (0.1 μM) exhibited any significant arrhythmogenic effects, but that the combination of high K+ (15 mM) and norepinephrine evoked arrhythmias when compared with time-matched hearts perfused with Krebs.
Table 4.
Arrhythmias elicited by PAF vehicle during perfusion with Krebs containing elevated K+ and norepinephrine (NA) in hearts previously exposed to PAF
| PAF (nmol) | |||||
|---|---|---|---|---|---|
| Perfusion solution modification | 0 | 0 | 0 | 0 | 0 |
| VPB (%) | |||||
| None | 17 | 8 | 17 | 17 | 0 |
| K elevated to 8 mM | 0 | 0 | 8 | 8 | 0 |
| K elevated to 15 mM K | 0 | 0 | 0 | 0 | 0 |
| + 0.1 μM NA | 25 | 8 | 8 | 17 | 0 |
| K elevated to 8 mM+0.1 μM NA | 25 | 8 | 0 | 0 | 17 |
| K elevated to 15 mM+0.1 μM NA | 50 | 75* | 75* | 75* | 75* |
| Bigeminy(%) | |||||
| None | 0 | 8 | 8 | 8 | 0 |
| K elevated to 8 mM | 0 | 0 | 0 | 0 | 0 |
| K elevated to 15 mM K | 0 | 0 | 0 | 0 | 0 |
| +0.1 μM NA | 8 | 0 | 8 | 8 | 0 |
| K elevated to 8 mM+0.1 μM NA | 0 | 0 | 0 | 0 | 0 |
| K elevated to 15 mM+0.1 μM NA | 42 | 58* | 42 | 58* | 58* |
| Salvo(%) | |||||
| None | 8 | 0 | 8 | 0 | 0 |
| K elevated to 8 mM | 0 | 0 | 0 | 0 | 0 |
| K elevated to 15 mM K | 0 | 0 | 0 | 0 | 0 |
| +0.1 μM NA | 8 | 0 | 8 | 0 | 0 |
| K elevated to 8 mM+0.1 μM NA | 0 | 0 | 0 | 0 | 0 |
| K elevated to 15 mM+0.1 μM NA | 42 | 50* | 42 | 42 | 58* |
| VT(%) | |||||
| None | 8 | 0 | 0 | 8 | 8 |
| K elevated to 8 mM | 0 | 0 | 0 | 0 | 0 |
| K elevated to 15 mM K | 0 | 0 | 0 | 0 | 0 |
| +0.1 μM NA | 8 | 0 | 8 | 0 | 0 |
| K elevated to 8 mM+0.1 μM NA | 8 | 0 | 0 | 0 | 0 |
| K elevated to 15 mM+0.1 μM NA | 42 | 33 | 50* | 42 | 58* |
| VF(%) | |||||
| None | 0 | 0 | 0 | 0 | 0 |
| K elevated to 8 mM | 0 | 0 | 0 | 0 | 0 |
| K elevated to 15 mM K | 0 | 0 | 0 | 0 | 0 |
| + 0.1 μM NA | 0 | 0 | 0 | 0 | 0 |
| K elevated to 8 mM+0.1 μM NA | 0 | 0 | 0 | 0 | 0 |
| K elevated to 15 mM+0.1 μM NA | 0 | 0 | 0 | 0 | 0 |
Hearts were initially exposed to PAF. The data represent group incidence (%) of arrhythmias during a subsequent exposure to PAF vehicle during which hearts were perfused with the solutions indicated. n=12 per group.
P<0.05 versus none (no addition to Krebs perfusion).
To summarise the key findings of the first and second studies, the final columns in Tables 3 and 4 have been reproduced in Table 5. The data confirm that PAF exposure alone is arrhythmogenic, that K+ (8 and 15 mM) antagonises this arrhythmogenic effect without itself eliciting arrhythmias, that 0.1 μM norepinephrine has little or no effect on the actions of PAF, while failing to evoke arrhythmias itself, and that the combination of 15 mM K+ and 0.1 μM norepinephrine evokes arrhythmias of a severity similar to that evoked by PAF alone, without adding to or diminishing the arrhythmogenic effects of PAF.
Table 5.
Arrhythmias elicited by PAF vehicle or by the final concentration of PAF in hearts perfused with Krebs containing elevated K+ and/or supplementation with norepinephrine (NA)
| Perfusion solution modification | PAF vehicle | 100 nmol PAF |
|---|---|---|
| VPB(%) | ||
| None | 0 | 91* |
| K elevated to 8 mM | 0 | 33 |
| K elevated to 15 mM K | 0 | 17 |
| +0.1 μM NA | 25 | 90* |
| K elevated to 8 mM +0.1 μM NA | 17 | 18 |
| K elevated to 15 mM +0.1 μM NA | 75 | 100 |
| Bigeminy(%) | ||
| None | 0 | 82* |
| K elevated to 8 mM | 0 | 0 |
| K elevated to 15 mM K | 0 | 17 |
| +0.1 μM NA | 0 | 60* |
| K elevated to 8 mM+0.1 μM NA | 0 | 0 |
| K elevated to 15 mM+0.1 μM NA | 58 | 75 |
| Salvo(%) | ||
| None | 0 | 55* |
| K elevated to 8 mM | 0 | 17 |
| K elevated to 15 mM K | 0 | 0 |
| +0.1 μM NA | 0 | 30 |
| K elevated to 8 mM+0.1 μM NA | 0 | 0 |
| K elevated to 15 mM+0.1 μM NA | 58 | 83 |
| VT(%) | ||
| None | 0 | 45* |
| K elevated to 8 mM | 0 | 0 |
| K elevated to 15 mM K | 0 | 0 |
| +0.1 μM NA | 0 | 20 |
| K elevated to 8 mM+0.1 μM NA | 0 | 0 |
| K elevated to 15 mM+0.1 μM NA | 45 | 58 |
| VF(%) | ||
| None | 0 | 9 |
| K elevated to 8 mM | 0 | 0 |
| K elevated to 15 mM K | 0 | 0 |
| +0.1 μM NA | 0 | 0 |
| K elevated to 8 mM+0.1 μM NA | 0 | 0 |
| K elevated to 15 mM+0.1 μM NA | 0 | 0 |
Hearts in all groups shown were initially exposed to PAF. The data represent group incidence (%) of arrhythmias during a second exposure to PAF or PAF vehicle. The results shown refer to administration of 100 nmol PAF or identically timed bolus of PAF vehicle (data from Tables 3 and 4). The hearts were perfused with K+ and/or NA added to the perfusion solution during the second exposure to PAF (or vehicle); n=12 per group.
P<0.05 versus PAF vehicle.
Effects of a second exposure to PAF in combination with norepinephrine and K+ on haemodynamics and the ECG
PAF reduced coronary flow during its first exposure selectively in the left coronary bed (data not shown) in a manner similar to that observed in the first protocol (above). K+ and norepinephrine tended to exacerbate the coronary vasoconstrictor effects of PAF. This reached significance during exposure to 100 nmol PAF (Table 6). In hearts exposed to PAF vehicle, K+ did not influence coronary flow, whereas norepinephrine evoked coronary vasoconstriction (Table 6).
Table 6.
Coronary flow duringexposure to PAF vehicle or the final concentration of PAF in hearts perfused with Krebs containingelevated K+ and/or supplementation with norepinephrine (NA)
| PAF vehicle | 100 nmol PAF | |
|---|---|---|
| Perfusion solution modification | ||
| None | −17±5 | −12±9 |
| K elevated to 8 mM | −18±5 | −35±10* |
| K elevated to 15 mM K | −22±5 | −47±3* |
| +0.1 μM NA | −16±7 | −47±6* |
| K elevated to 8 mM+0.1 μM NA | −21±8 | −47±4* |
| K elevated to 15 mM+0.1 μM NA | −15±9 | −46±3* |
All groups shown were initially exposed to PAF (0–100 nmol). The data represent coronary flow (ml min−1 g−1, expressed as a % change from baseline), in the left coronary bed, during a second exposure to PAF (only data for the highest dose, 100 nmol PAF, or a time-matched bolus of PAF vehicle, are shown). The hearts were perfused with K+ and/or norepinephrine added to the perfusion solution during the second exposure to PAF or vehicle; n=12 per group. Values are mean±s.e.m.
P<0.05 versus PAF vehicle.
The QT-widening effects of PAF were not exacerbated or diminished by perfusion with K+ and norepinephrine and, in time-matched groups, K+ and norepinephrine by themselves did not affect the QT interval in the absence of PAF (Table 7).
Table 7.
QT interval duringexposure to PAF vehicle or the final concentration of PAF in hearts perfused with Krebs containingelevated K+ and/or supplementation with norepinephrine (NA)
| PAF vehicle | 100 nmol PAF | |
|---|---|---|
| Perfusion solution modification | ||
| None | 62±3 | 74±3* |
| K elevated to 8 mM | 61±9 | 79±5* |
| K elevated to 15 mM K | 66±5 | 76±3* |
| + 0.1 μM NA | 67±6 | 77±2* |
| K elevated to 8 mM +0.1 μM NA | 60±4 | 75±3* |
| K elevated to 15 mM +0.1 μM NA | 57±5 | 71±7* |
All groups shown were initially exposed to PAF (0–100 nmol). The data represent QT interval (ms; measured at 90% repolarisation), during the second exposure to PAF (only data for the highest dose, 100 nmol PAF, or a time-matched bolus of PAF vehicle, are shown). The hearts were perfused with K+ and/or norepinephrine added to the perfusion solution during the second exposure to PAF or vehicle; n=12 per group. Values are mean±s.e.m.
P<0.05 versus PAF vehicle.
Perfusion with K+ (8 or 15 mM) did not change the heart rate during exposure to PAF, whereas heart rate was increased in the groups perfused with norepinephrine-containing solutions (data not shown).
Effects of BN-50739 on PAF-induced arrhythmias, haemodynamics and QT interval
BN-50739 (10 μM) significantly reduced the ability of a second exposure to PAF to induce VPBs and bigeminy (Table 8). PAF-induced salvos, VT and VF were not influenced significantly by BN-50739, although they were generally reduced as a trend (Table 8).
Table 8.
Effect of BN-50739 on the arrhythmogenic actions of PAF
| PAF (nmol) | ||||||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 1 | 10 | 100 | ||
| VPBs | DMSO | 33 | 50 | 67 | 83 | 91 |
| BN-50739 | 17 | 17 | 33 | 17* | 33* | |
| Bigeminy | DMSO | 0 | 50 | 67 | 50 | 82 |
| BN-50739 | 0 | 33 | 17* | 0* | 42 | |
| Salvos | DMSO | 17 | 17 | 33 | 33 | 0 |
| BN-50739 | 17 | 17 | 17 | 0 | 0 | |
| VT | DMSO | 0 | 33 | 17 | 50 | 33 |
| BN-50739 | 17 | 33 | 17 | 33 | 0 | |
| VF | DMSO | 0 | 17 | 0 | 17 | 0 |
| BN-50739 | 17 | 0 | 0 | 0 | 0 | |
All groups shown were initially exposed to PAF (0–100 nmol). The data represent arrhythmia incidence (%), during a second exposure to PAF (0–100 nmol). During this second exposure, PAF was delivered selectively to the left coronary bed which was perfused continuously with Krebs containing BN-50739 vehicle (DMSO) or BN-50739 (10 μM). 0 nmol refers to PAF vehicle; n=12 per group.
P<0.05 versus exposure-matched DMSO group.
BN-50739 reduced the coronary vasoconstrictor activity of PAF, an effect that was observed during first exposure to PAF, during second exposure to PAF and during the 20 min recovery period prior to second PAF exposure (Table 9). However, BN-50739 itself evoked significant vasodilatation prior to the first exposure to PAF (Table 9).
Table 9.
Effect of BN-50739 on the coronary vascular actions of PAF
| PAF (nmol) | |||||||
|---|---|---|---|---|---|---|---|
| Prior to PAF | 0 | 0.1 | 1 | 10 | 100 | ||
| First Exposure to PAF | |||||||
| +DMSO | −15±2 | −14±2 | −25±4 | −26±2 | −26±3 | −22±8 | |
| +BN-50739 | 9±4* | 11±4* | 12±4* | 9±3* | 11±6* | 2±8* | |
| Second Exposure to PAF | |||||||
| +DMSO | −27±3 | −29±3 | −34±3 | −47±4 | −46±4 | −47±7 | |
| +BN-50739 | −7±6* | −20±2* | −22±10* | −24±10* | −30±8* | −35±7 | |
The data represent coronary flow (% change from baseline) in the left coronary bed 1 min prior to PAF exposure and during the first and second exposures to PAF. PAF was delivered selectively to the left coronary bed with DMSO or BN-50739. 0 nmol refers to PAF vehicle; n=12 per group.
P<0.05 versus exposure-matched DMSO group.
BN-50739 widened the QT interval in PAF-treated hearts. As with coronary flow, significant effects were observed before as well as during the first and second exposures to PAF (Table 10).
Table 10.
Effect of BN-50739 on the actions of PAF on the QT interval
| PAF (nmol) | |||||||
|---|---|---|---|---|---|---|---|
| Prior to PAF | 0 | 0.1 | 1 | 10 | 100 | ||
| First Exposure to PAF | |||||||
| +DMSO | 59±2 | 56±1 | 61±4 | 61±4 | 59±3 | 62±3 | |
| +BN-50739 | 78±8* | 75±10 | 76±10 | 76±9 | 91±11* | 83±11* | |
| Second Exposure to PAF | |||||||
| +DMSO | 63±4 | 63±4 | 68±5 | 70±5 | 68±5 | 67±4 | |
| +BN-50739 | 87±9* | 77±13 | 82±10 | 79±10 | 97±14* | 93±11* | |
The data represent QT interval (ms; measured at 90% repolarisation), 1 min prior to PAF exposure and during the first and second exposures to PAF. PAF was delivered selectively to the left coronary bed with DMSO or BN-50739. 0 nmol refers to PAF vehicle; n=12 per group.
P<0.05 versus exposure-matched DMSO group.
BN-50739 did not influence the heart rate or PR interval, before or during first or second exposures to PAF (data not shown).
Discussion
Left coronary administration of PAF is arrhythmogenic
The most important finding was that perfusion of the left coronary bed with PAF led to the manifestation of ventricular arrhythmias without the occurrence of any supraventricular arrhythmias, supporting the notion that PAF participates locally in acute ischaemia as an arrhythmogenic mediator. However, consideration of ancillary variables is required in order to ensure that the effects are truly local – necessary if PAF is a genuine endogenous mediator of arrhythmias. Additionally, the data require careful interpretation in order to gauge the extent of this participation and how it is affected by other components of the ischaemic milieu.
Arrhythmogenic effects of PAF – the need for two exposures
The ability of PAF to evoke ventricular arrhythmias required two consecutive cumulative exposures. One possible explanation for this is that PAF ‘primes' the heart to respond in an exaggerated manner to a second exposure to PAF through some intrinsic alteration in the molecular response to PAF. An equivalent priming effect of PAF has been observed in neutrophils (Doebber & Wu, 1987) and endothelial cells (Heller et al., 1992), and this has been attributed to the ability of PAF to induce its own synthesis. Newly synthesised PAF is thought to be retained within the cell, leading to an exaggerated response to subsequent PAF administration. It has been shown that 1–100 nM PAF induced a dose- and time-dependent increase of PAF synthesis in human umbilical vein endothelial cells, within 5 min of its application, and 93% of this newly synthesised PAF was retained within cells, leading to speculation that PAF could act both as an intracellular and extracellular mediator, via the activation of specific cell surface and intracellular receptors (Heller et al., 1992). However, this is not the only possible explanation for the present findings.
The enhanced arrhythmogenic action of PAF during the second exposure may be explained simply on the basis of accumulation of PAF within the cardiac tissue during first exposure, such that the second exposure to PAF leads to a greater ambient (tissue) concentration and hence a greater response. The evidence for this is provided by the detection of 3H-labeled PAF accumulation and retention in the heart 5 min after a bolus administration of 3H-PAF.
Regardless of the mechanism responsible for the priming phenomenon, it is clear from the data that PAF can evoke ventricular arrhythmias (VPBs, bigeminy, salvos and VT) when administered selectively to the left coronary bed. Although PAF did not evoke VF in a sufficient number of hearts for this to reach statistical significance, the appearance of VF in one or two hearts is likely to be of biological significance because healthy hearts do not fibrillate during control perfusion. However, the data suggest that PAF actions are not sufficient alone to account fully for ischaemia-induced arrhythmias, especially VF, since regional ischaemia itself evokes VF in the majority of perfused rat hearts in our hands (e.g., Baker & Curtis, 1999).
Possible electrophysiological mechanisms by which PAF elicits ventricular arrhythmias
There are several potential electrophysiological mechanisms by which PAF could induce arrhythmias. Although this topic is beyond the scope of the present study and has been studied previously, some discussion is warranted. One mechanism that may have relevance is the induction of early afterdepolarizations (EADs). EADs are associated with prolongation of action potential duration (APD) and blockade of IK1 (Isenberg & Trautwein, 1974; Wit & Rosen, 1992). Exogenous PAF has been shown to produce prolonged depression of IK1 in both guinea-pig (Wahler et al., 1990) and canine (Hoffman et al., 1996) myocytes, an effect that was blocked by the PAF antagonist CV-6209 (Hoffman et al., 1996). In the present study, PAF widened the QT interval, and two exposures to PAF were required to increase the QT interval and evoke arrhythmias. Since, repolarization is the main determinant of the QT interval in isolated perfused rat hearts (Farkas & Curtis, 2003) as it is in general (Singh & Nademanee, 1985), it is possible that PAF induced arrhythmias by delay of repolarization and evocation of EADs. An argument against this is that the PAF antagonist BN-50739 also widened QT in the present study, yet it did not evoke arrhythmias itself and actually antagonised the arrhythmogenic effects of PAF.
Another possibility that cannot be discounted is re-entry occurring as a result of regional dispersion of repolarization. The regional QT widening caused by PAF in the present study may be sufficient to trigger re-entry by the creation of regional systolic and diastolic injury currents, as seen in pig and dog isolated regionally ischaemic hearts (Janse et al., 1980).
Relationship between PAF and other putative arrhythmogenic mediators
As numerous biochemicals accumulate (or are depleted) in the ischaemic milieu (Curtis et al., 1993) it would be naïve to assume that just one mediator is sufficient and necessary for evoking ischaemia-induced arrhythmias. We selected two candidate mediators in order to explore possible interactions with the effects of PAF. The arrhythmogenic effects of PAF were significantly reduced by perfusion with K+, alone or in combination with norepinephrine. The ability of K+ to reduce the ability of PAF to evoke arrhythmias in hearts that were previously exposed to PAF is the first report of a possible paradoxical antagonistic relationship between two putative mediators of ischaemia-induced arrhythmias. One explanation may be that PAF-induced EADs (discussed above) were inhibited by an elevation in extracellular K+ concentration, since K+ elevation has been shown previously to suppress EADs (Coetzee & Opie, 1987).
Despite numerous reports that regional increases in extracellular K+ can facilitate the induction of ventricular arrhythmias in the absence of ischaemia, in species including dog (Ettinger et al., 1973; Pelleg et al., 1989) and rabbit (Curtis, 1991), in the present study extracellular K+ elevation, alone, did not exhibit any significant arrhythmogenic activity in rat hearts. One possible explanation for this could be the influence of differences in baseline QT values (and hence APD) between species. In the rabbit heart, in the absence of ischaemia, the baseline QT interval is much wider than that of the rat (e.g., 139±4 versus 88±5 ms, respectively, measured to the point of 100% repolarization; Rees & Curtis, 1993a) and, as such, the rabbit may be more susceptible to APD-shortening interventions (such as elevated concentrations of extracellular K+) that would increase the likelihood of development of re-entry circuits and arrhythmias. As an adjunct to this, any intervention capable of increasing inward currents would therefore unmask or facilitate the arrhythmogenic activity of regional elevations of extracellular K+. This would explain why the combination of norepinephrine with K+ was significantly arrhythmogenic. However, this has no direct bearing on the arrhythmogenicity of PAF.
The importance of norepinephrine itself (alone) to arrhythmogenesis during ischaemia is unclear. Norepinephrine alone did not exhibit any significant arrhythmogenic activity when delivered selectively to the left coronary bed. Norepinephrine perfusion solution necessarily contained 5 μM ascorbate. Although it has no effect on heart rate, PR and QT interval or coronary flow in isolated rat hearts, and does not evoke arrhythmias itself (Clements-Jewery et al., 2002), we cannot discount the possibility that ascorbate ameliorated the arrhythmogenic effects of norepinephrine. Unfortunately, it is not possible to test this (it is possible to substitute ascorbate for a range of other antioxidants, but an antioxidant-free norepinephrine solution would need to be included in any study, and this is not achievable). Although there are many reports linking increased norepinephrine levels to increased severity of arrhythmias during ischaemia (see Methods section), there are contradictory reports suggesting that catecholamines are not substantially involved in the development of ischaemia-induced arrhythmias. For example, in the anaesthetised rat, catecholamine depletion (by adrenalectomy or 6-hydroxy-dopamine) did not protect against ischaemia-induced arrhythmias (Daugherty et al., 1986). Similarly, in intact conscious rats, the adrenergic receptor antagonists labetalol and propanolol failed to reduce the incidence of ischaemia-induced arrhythmias (Botting et al., 1983). Furthermore, forskolin-induced stimulation of adenylyl cyclase (albeit, global as opposed to regional) did not exacerbate ischaemia-induced arrhythmias in the rat, despite markedly elevating myocardial cAMP concentrations (Manning et al., 1985). Although Kurz et al. (1995) demonstrated pro-fibrillatory effects of exogenous norepinephrine in the isolated rat heart in the absence of ischaemia, the pathophysiological relevance of these data is questionable, owing to the fact that norepinephrine was delivered globally rather than regionally (different from the present study), and administered in conjunction with desipramine, which would increase local norepinephrine concentrations by preventing norepinephrine uptake.
Thus, the interaction between PAF and other putative mediators of ischaemia-induced arrhythmias is complex.
PAF and coronary flow
Initial exposure to PAF resulted in coronary vasoconstriction, as detected by a reduction in coronary flow. This was restricted to the left coronary bed, that to which PAF was administered.
The coronary vasoconstrictor effects of PAF are believed to be mediated indirectly via the release of leukotrienes and other arachidonic acid metabolites (Hu & Man, 1991). Indeed, Piper & Stewart (1986) demonstrated that the vasoconstrictor effects of PAF in the isolated rat heart were mediated via the release of leukotriene C4. In the same study, PAF was shown to increase the production of thromboxane B2 (Piper & Stewart, 1986). Both leukotriene C4 and thromboxanes are potent vasoconstrictors in the coronary circulation (Allan & Levi, 1981; Piper & Stewart, 1986).
The vasoconstrictor activity of PAF, although significant during first exposure, was more pronounced upon the second exposure. This is consistent with previously described evidence of accumulation of PAF. As an alternative (or additional) explanation, PAF has been shown to actively disrupt the cytoskeleton of cultured human endothelial cells, leading to cell retraction and formation of intercellular gaps (Bussolino et al., 1987). The vascular endothelium actively controls the tone of underlying smooth muscle via the release of numerous vasoactive mediators (Furchgott & Vanhoutte, 1989; Vane et al., 1990). Endothelial damage could therefore have reduced the vasodilator effects of endogenous vasodilatory mediators such as prostacyclin (Bunting et al., 1976) and nitric oxide (Sausbier et al., 2000), thus increasing the potential for PAF to increase vascular tone and cause coronary vasoconstriction. Removal of the endothelium has previously been shown to augment the contractile response to norepinephrine in rat isolated thoracic aorta (Ito et al., 1994). This effect was believed to result from the inhibition of nitric oxide generation since, in endothelium intact cells, the contractions elicited by norepinephrine were also potentiated by methylene blue, a guanylyl cyclase inhibitor (Ito et al., 1994). In the present experiments, norepinephrine alone caused vasoconstriction, an effect that also tended to be more pronounced after administration of PAF. The relevance of the observed vasoconstriction to arrhythmogenesis is discussed later (see below).
As with its arrhythmogenic actions, the vasoconstrictor effects of PAF also appeared to be influenced by changes in extracellular K+. However, the elevation of K+ tended to facilitate, rather than reduce, PAF's vasoconstrictor activity (the opposite of the effect on PAF's arrhythmogenic actions). It is possible that changes in the affinity of the PAF receptor for PAF could account in part for this. The PAF receptor is believed to exist in several conformational states, each with differing affinities for PAF. In rabbit platelet membranes, the differential binding of 3H-PAF and 3H-L-659-989 is believed to result from changes in conformation of the receptor according to the composition of the ionic milieu. Since 3H-PAF and 3H-L-659-989 share a common binding site on the PAF receptor, the reduced binding of 3H-PAF, but not 3H-L-659-989, indicates conformational changes in the receptor such that its affinity for 3H-PAF is decreased (Hwang et al., 1988). In the same study, K+ ions and divalent cations such as Mg2+ and Ca2+, were shown to potentiate the binding of both 3H-PAF and 3H-L-659-989, suggesting that these ions convert the receptor into a state where it exhibits high affinity for both PAF and its antagonist (Hwang et al., 1988). Thus, under conditions of regional hyperkalaemia, K+ may facilitate the vasoconstrictor activity of PAF by inducing a conformational change to the PAF receptor that leads to a higher affinity for PAF. This does not contradict the interpretation of the antagonistic effect of K+ on the arrhythmogenic action of PAF (see above), since nonspecific antagonism (antagonism of post-receptor events) was deduced.
The ability of norepinephrine and K+ to facilitate the vasoconstrictor effects of PAF without enhancing its ability to induce arrhythmias would suggest that the effects of the second exposure to PAF on arrhythmia susceptibility are independent of its effects on coronary flow. If the arrhythmogenic activity of PAF were secondary to a reduction in coronary flow, then an increased incidence of arrhythmias would be expected during the second exposure to PAF in the presence of K+, as well as norepinephrine, and this did not occur.
It is also important to note that the flow reductions seen during PAF exposure are not sufficiently large in themselves to evoke manifestations of ischaemia in the isolated rat heart (Maxwell et al., 1987). Therefore the arrhythmogenic effects of PAF were mediated directly, and were not secondary to induced ischaemia.
Effects of BN-50739 on PAF-induced arrhythmias, coronary flow and QT interval
PAF receptors have been detected in human cardiomyocytes (Sugimoto et al., 1992) and in frozen sections of rat heart (Predescu et al., 1996; Wang et al., 1997). The PAF receptor antagonist BN-50739 significantly reduced the ability of PAF to evoke arrhythmias, providing evidence that PAF-induced arrhythmias may result from direct effects of PAF on its receptor.
BN-50739 also opposed the vasoconstrictor actions of PAF. However, the fact that BN-50739 caused significant vasodilatation before the first exposure to exogenous PAF suggests that either there is basal release of endogenous PAF in the isolated rat heart, or that BN-50739 increased coronary flow, in part, independently of the PAF receptor. Some PAF receptor antagonists have only limited selectivity for PAF receptors. For example, kadsurenone was shown to inhibit both PAF and histamine-induced oedema in rat skin (Hwang et al., 1985). In previous studies (Baker & Curtis, 1999), BN-50739 was found to exhibit PAF receptor-independent effects in rat hearts.
BN-50739 did not antagonise the ability of PAF to widen the QT interval. In fact, BN-50739 itself widened the QT interval, an effect that was significant both before and during PAF exposure. The ability of BN-50739 to widen the QT interval without affecting the ability of PAF to widen the QT interval is further evidence for PAF receptor-independent actions. Science QT widening is indicative of repolarisation delay, it is possible that BN-50739 exerted direct K+ channel-blocking actions. In some previous published studies with the drug, QT measurements were not made (Hu & Man, 1991; Koltai et al., 1991). However, we have observed QT widening in previous studies with the drug, and argued that PAF receptor-independent actions on K+ currents is the most likely explanation (Baker & Curtis, 1999). Thus, the apparent lack of selectivity of BN-50739 means that the ability of the drug to antagonise the arrhythmogenic effects of PAF does not necessarily prove that PAF's arrhythmogenic effects were PAF receptor-mediated.
Conclusion
Exogenous PAF can elicit cardiac arrhythmias in the absence of ischaemia. These are not as severe as those elicited by ischaemia itself, and exogenous PAF rarely evoked VF. These data therefore suggest that the effects of PAF alone are not sufficient to account in full for ischaemia-induced VF. The findings also suggest a complex interplay between PAF and other substances known to accumulate in acutely ischaemic myocardium, with facilitation and antagonism of arrhythmogenicity by various components of the milieu. Clarification of the role of PAF may be facilitated in future studies by the use of additional species such as rabbit (which exhibits more human-like QT intervals) and more selective PAF antagonists, should they become available, but the present findings would suggest that selective targeting of PAF would by itself have little impact on clinical VF and SCD.
Acknowledgments
This work was funded by the British Heart Foundation (FS/95040). We thank Dr Hugh Clements-Jewery for his valuable comments.
Abbreviations
- APD
action potential duration
- BSA
bovine serum albumin
- DMSO
dimethyl sulphoxide
- DPMs
disintegrations per minute
- EADs
early afterdepolarizations
- ECG
electrocardiogram
- i.p.
intraperitoneal
- i.u.
international units
- PAF
platelet activating factor
- SCD
sudden cardiac death
- VF
ventricular fibrillation
- VPBs
ventricular premature beats
- VT
ventricular tachycardia
References
- ALAM I., SMITH J.B., SLIVER M.J. Human and rabbit platelets form platelet activating factor in response to calcium ionophore. Thrombosis Res. 1983;30:71–79. doi: 10.1016/0049-3848(83)90398-5. [DOI] [PubMed] [Google Scholar]
- ALLAN G., LEVI R. Thromboxane and prostacyclin release during cardiac immediate hypersensitivity reactions in vitro. J. Pharmacol. Exp. Therap. 1981;217:157–161. [PubMed] [Google Scholar]
- ARRIBAS S., MARIN J., PONTE A., BALFAGON G., SALAICES M. Norepinephrine-induced relaxations in rat aorta mediated by endothelial beta-adrenoceptors – impairment by aging and hypertension. J. Pharmacol. Exp. Therap. 1994;270:520–527. [PubMed] [Google Scholar]
- AVKIRAN M., CURTIS M.J. Independent dual perfusion of left and right coronary arteries in isolated rat hearts. Am. J. Physiol. 1991;261:H2082–H2090. doi: 10.1152/ajpheart.1991.261.6.H2082. [DOI] [PubMed] [Google Scholar]
- BAKER K.E., CURTIS M.J. Protection against ventricular fibrillation by the PAF antagonist, BN-50739, involves an ischaemia-selective mechanism. J. Cardiovasc. Pharmacol. 1999;34:394–401. doi: 10.1097/00005344-199909000-00012. [DOI] [PubMed] [Google Scholar]
- BERTI F., MAGNI F., ROSSONI G., DEANGELIS L., GALLI G. Production and biologic interactions of prostacyclin and platelet-activating-factor in acute myocardial-ischemia in the perfused rabbit heart. J. Cardiovasc. Pharmacol. 1990;16:727–732. doi: 10.1097/00005344-199011000-00006. [DOI] [PubMed] [Google Scholar]
- BOTTING J.H., JOHNSTON K.M., MACLEOD B.A., WALKER M.J.A. The effect of modification of sympathetic activity on responses to ligation of a coronary-artery in the conscious rat. Br. J. Pharmacol. 1983;79:265–271. doi: 10.1111/j.1476-5381.1983.tb10520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRITISH HEART FOUNDATION Age-sex specific death rates per 100,000 population from CHD, 1968–1999, United Kingdom. 2003.
- BUNTING S., GRYGLEWSKI R., MONCADA S., VANE J.R. Arterial walls generate from prostaglandin endoperoxides a substance which relaxes strips of mesenteric and coeliac arteries and inhibits platelet aggregation. Prostaglandins. 1976;12:897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- BUSSOLINO F., CAMUSSI G., AGLIETTA M., BRAQUET P., BOSIA A., PESCARMONA G., SANAVIO F., DURSO N., MARCHISIO P.C. Human-endothelial cells are target for platelet-activating-factor. 1 platelet-activating-factor induces changes in cytoskeleton structures. J. Immunol. 1987;139:2439–2446. [PubMed] [Google Scholar]
- CASCIO W.E., JOHNSON T.A., GETTES L.S. Electrophysiologic changes in ischemic ventricular myocardium. 1. Influence of ionic, metabolic, and energetic changes. J. Cardiovasc. Electrophysiol. 1995;6:1039–1062. doi: 10.1111/j.1540-8167.1995.tb00381.x. [DOI] [PubMed] [Google Scholar]
- CAST trial Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N. Engl. J. Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- CLEMENTS-JEWERY H., CURTIS M.J.Biochemical mediators of ventricular arrhythmias in ischaemic heart disease Cardiac Drug Development Guide 2003U.S.A.: The Humana Press Inc; 203–226.ed. Pugsley, M.K. Totowa, NJ [Google Scholar]
- CLEMENTS-JEWERY H., HEARSE D.J., CURTIS M.J. Independent contribution of catecholamines to arrhythmogenesis during evolving infarction in the isolated rat heart. Br. J. Pharmacol. 2002;135:807–815. doi: 10.1038/sj.bjp.0704509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COETZEE W.A., OPIE L.H. Effects of components of ischemia and metabolic inhibition on delayed afterdepolarizations in guinea-pig papillary-muscle. Circ. Res. 1987;61:157–165. doi: 10.1161/01.res.61.2.157. [DOI] [PubMed] [Google Scholar]
- CULLING W., PENNY W.J., LEWIS M.J., MIDDLETON K., SHERIDAN D.J. Effects of myocardial catecholamine depletion on cellular electrophysiology and arrhythmias during ischemia and reperfusion. Cardiovasc. Res. 1984;18:675–682. doi: 10.1093/cvr/18.11.675. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J. The rabbit dual coronary perfusion model – a new method for assessing the pathological relevance of individual products of the ischemic milieu – role of potassium in arrhythmogenesis. Cardiovasc. Res. 1991;25:1010–1022. doi: 10.1093/cvr/25.12.1010. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., HEARSE D.J. Ischaemia induced and reperfusion induced arrhythmias differ in their sensitivity to potassium: implications for mechanism of initiation and maintenance of ventricular fibrillation. J. Mol. Cel. Cardiol. 1989a;21:21–40. doi: 10.1016/0022-2828(89)91490-9. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., HEARSE D.J. Reperfusion induced arrhythmias are critically dependent upon occluded zone size: relevance to the mechanism of arrhythmogenesis. J. Mol. Cel. Cardiol. 1989b;21:625–637. doi: 10.1016/0022-2828(89)90828-6. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., PUGSLEY M.K., WALKER M.J.A. Endogenous chemical mediators of ventricular arrhythmias in ischaemic heart disease. Cardiovasc. Res. 1993;27:703–719. doi: 10.1093/cvr/27.5.703. [DOI] [PubMed] [Google Scholar]
- DAUGHERTY A., FRAYN K.N., REDFERN W.S., WOODWARD B. The role of catecholamines in the production of ischemia-induced ventricular arrhythmias in the rat in vivo and in vitro. Br. J. Pharmacol. 1986;87:265–277. doi: 10.1111/j.1476-5381.1986.tb10180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOEBBER T.W., WU M.S. Platelet-activating-factor (PAF) stimulates the PAF-synthesizing enzyme acetyl-CoA-1-alkyl-sn-glycero-3-phosphocholine O-2-acetyltransferase and PAF synthesis in neutrophils. Proc. Nat. Acad. Sci. U.S.A. 1987;84:7557–7561. doi: 10.1073/pnas.84.21.7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU X.J., WOODCOCK E.A., LITTLE P.J., ESLER M.D., DART A.M. Protection of neuronal uptake-1 inhibitors in ischemic and anoxic hearts by norepinephrine-dependent and independent mechanisms. J. Cardiovasc. Pharmacol. 1998;32:621–628. doi: 10.1097/00005344-199810000-00015. [DOI] [PubMed] [Google Scholar]
- ETTINGER P.O., REGAN T.J., OLDEWURTEL H.A., KHAN M.I. Ventricular conduction delay and arrhythmias during regional hyperkalaemia in the dog. Circ. Res. 1973;33:521–531. doi: 10.1161/01.res.33.5.521. [DOI] [PubMed] [Google Scholar]
- FARKAS A., CURTIS M.J. Does QT widening in the Langendorff-perfused rat heart represent the effect of repolarization delay or conduction slowing. J. Cardiovasc. Pharmacol. 2003;42:612–621. doi: 10.1097/00005344-200311000-00006. [DOI] [PubMed] [Google Scholar]
- FLORES N.A., SHERIDAN D.J. Electrophysiological and arrhythmogenic effects of PAF during normal perfusion, myocardial ischaemia and reperfusion in the guinea pig. Br.J. Pharmacol. 1990;101:734–738. doi: 10.1111/j.1476-5381.1990.tb14149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R.F., VANHOUTTE P.M. Endothelium derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- HELLER R., BUSSOLINO F., GHIGO D., GARBARINO G., PESCARMONA G., TILL U., BOSIA A. Human endothelial-cells are target for platelet-activating-factor 2. Platelet-activating-factor induces platelet-activating-factor synthesis in human umbilical vein endothelial-cells. J. Immunol. 1992;149:3682–3688. [PubMed] [Google Scholar]
- HIRCHE H.J., FRANZ C., BOS L., BISSIG R., LANG R., SCHRAMM M. Myocardial extracellular K+ and H+ increase and noradrenaline release as possible cause of early arrhythmias following acute coronary artery occlusion in pigs. J. Mol. Cell. Cardiol. 1980;12:579–593. doi: 10.1016/0022-2828(80)90016-4. [DOI] [PubMed] [Google Scholar]
- HOFFMAN B.F., GUO S.D., FEINMARK S.J. Arrhythmias caused by platelet activating factor. J. Cardiovasc. Electrophysiol. 1996;7:120–133. doi: 10.1111/j.1540-8167.1996.tb00507.x. [DOI] [PubMed] [Google Scholar]
- HU W.M., MAN R.Y.K. Differential actions of PAF receptor antagonists on the vasodilator and vasoconstrictor effects of PAF in the rat perfused heart. Br. J. Pharmacol. 1991;104:773–775. doi: 10.1111/j.1476-5381.1991.tb12504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWANG S.B., LAM M.H., HSU H.M. Characterisation of platelet activating (PAF) receptor by specific binding of [3H]-L-659,989, a PAF receptor antagonist to rabbit platelet membranes: possible multiple conformational states of a single type of PAF receptor. Mol. Pharmacol. 1988;35:48–58. [PubMed] [Google Scholar]
- HWANG S.B., LI C.L., LAM M.H., SHEN T.Y. Characterization of cutaneous vascular-permeability induced by platelet-activating factor in guinea-pigs and rats and its inhibition by a platelet-activating factor receptor antagonist. Lab. Investig. 1985;52:617–630. [PubMed] [Google Scholar]
- ISENBERG G., TRAUTWEIN W. Cardiac Purkinje fibres: caesium as a tool to block inward rectifying potassium currents. Pflugers Arch. 1974;365:99–106. doi: 10.1007/BF01067006. [DOI] [PubMed] [Google Scholar]
- ITO M., NARUSE A., YAMAMOTO I., OGURI M., SUZUKI Y., SATAKE N., SHIBATA S. Nephrosis augments contractile response to adrenoceptor agonists by the decrease in release of endothelium-derived relaxing factor from the endothelial-cells. J. Pharmacol. Exp. Therap. 1994;269:589–595. [PubMed] [Google Scholar]
- JANSE M.J. A brief history of sudden cardiac death and its therapy. Pharmacol. Therap. 2003;100:89–99. doi: 10.1016/s0163-7258(03)00104-9. [DOI] [PubMed] [Google Scholar]
- JANSE M.J., CAPELLE F.J.L., VAN MORSINK H., KLEBER A.G., WILMS-SCHOPMAN F., CARDINAL R., D'ALINONCOURT C.N., DURRER D. Flow of injury current and patterns of excitation during early ventricular arrhythmias in acute regional myocardial ischemia in isolated porcine and canine hearts. Circ. Res. 1980;44:576–588. doi: 10.1161/01.res.47.2.151. [DOI] [PubMed] [Google Scholar]
- KOLTAI M., TOSAKI A., HOSFORD D., ESANU A., BRAQUET P. Effect of bn-50739, a new platelet-activating-factor antagonist, on ischemia induced ventricular arrhythmias in isolated working rat hearts. Cardiovasc. Res. 1991;25:391–397. doi: 10.1093/cvr/25.5.391. [DOI] [PubMed] [Google Scholar]
- KURZ T., OFFNER B., SCHREIECK J., RICHARDT G., TOLG R., SCHÖMIG A. Nonexocytotic noradrenaline release and ventricular fibrillation in ischemic rat hearts. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:491–496. doi: 10.1007/BF00169382. [DOI] [PubMed] [Google Scholar]
- KUSUOKA H., HOFFMAN J.I.E. Advice on statistical analysis for circulation research. Circ. Res. 2002;91:662–671. doi: 10.1161/01.res.0000037427.73184.c1. [DOI] [PubMed] [Google Scholar]
- MANNING A.S., KINOSHITA K., BUSCHMANS E., COLTART D.J., HEARSE D.J. The genesis of arrhythmias during myocardial ischemia – dissociation between changes in cyclic adenosine-monophosphate and electrical instability in the rat. Circ. Res. 1985;57:668–675. doi: 10.1161/01.res.57.5.668. [DOI] [PubMed] [Google Scholar]
- MAXWELL M.P., HEARSE D.J., YELLON D.M. Species variation in the coronary circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc. Res. 1987;21:737–746. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- MAZENOT C., RIBUOT C., DURAND R., JOULIN Y., DEMENGE P., GODIN-RIBUOT D. In vivo demonstration of H-3-histaminergic inhibition of cardiac sympathetic stimulation by R-alpha-methyl-histamine and its prodrug BP 2.94 in the dog. Br. J. Pharmacol. 1999;126:264–268. doi: 10.1038/sj.bjp.0702257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORENA H., JANSE M.J., FIOLET J.W.T., KRIEGER W.J.G., CRIJNS H., DURRER D. Comparison of the effects of regional ischaemia, hypoxia, hyperkalaemia, and acidosis on intracellular and extracellular potentials and metabolism in the isolated porcine heart. Circ. Res. 1980;46:624–646. doi: 10.1161/01.res.46.5.634. [DOI] [PubMed] [Google Scholar]
- PELLEG A., MITAMURA H., PRICE R., KAPLINSKY E., MENDUKE H., DREIFUS L.S., MICHELSON E.L. Extracellular potassium-ion dynamics and ventricular arrhythmias in the canine heart. J. Am. College Cardiol. 1989;13:941–950. doi: 10.1016/0735-1097(89)90240-4. [DOI] [PubMed] [Google Scholar]
- PIPER P.J., STEWART A.G. Coronary vasoconstriction in the rat, isolated perfused heart induced by PAF is mediated by LTC4. Br. J. Pharmacol. 1986;88:595–605. doi: 10.1111/j.1476-5381.1986.tb10240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PREDESCU D., IHIDA K., PREDESCU S., PALADE G.E. The vascular distribution of the platelet activating factor receptor. Eur. J. Cell Biol. 1996;69:86–98. [PubMed] [Google Scholar]
- REES S.A., CURTIS M.J. Selective I(k) blockade as an antiarrhythmic mechanism – effects of UK66,914 on ischemia and reperfusion arrhythmias in rat and rabbit hearts. Br. J. Pharmacol. 1993a;108:139–145. doi: 10.1111/j.1476-5381.1993.tb13453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES S., CURTIS M.J. Tacrine inhibits ventricular fibrillation induced by ischaemia and reperfusion and widens QT interval in rat. Cardiovasc. Res. 1993b;27:453–458. doi: 10.1093/cvr/27.3.453. [DOI] [PubMed] [Google Scholar]
- REES S.A., CURTIS M.J. Specific IK1 blockade, a new antiarrhythmic mechanism. Effect of RP58866 on ventricular arrhythmias in rat, rabbit and primate. Circulation. 1993c;87:1979–1989. doi: 10.1161/01.cir.87.6.1979. [DOI] [PubMed] [Google Scholar]
- REES S.A., CURTIS M.J. Further investigations into the mechanism of antifibrillatory action of the specific IK1 blocker, RP58866, assessed using the rat dual coronary perfusion model. J. Mol. Cell. Cardiol. 1995a;27:2595–2606. doi: 10.1006/jmcc.1995.0046. [DOI] [PubMed] [Google Scholar]
- REES S.A., CURTIS M.J. Pharmacological analysis in rat of the role of the ATP-sensitive potassium channel as a potential target for antifibrillatory intervention in acute myocardial ischaemia. J. Cardiovasc. Pharmacol. 1995b;26:280–288. doi: 10.1097/00005344-199508000-00014. [DOI] [PubMed] [Google Scholar]
- REES S.A., TSUCHIHASHI K., HEARSE D.J., CURTIS M.J. Combined administration of an IK(ATP) activator and Ito blocker increase coronary flow independently of effects on heart rate, QT interval, and ischaemia induced ventricular fibrillation in rats. J. Cardiovasc. Pharmacol. 1993;22:343–349. doi: 10.1097/00005344-199309000-00001. [DOI] [PubMed] [Google Scholar]
- RIDLEY P.H., YACOUB M.H., CURTIS M.J. A modified model of global ischaemia: application to the study of syncytial mechanisms of arrhythmogenesis. Cardiovasc. Res. 1992;26:313–323. doi: 10.1093/cvr/26.4.309. [DOI] [PubMed] [Google Scholar]
- SAUSBIER M., SCHUBERT R., VOIGT V., HIRNEISS C., PFEIFER A., KORTH M., KLEPPISCH T., RUTH P., HOFMANN F. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ. Res. 2000;87:825–830. doi: 10.1161/01.res.87.9.825. [DOI] [PubMed] [Google Scholar]
- SHERIDAN D.J., PENKOSKE P.A., SOBEL B.E., CORR P.B. Alpha adrenergic contributions to dysrhythmia during myocardial ischaemia and reperfusion in cats. J. Clin. Investig. 1980;65:161–171. doi: 10.1172/JCI109647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH B.N., NADEMANEE K. Control of cardiac arrhythmias by selective lengthening of repolarisation: theoretic considerations and clinical observations. Am. Heart J. 1985;109:421–430. doi: 10.1016/0002-8703(85)90629-5. [DOI] [PubMed] [Google Scholar]
- SUGIMOTO T., TSUCHINOCHI H., MCGREGOR C.G., MUTOH H., SHIMIZU T., KURACHI D.Y. Molecular cloning and characterisation of the platelet activating factor receptor gene expressed in the human heart. Biochem. Biophys. Res. Commun. 1992;189:617–624. doi: 10.1016/0006-291x(92)92245-s. [DOI] [PubMed] [Google Scholar]
- TSUCHIHASHI K., CURTIS M.J. Influence of tedisamil on the initiation and maintenance of ventricular fibrillation: chemical defibrillation by Ito blockade. J. Cardiovasc. Pharmacol. 1991;18:455–456. doi: 10.1097/00005344-199109000-00018. [DOI] [PubMed] [Google Scholar]
- VANE J.R., ANGGARD E.E., BOTTING R.M. Regulatory functions of the vascular endothelium. N. Eng. J. Med. 1990;323:27–35. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- WAHLER G.M., COYLE D.E., SPERELAKIS N. Effects of platelet-activating factor on single potassium channel currents in guinea-pig ventricular myocytes. Mol. Cell. Biochem. 1990;93:69–76. doi: 10.1007/BF00223494. [DOI] [PubMed] [Google Scholar]
- WALDO A.L., CAMM A.J., DERUYTER H., FRIEDMAN P.L., MACNEIL D.J., PAULS J.F., PITT B., PRATT C.M., SCHWARTZ P.J., VELTRI E.P. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival with oral d-sotalol. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- WALKER M.J.A., CURTIS M.J., HEARSE D.J., CAMPBELL R.W.F., JANSE M.J., YELLON D.M., COBBE S.M., COKER S.J., HARNESS J.B., HARRON D.W.G., HIGGINS A.J., JULIAN D.G., LAB M.J., MANNING A.S., NORTHOVER B.J., PARRATT J.R., RIEMERSMA R.A., RIVA E., RUSSELL D.C., SHERIDAN D.J., WINSLOW E., WOODWARD B. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovasc. Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- WANG H., TAN X.D., CHANG H., GONZALEZCRUSSI F., REMICK D.G., HSUEH W. Regulation of platelet-activating factor receptor gene expression in vivo by endotoxin, platelet-activating factor and endogenous tumour necrosis factor. Biochemi. J. 1997;322:603–608. doi: 10.1042/bj3220603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIT A.L., ROSEN M.R.Afterdepolarizations and triggered activity: distinction from automaticity as an arrhythmogenic mechanism The Heart and Cardiovascular System 1992U.S.A.: Scientific Foundations, Raven Press; 2113–2164.In: Fozzard, H.A., Haber, E., Jennings, R.B. et al. eds1st edn [Google Scholar]