Figure 1.
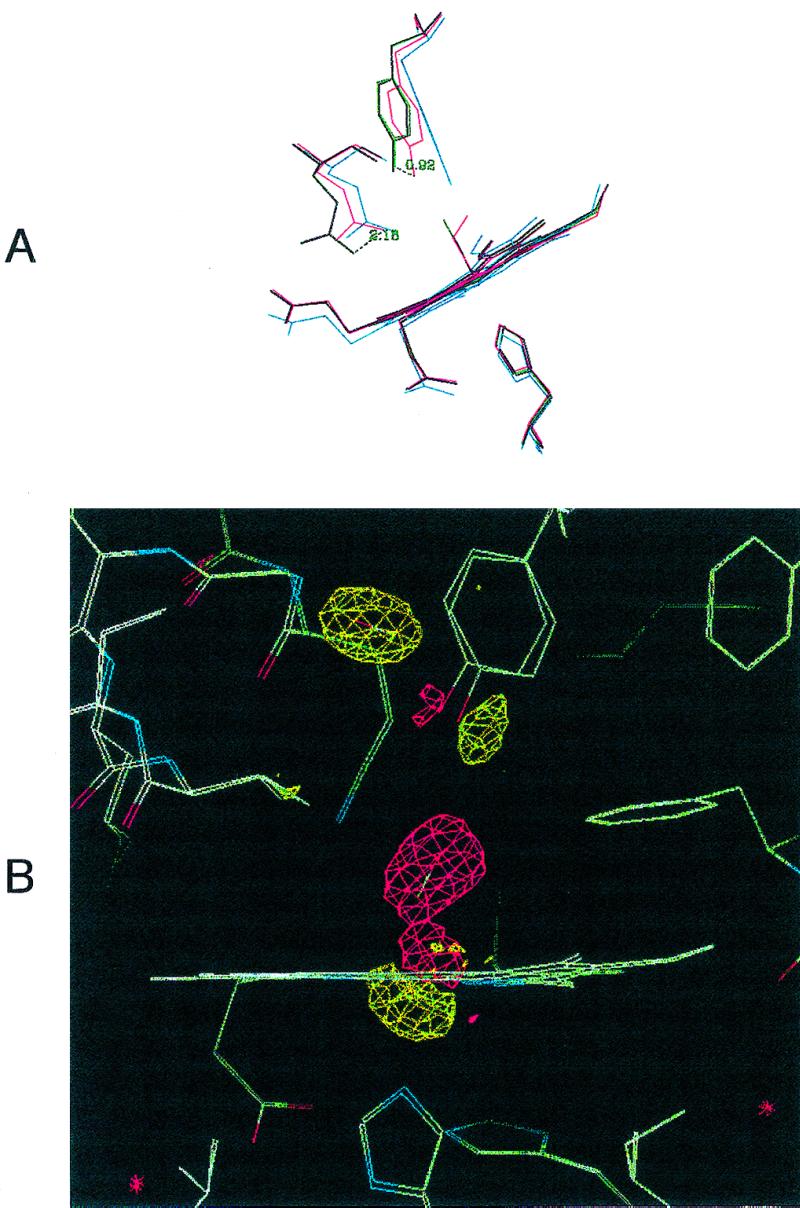
(A) Distal site pocket in Mb-YQR. The heme, its ligands (O2 and CO), and residues Tyr(B10)29, Gln(E7)64, and His(F8)93 are shown in the following ligation states: deoxygenated, blue; oxygenated, red; carbonmonoxy, purple; CO* photolyzed at 20 K, green. The displacements of the hydroxyl group of Tyr(B10)29 and the amino group of Gln(E7)64, respectively, in going from the oxygenated to the carbonmonoxy state, are given in Å. It also may be noted that the side chain of Tyr(B10)29 slightly “bounces” toward the outside of the pocket in the photolyzed state with respect to the CO-bound state. (B) Electron density maps in the heme region calculated by using the measured structure factor amplitudes for Mb-YQR⋯CO* at 20 K minus the ones from Mb-YQR-CO bound. The map is contoured at 3-σ electron density. Shown are the bound CO of Mb-YQR-CO in a negative density region (red) and CO* clearly positioned in an elongated sphere of positive density (yellow). In addition, the position of other residues in the structure of the CO bound and the photolyzed states change: the iron moves out the porphyrin plane, and the proximal His(F8)93 (Lower) and Tyr(B10)29 (Upper) shift.
