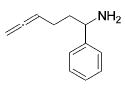
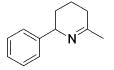
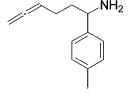
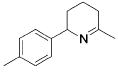
Table 3.
Hydroamination of Aryl-Substituted Aminoallenes (5 Mol % of Catalyst in C6D6, 75°C)
| isolated yielda |
||||||
|---|---|---|---|---|---|---|
| entry | substrate | major product | cat. | t / h | imine | allyamine |
| 1 |
|
|
3 | 22 | (95) | (5) |
| 2 | 13 | 1 | 84 | --- | ||
| 3 |
|
|
3 | 9 | (90) | (10) |
| 4 | 13 | 5 | 79 | --- | ||
| 5 |
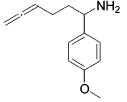
|
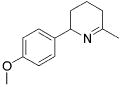
|
3 | 4 | (92) | (8) |
| 6 | 13 | 1.5 | 95 | --- | ||
| 7 |
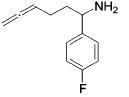
|
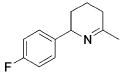
|
13 | 10 | 93 | --- |
| 8 |
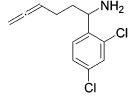
|
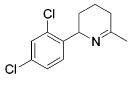
|
13 | 2 | 88 | --- |
| 9 |
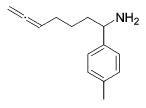
|
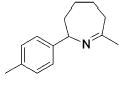
|
13b | 3 | (93) | (7) |
| 10 |
|
--- | 13b | 24 | --- | --- |
Yields in parentheses refer to ratio of imine/allylamine by NMR after complete and selective conversion of the starting material (versus internal standard)
10 mol %.
