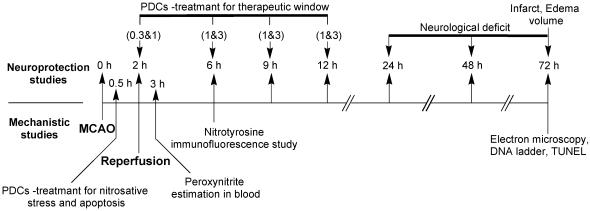
Figure 2.
Study design for neuroprotective efficacy, therapeutic time window and mechanism of PDCs action: For neuroprotective efficacy and therapeutic time window, FeTMPyP and FeTPPS were tested at two dose levels (either 0.3 and 1 or 1 and 3 mg kg−1) at 2, 6, 9, 12 h post MCAO along with a vehicle-treated control, which accounted for a total of 17 different groups of rats. The numbers mentioned in parenthesis are the doses (mg kg−1). The times scheme, at which FeTMPyP and FeTPPS treatments were done after MCAO for different groups of animals, is indicated by their doses in parenthesis. Rats were killed 70 h post MCAO and extent of neurological damage was measured. For mechanistic studies PDCs were administered 30 min post MCAO. Peroxynitrite levels were estimated (dihydrorhodamine123 method) 3 h post MCAO and nitrotyrosine immunofluorescence analysis was performed 6 h post MCAO. DNA ladder, TUNEL and electron microscopy were conducted 72 h post MCAO.