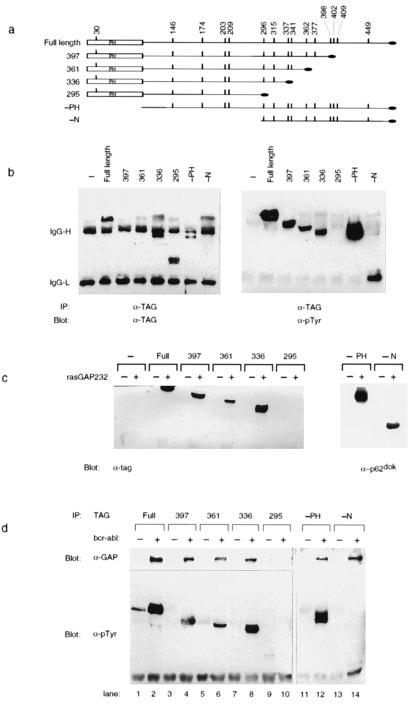
Figure 3.
Removal of N- and C-terminal residues from p62dok does not prevent its in vitro and in vivo association with RasGAP. (a) Schematic diagram of p62dok truncations. Four C-terminal Dok-1 truncations were constructed. In each case, a different tyrosine (residues 398, 362, 337, and 296) and all amino acids C terminal to the selected tyrosine were replaced with a C-terminal antigenic tag (RYIRS, black oval). Additionally, two N-terminal truncations were constructed, lacking either the PH domain (−PH) or the entire N-terminal region (−N). The vertical bars mark the location of the tyrosines within p62dok. The PH domain is indicated (PH). (b) Tyrosine phosphorylation of p62dok truncations. The indicated Dok-1 truncations and p210bcr-abl were expressed in 293 cells. Antibody directed against the C-terminal tag was used to precipitate Dok-1 proteins. Immune complexes were analyzed by immunoblotting by using antibodies against the C-terminal tag (TAG, Left) or phosphotyrosine (pTyr, Right). Bands derived from IgG are indicated as IgG-H and IgG-L. The only Dok-1 mutant to lack detectable tyrosine phosphorylation in the presence of p210bcr-abl was that which lacked all 10 C-terminal tyrosines (295). (c) Removal of N-terminal and C-terminal residues from p62dok does not prevent its in vitro association with RasGAP. The indicated Dok-1 truncations and Bcr-Abl were expressed in 293 cells. Cell lysates were prepared and utilized in an in vitro binding reaction to glutathione-Sepharose beads to which were bound GST (−) or GST-GAP SH2-SH3-SH2 region. Bound proteins were separated by SDS/PAGE, blotted to nitrocellulose, and detected by immunoblotting with antibodies to the C-terminal tag (Left) or to p62dok (Right). The only Dok-1 mutant unable to associate with GAP was that which lacked all 10 C-terminal tyrosines (295). (d) Removal of N-terminal and C-terminal residues from p62dok does not prevent its in vivo association with RasGAP. Dok-1 truncations and p210bcr-abl were expressed in 293 cells as described above.