Abstract
A novel muscarinic receptor antagonist, solifenacin succinate, inhibited specific binding of [N-methyl-3H]-scopolamine ([3H]-NMS) in the mouse bladder, submaxillary gland and heart in a concentration-dependent manner. This inhibitory effect was greatest in the submaxillary gland, followed by the bladder and heart.
After oral administration of oxybutynin (76.1 μmol kg−1) or solifenacin (62.4, 208 μmol kg−1), a significant dose- and time-dependent increase in KD values for specific [3H]-NMS binding was seen in the bladder, prostate, submaxillary gland, heart, colon and lung, compared with control values. The increase in KD induced by oxybutynin in each tissue reached a maximum 0.5 h after oral administration and then rapidly declined, while that induced by solifenacin was greatest 2 h after administration and was maintained for at least 6 or 12 h, depending on the dose. The muscarinic receptor binding of oral solifenacin was slower in onset and of a longer duration than that of oxybutynin.
Plasma concentrations of oxybutynin and its active metabolite (N-desethyl-oxybutynin, DEOB) were maximum 0.5 h after its oral administration and then declined rapidly. Oral solifenacin persisted in the blood for longer than oxybutynin.
Pilocarpine-induced salivary secretion in mice was significantly reduced by oral administration of solifenacin and was completely abolished 0.5 h after oral oxybutynin. Although the suppression induced by solifenacin was more persistent than that due to oxybutynin, the antagonistic effect of solifenacin on the dose–response curves to pilocarpine was significantly weaker than that of oxybutynin. It is concluded that oral solifenacin persistently binds to muscarinic receptors in tissues expressing the M3 subtype, such as the bladder.
Keywords: Bladder, submaxillary gland, oxybutynin, solifenacin, muscarinic receptor binding, plasma concentration, salivary secretion
Introduction
An overactive bladder is characterized by symptoms of increased frequency of micturition, urgency and urge incontinence (Bulmer & Abrams, 2000; Abrams et al., 2002). Muscarinic receptor antagonists such as oxybutynin have been used for some time to treat overactive bladder (Andersson, 1988; Wein, 1990). However, the oral use of oxybutynin is often limited by frequent and serious systemic side effects such as dry mouth, blurred vision, constipation and tachycardia (Yarker et al., 1995). To reduce or even eliminate this problem, novel antimuscarinic agents that exhibit pharmacological selectivity in the bladder relative to other tissues such as the salivary gland have been developed (Nilvebrant et al., 1997; Abrams et al., 1998; Anderson et al., 1999; Gupta & Sathyan, 1999; Chapple, 2000).
Muscarinic cholinoceptors have been classified into five subtypes (M1–M5) based on genetic and pharmacological characteristics (Hulme et al., 1990; Caulfield, 1993). Although both M2 and M3 subtypes coexist in smooth muscle, the M2 subtype predominates but functional in vitro data with a number of selective antagonists indicate that the contraction of most smooth muscle, including the urinary bladder, is mediated by the M3 subtype (Caulfield, 1993; Eglen et al., 1994). On the basis of these results, muscarinic receptor antagonists with a higher affinity for M3 than M2 subtypes should be more beneficial in the treatment of overactive bladder.
Solifenacine succinate (YM905; [(+)-(1S,3′R)-quinuclidin-3′-yl-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylate monosuccinate]) is a novel muscarinic receptor antagonist developed for the treatment of urinary incontinence and other symptoms of overactive bladder (Ikeda et al., 2002; Kobayashi et al., 2004). In vitro radioligand studies with human recombinant muscarinic subtypes have revealed that solifenacin exhibits high affinity and specificity for the muscarinic M3 subtype relative to the M1 and M2 subtypes; the affinity of solifenacin for these subtypes is 6–10 times less than that of oxybutynin (Ikeda et al., 2002). Solifenacin has been found to be equipotent with oxybutynin at inhibiting carbachol-stimulated Ca2+ mobilization in detrusor cells, but less potent in submandibular gland cells (Ikeda et al., 2002; Kobayashi et al., 2004). In vivo studies in anaesthetized rats have shown that solifenacin, unlike oxybutynin, is more potent at inhibiting bladder contraction than salivation (Ikeda et al., 2002; Ohtake et al., 2004).
A number of authors have indicated the importance of characterizing the binding of ligands to receptors in the presence of various pharmacokinetic and pharmacodynamic factors (Beauchamp et al., 1995; Uchida et al., 1995; Ohkura et al., 1998; Yamada et al., 2001; 2002; 2003). The aim of the present study was to characterize the in vivo muscarinic receptor binding properties of solifenacin (after oral administration) in the bladder and submaxillary gland, compare them with those of oxybutynin and relate them to their plasma levels and inhibitory effects on salivary secretion.
Methods
Animals
Male ddY strain mice aged 11 to 16 weeks (Japan SLC Inc., Shizuoka, Japan) were used. They were housed under a 12-h light–dark cycle and fed laboratory chow and water ad libitum.
Administration of oxybutynin and solifenacin
Mice were fasted for 16 h, then orally administered oxybutynin (76.1 μmol kg−1) or solifenacin (62.4 or 208 μmol kg−1) dissolved in distilled water. Control animals received vehicle alone. The study was conducted in accordance with the guidelines of the Experimental Animal Ethical Committee of the University of Shizuoka.
Tissue preparation
At 0.5 to 24 h after drug administration, the mice were anaesthetized with diethyl ether and exsanguinated by taking the blood from the descending aorta. The tissues were then perfused with cold saline via the aorta. The bladder, prostate, submaxillary gland, heart, lung and colon were dissected out, and any fat and blood vessels removed. As the individual organs did not weigh very much, samples from three mice were pooled for a determination of the results. The tissues were minced with scissors and homogenized with a Kinematica Polytron homogenizer in 19 volumes of ice-cold 30 mM Na+/HEPES buffer (pH 7.5). The homogenates were then centrifuged at 40,000 × g for 20 min and the resulting pellet was suspended in ice-cold buffer for the binding assay. In the ex vivo experiments, it is possible that oxybutynin and solifenacin can dissociate from the receptor sites during tissue preparation (homogenization and suspension). Yamada et al. (1980) have shown that such dissociation is extremely slow at 4°C, hence all preparation was conducted at this temperature. Protein concentrations were measured according to the method of Lowry et al. (1951). Mouse plasma was isolated from blood by centrifugation and stored at −80°C until analysis.
Muscarinic receptor binding assay
The binding assay for muscarinic receptors was performed using [N-methyl-3H]-scopolamine methyl chloride ([3H]-NMS) as previously described (Ehlert & Tran, 1990; Oki et al., 2004). The mouse tissue homogenates (60–860 μg protein) were incubated with different concentrations (0.06–1.0 nM) of [3H]-NMS in 30 mM Na+/HEPES buffer (pH 7.5) for 60 min at 25°C. The reaction was terminated by rapid filtration (Cell Harvester, Brandel Co., Gaithersburg, MD, U.S.A.) through Whatman GF/B glass fibre filters, and the filters were then rinsed three times with 3 ml of ice-cold buffer. Tissue-bound radioactivity was extracted from the filters by overnight immersion in scintillation fluid (2 1 toluene, 1 1 Triton X-100, 15 g 2,5-diphenyloxazole, 0.3 g 1,4-bis[2-(5-phenyloxazolyl)]benzene), and radioactivity was determined with a liquid scintillation counter. Specific [3H]-NMS binding was determined as the difference between counts in the absence and presence of 1 μM atropine. All assays were performed in duplicate.
Measurement of plasma concentrations
Concentrations of oxybutynin and its active metabolite (N-desethyl-oxybutynin: DEOB) in mouse plasma were determined by gas chromatography and mass spectrometry (GC/MS) as described previously (Oki et al., 2004). A plasma sample (0.1–0.5 ml) was mixed with internal standard ([2H13]-oxybutynin·HCl and [2H13]-DEOB·HCl) and, after alkalinization with 0.5 ml of 0.5 M carbonate buffer (pH 9.5), extracted with 6 ml of n-hexane. After centrifugation at 1500 × g for 5 min, the supernatant was evaporated to dryness under reduced pressure. The residue was dissolved in 100 μl of CH3CN, and 0.5–1 μl was injected into a GC/MS system consisting of a 5890 Series II gas chromatograph, a 5792 Series mass selective detector, a 7673 GC/SFC injector, a VECTRA 486/66U computer and a LaserJet 4 printer (Hewlett Packard Co.). Chromatographic separation was carried out using a 15 m × 0.25 mm i.d. × 0.25 μm film UA+-1 HT (Frontier Lab Ltd.). The carrier gas was helium at a flow rate of 1.0 ml min−1. Oven temperature was held at 150°C for 1 min, then programmed from 150 to 220°C at 20°C min−1 for the first ramp, from 220 to 260°C at 10°C min−1 for the second ramp, and from 260 to 300°C at 30°C min−1 for the third ramp. It was held at 300°C for 2 min and then returned to the initial starting temperature of 150°C. The injection temperature was 200°C. Fragmentation was accomplished by electron impact at 70 eV ionizing voltage and 300 μA ionizing current. Selected ion monitoring was performed at m/z 342 (oxybutynin), m/z 355 (internal standard: [2H13]-oxybutynin), m/z 189 (DEOB) and m/z 200 (internal standard: [2H13]-DEOB). The limits of detection of oxybutynin and DEOB in plasma were 1.40 and 3.04 nM, respectively.
The concentration of solifenacin in mouse plasma was determined by a validated high-performance liquid chromatographic (HPLC) method. Briefly, solifenacin was extracted from mouse plasma by a two-step liquid–liquid extraction and analysed by semi-micro HPLC with UV detection. The lower limit of detection was 20.8 nM.
Measurement of salivary secretion
Mice were anaesthetized with pentobarbitone (161 μmol kg−1, intraperitoneally (i.p.)). Any saliva remaining in the oral cavity was removed with a cotton ball before measuring the total saliva collected in the cavity for a 10-min period. The saliva was absorbed onto three to five cotton balls for 10 min and the balls were weighed on an electric balance immediately after the collection period to prevent moisture loss. To examine the effects of oral administration of oxybutynin and solifenacin on pilocarpine-induced salivary secretion, pilocarpine (4.09 μmol kg−1, dissolved in physiological saline) was administered (intravenous, i.v.) 0.5, 2, 6, 12 and 24 h after oral administration of the drugs, and saliva was collected for 10 min. The effect of oral administration of oxybutynin and solifenacin on total salivary secretion induced by cumulative doses (0.41–40.9 μmol kg−1, i.v.) of pilocarpine given at 5-min intervals was also measured.
Data analysis
Analysis of [3H]-NMS binding data was performed as described previously (Yamada et al., 1980). The apparent dissociation constant (KD) and maximal number of binding sites (Bmax) for [3H]-NMS were obtained by Rosenthal analysis of the saturation data. The ability of muscarinic receptor antagonists to inhibit specific [3H]-NMS binding was determined from the IC50 values, namely the molar concentration of unlabelled drug needed to displace 50% of the specific binding of [3H]-NMS (determined by log probit analysis). The inhibition constant, Ki, was calculated from the equation Ki=IC50/(1+L/KD), where L is the concentration of [3H]-NMS (0.13 nM), and pKi (−log Ki) values determined. Also, Hill slopes for the inhibition by muscarinic receptor antagonists were calculated.
For the analysis of salivary secretion data, pKB values were calculated using the equation pKB=log (CR−1)−log [antagonist], where CR is the ratio of ED50 values (the dose producing 50% of the maximum response) of the agonist with and without antagonist (van Rossum et al., 1963).
Statistical analysis of the data was performed by one-way analysis of variance (ANOVA) followed by Dunnett's test for multiple comparisons. A value of P<0.05 was considered significant.
Materials
[3H]-NMS (3.03 TBq mmol−1) was purchased from Perkin-Elmer Life Sciences, Inc. (Boston, MA, U.S.A.). Solifenacin succinate (YM905) was donated by Yamanouchi Pharmaceutical Co. Ltd (Tsukuba, Japan). Oxybutynin hydrochloride and its active metabolite (DEOB) were donated by Meiji Milk Products Co. Ltd (Odawara, Japan). All other chemicals were purchased from commercial sources.
Results
In vitro effects of oxybutynin, DEOB and solifenacin on muscarinic receptors in mouse tissues
Oxybutynin (1–1000 nM) and DEOB (0.3–100 nM) inhibited specific [3H]-NMS binding in the bladder, submaxillary gland and heart in a concentration-dependent manner in vitro. Respective pKi values for oxybutynin in these tissues were 7.80, 8.22 and 7.39, while those for DEOB were 8.30, 9.00 and 7.80 (Table 1). The inhibitory potencies for DEOB in the bladder, submaxillary gland and heart were thus significantly (P<0.01) higher (3.1, 6.0 and 2.6 times, respectively) than those obtained for oxybutynin.
Table 1.
pKi values for in vitro inhibition by oxybutynin, DEOB and solifenacin of specific [3H]-NMS binding in the bladder, submaxillary gland and heart of mice
| Drug | pKi value | ||
|---|---|---|---|
| Bladder | Submaxillary gland | Heart | |
| Oxybutynin | 7.80±0.03†† | 8.22±0.03**†† | 7.39±0.06**†† |
| (0.98±0.03) | (0.87±0.07) | (1.03±0.05) | |
| DEOB | 8.30±0.08†† | 9.00±0.05**†† | 7.80±0.02**†† |
| (0.84±0.09) | (0.78±0.04) | (1.08±0.03) | |
| Solifenacin | 7.38±0.03 | 7.89±0.03** | 7.00±0.03** |
| (0.95±0.03) | (0.90±0.02) | (1.00±0.06) |
Values are mean±s.e.m. of three or four mice. Values in parentheses represent Hill slopes. Asterisks show a significant difference from values in the bladder,
P<0.01. Daggers show a significant difference from the values with solifenacin,
P<0.01.
Solifenacin (3–1000 nM) inhibited specific [3H]-NMS binding in the bladder, submaxillary gland and heart, and the associated pKi values were 7.38, 7.89 and 7.00, respectively (Table 1). The inhibitory effect of solifenacin was significantly lower (1/3.2) in the submaxillary gland and higher (2.4 times) in the heart than in the bladder. Further, the inhibitory effects of solifenacin in the bladder, submaxillary gland and heart were significantly lower (1/2.6, 1/2.1 and 1/2.4 respectively) than those of oxybutynin.
The Hill slopes for oxybutynin, DEOB and solifenacin in these tissues were close to unity except that for DEOB in the submaxillary gland which was 0.78.
Effects of oral administration of oxybutynin and solifenacin on muscarinic receptors in mouse tissues
At 0.5 and/or 2 h after oral administration of oxybutynin (76.1 μmol kg−1), there was a significant increase in the KD value for specific [3H]-NMS binding in the bladder, prostate, submaxillary gland, heart, lung and colon compared with the corresponding control values (Table 2). The increases were maximal after 0.5 h being 54.5, 220, 668, 91.7, 391 and 269%, respectively, while those at 2 h were 44.3, 218, 215, 16.9, 115 and 109%, respectively. The increase at 0.5 h was least in the bladder and most in the submaxillary gland. The KD in each tissue was not significantly different from the control value at 6, 12 and 24 h. Oxybutynin had no significant effect on Bmax values for specific [3H]-NMS binding in any of the tissues.
Table 2.
KD and Bmax for specific [3H]-NMS binding in the bladder, prostate, submaxillary gland, heart, lung and colon of mice 0.5–24 h after oral administration of oxybutynin (76.1 μmol kg−1)
| Organ | Time after oraloxybutynin (h) | KD (pM) | Bmax (fmol mg protein−1) |
|---|---|---|---|
| Bladder | Control | 176±4 | 152±8 |
| 0.5 | 272±24 (1.55)** | 120±12 | |
| 2 | 254±27 (1.44)** | 141±8 | |
| 6 | 221±5 | 138±14 | |
| 12 | 190±10 | 139±14 | |
| 24 | 178±8 | 135±5 | |
| Prostate | Control | 124±3 | 160±17 |
| 0.5 | 397±53 (3.20)*** | 120±25 | |
| 2 | 394±78 (3.18)*** | 159±30 | |
| 6 | 246±33 | 137±14 | |
| 12 | 182±21 | 151±24 | |
| 24 | 129±5 | 166±20 | |
| Submaxillary gland | Control | 111±2 | 141±8 |
| 0.5 | 852±149 (7.68)*** | 140±6 | |
| 2 | 350±17 (3.15)* | 119±10 | |
| 6 | 181±14 | 166±17 | |
| 12 | 138±4 | 146±5 | |
| 24 | 113±1 | 154±8 | |
| Heart | Control | 278±12 | 43.1±1.1 |
| 0.5 | 533±67 (1.92)*** | 46.2±0.8 | |
| 2 | 325±13 | 47.9±2.8 | |
| 6 | 315±8 | 47.8±1.1 | |
| 12 | 276±10 | 40.4±1.5 | |
| 24 | 257±9 | 44.8±3.2 | |
| Lung | Control | 185±10 | 84.2±4.9 |
| 0.5 | 909±153 (4.91)*** | 84.1±15.3 | |
| 2 | 398±29 (2.15)* | 89.2±5.3 | |
| 6 | 273±13 | 81.0±4.6 | |
| 12 | 202±6 | 102±10 | |
| 24 | 186±5 | 110±5 | |
| Colon | Control | 170±3 | 150±7 |
| 0.5 | 628±100 (3.69)*** | 165±18 | |
| 2 | 355±25 (2.09)* | 143±9 | |
| 6 | 321±35 | 162±8 | |
| 12 | 234±19 | 169±6 | |
| 24 | 182±8 | 160±5 | |
Rosenthal analysis was performed with [3H]-NMS (0.06–1.0 nM) binding in mouse tissues after oral oxybutynin (76.1 μmol kg−1). Values are mean±s.e.m. of three to five mice. Values in parentheses represent the fold-increase in KD values relative to controls. Asterisks show a significant difference from the control values,
P<0.05,
P<0.01,
P<0.001.
Oral administration of solifenacin (62.4 μmol kg−1) significantly increased the KD value for specific [3H]-NMS binding in all the tissues when compared with the corresponding control values (Table 3). These effects were significant in the heart (24.0 and 25.1%) and lung (244 and 182%) at 0.5 and 2 h, in the bladder (18.2–29.0%) and submaxillary gland (106–363%) at 0.5 to 6 h, and in the prostate (72.7–120%) and colon (83.6–85.6%) at 0.5 to 12 h. In addition, the maximum effect in each tissue was seen at 0.5, 2 h or both. Further, oral administration of solifenacin at an approximately three-fold higher dose (208 μmol kg−1) further increased the KD values for [3H]-NMS binding in each tissue (Table 4), with increases being significant in the heart (56.5 and 47.0%, respectively) at 0.5 and 2 h, and in the bladder (22.2–49.4%), prostate (166–320%), submaxillary gland (234–613%) and colon (116–131%) at 0.5 to 12 h. Solifenacin had no significant effect on the Bmax value for any of the tissues, except in the heart where a significant (38.1%) increase occurred at 2 h (at the dose of 208 μmol kg−1).
Table 3.
KD and Bmax for specific [3H]-NMS binding in the bladder, prostate, submaxillary gland, heart, lung and colon of mice 0.5–24 h after oral administration of solifenacin (62.4 μmol kg−1)
| Organ | Time after oralsolifenacin (h) | KD (pM) | Bmax (fmol mg protein−1) |
|---|---|---|---|
| Bladder | Control | 176±6 | 169±22 |
| 0.5 | 208±9 (1.18)* | 156±25 | |
| 2 | 227±6 (1.29)*** | 139±17 | |
| 6 | 208±11(1.18)* | 126±10 | |
| 12 | 188±7 | 123±26 | |
| 24 | 182±4 | 123±18 | |
| Prostate | Control | 110±4 | 170±23 |
| 0.5 | 217±34 (1.97)*** | 221±33 | |
| 2 | 242±8 (2.20)*** | 191±23 | |
| 6 | 203±3 (1.85)*** | 203±8 | |
| 12 | 190±14 (1.73)*** | 219±24 | |
| 24 | 129±2 | 186±9 | |
| Submaxillary gland | Control | 96.7±3.1 | 122±9 |
| 0.5 | 233±35 (2.41)*** | 116±13 | |
| 2 | 448±36 (4.63)*** | 116±8 | |
| 6 | 199±12 (2.06)** | 115±14 | |
| 12 | 145±16 | 109±4 | |
| 24 | 96.6±0.8 | 112±8 | |
| Heart | Control | 283±12 | 35.4±0.9 |
| 0.5 | 351±11 (1.24)** | 43.2±4.6 | |
| 2 | 354±9 (1.25)** | 39.9±4.2 | |
| 6 | 305±12 | 46.1±3.7 | |
| 12 | 301±15 | 44.1±3.1 | |
| 24 | 288±11 | 44.2±2.8 | |
| Lung | Control | 177±7 | 80.4±3.1 |
| 0.5 | 608±86 (3.44)*** | 80.9±6.9 | |
| 2 | 500±41 (2.82)*** | 80.8±15.0 | |
| 6 | 274±7 | 69.1±4.3 | |
| 12 | 225±24 | 66.5±3.8 | |
| 24 | 186±6 | 60.5±0.8 | |
| Colon | Control | 146±12 | 131±14 |
| 0.5 | 271±34 (1.86)*** | 121±9 | |
| 2 | 268±12 (1.84)*** | 150±18 | |
| 6 | 270±10 (1.85)*** | 123±8 | |
| 12 | 271±10 (1.86)*** | 139±10 | |
| 24 | 162±7 | 141±4 | |
Rosenthal analysis was performed with [3H]-NMS (0.06–1.0 nM) binding in mouse tissues after oral solifenacin (62.4 μmol kg−1). Values are mean±s.e.m. of three to seven mice. Values in parentheses represent the fold-increase in KD values relative to controls. Asterisks show a significant difference from the control values,
P<0.05,
P<0.01,
P<0.001.
Table 4.
KD and Bmax for specific [3H]-NMS binding in the bladder, prostate, submaxillary gland, heart, lung and colon of mice 0.5–24 h after oral administration of solifenacin (208 μmol kg−1)
| Organ | Time after oralsolifenacin (h) | KD (pM) | Bmax (fmol mg protein−1) |
|---|---|---|---|
| Bladder | Control | 176±6 | 169±22 |
| 0.5 | 240±1 (1.36)*** | 138±21 | |
| 2 | 263±15 (1.49)*** | 156±11 | |
| 6 | 243±16 (1.38)*** | 141±26 | |
| 12 | 215±9 (1.22)* | 139±15 | |
| 24 | 175±8 | 124±19 | |
| Prostate | Control | 110±4 | 170±23 |
| 0.5 | 293±28 (2.66)*** | 215±33 | |
| 2 | 462±37 (4.20)*** | 168±15 | |
| 6 | 396±37 (3.60)*** | 170±34 | |
| 12 | 340±11 (3.09)*** | 234±34 | |
| 24 | 142±11 | 197±18 | |
| Submaxillary gland | Control | 96.7±3.1 | 122±9 |
| 0.5 | 455±27 (4.71)*** | 129±9 | |
| 2 | 689±79 (7.13)*** | 97.2±2.5 | |
| 6 | 469±18 (4.85)*** | 120±16 | |
| 12 | 323±15 (3.34)*** | 111±11 | |
| 24 | 110±4 | 136±13 | |
| Heart | Control | 283±12 | 35.4±0.9 |
| 0.5 | 443±22 (1.57)*** | 42.2±3.0 | |
| 2 | 416±21 (1.47)*** | 48.9±4.1* | |
| 6 | 321±25 | 40.7±6.7 | |
| 12 | 319±7 | 46.6±2.3 | |
| 24 | 278±13 | 43.4±2.6 | |
| Lung | Control | 177±7 | 80.4±3.1 |
| 0.5 | 468±278 | 67.8±8.3 | |
| 2 | 806±54 (4.55)** | 60.0±5.9 | |
| 6 | 488±25 | 67.7±0.6 | |
| 12 | 408±15 | 78.4±11.5 | |
| 24 | 196±7 | 62.1±2.3 | |
| Colon | Control | 146±12 | 131±14 |
| 0.5 | 334±38 (2.29)*** | 126±8 | |
| 2 | 315±8 (2.16)*** | 167±13 | |
| 6 | 337±18 (2.31)*** | 126±11 | |
| 12 | 320±25 (2.19)*** | 147±13 | |
| 24 | 172±16 | 156±24 | |
Rosenthal analysis was performed with [3H]-NMS (0.06–1.0 nM) binding in mouse tissues after oral solifenacin (208 μmol kg−1). Values are mean±s.e.m. of three to seven mice. Values in parentheses represent the fold-increase in KD values relative to controls. Asterisks show a significant difference from the control values,
P<0.05,
P<0.01,
P<0.001.
Plasma levels of oxybutynin, DEOB and solifenacin
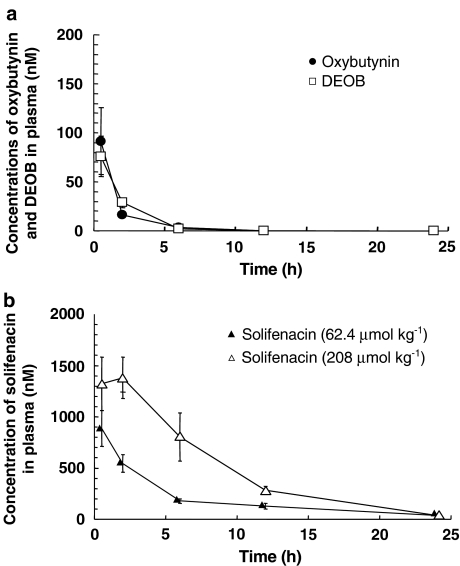
Figure 1 shows the plasma concentrations of oxybutynin, DEOB and solifenacin over time after oral administration of oxybutynin and solifenacin. The concentrations of oxybutynin and DEOB reached maximum levels (oxybutynin, 91.5±34.3 nM; DEOB, 75.8±20.6 nM; n=7–9) 0.5 h after administration of oxybutynin (76.1 μmol kg−1) and then they rapidly declined (Figure 1a). Respective plasma concentrations of oxybutynin at 2 and 6 h were 16.3±2.7 and 3.15±1.54 nM, while those of DEOB were 28.8±5.3 and 2.18±2.18 nM.
Figure 1.
Plasma concentrations of oxybutynin, DEOB and solifenacin over time after oral administration of oxybutynin (a) and solifenacin (b) in mice. Mice received oxybutynin (76.1 μmol kg−1) or solifenacin (62.4, 208 μmol kg−1) orally, and were then killed. Blood samples were taken from the descending aorta of each mouse. Each point represents the mean±s.e.m. of one to nine mice.
Solifenacin at doses of 62.4 and 208 μmol kg−1 produced dose-dependent increases in its plasma concentration. Maximum levels were reached at 0.5 h (886±176 nM; n=6) and 2 h (1381±201 nM; n=6), respectively, followed by a gradual decline (Figure 1b) to 546±84, 178±20, 127±29 and 38.5 nM at 2, 6, 12 and 24 h, respectively, and to 804±233, 283±40 and 38.7±6.0 nM at 6, 12 and 24 h, respectively.
Salivary secretion
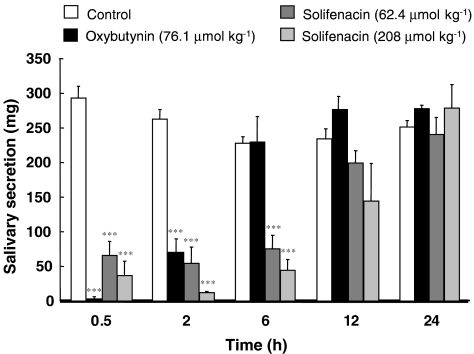
Figure 2 shows the effect of oral administration of oxybutynin and solifenacin on pilocarpine-induced salivary secretion in mice. Salivary secretion induced by an i.v. injection of pilocarpine in control mice showed good reproducibility, with similar amounts of saliva being secreted during the 10-min collection period at 0.5, 2, 6, 12 and 24 h after stimulation (293±18, 263±14, 228±9, 234±15 and 252±9 mg, respectively (n=12)). Pilocarpine-induced secretion was significantly decreased at 0.5 and 2 h after oral administration of oxybutynin (76.1 μmol kg−1), compared with the control value; the response to pilocarpine was almost abolished at 0.5 h but secretion recovered to the control level at 6 h.
Figure 2.
Effects of oral administration of oxybutynin and solifenacin on pilocarpine-induced salivary secretion in mice. Mice received oxybutynin (76.1 μmol kg−1) or solifenacin (62.4, 208 μmol kg−1) orally, and then the total saliva was collected for 10 min with absorbent cotton balls following pilocarpine stimulation (4.09 μmol kg−1, i.v.). Each column represents the mean±s.e.m. of 4–12 mice. Asterisks show a significant difference from control values, ***P<0.001.
Similarly, pilocarpine-induced salivary secretion was markedly reduced but not abolished at 0.5–6 h after oral administration of solifenacin (62.4 and 208 μmol kg−1), as shown by residual saliva (62.4 μmol kg−1: 0.5 h, 66.0±20.4 mg; 2 h, 54.6±23.3 mg; 6 h, 75.2±19.6 mg; and 208 μmol kg−1: 0.5 h, 36.6±20.8 mg; 2 h, 11.8±2.3 mg; 6 h, 44.2±15.6 mg). There was little difference in the secretion responses between the control and solifenacin treatment groups at 12 and 24 h.
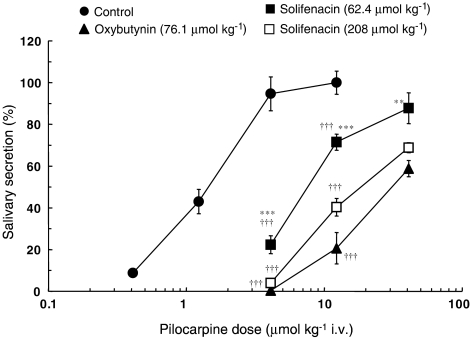
As shown in Figure 3, cumulative doses of pilocarpine (0.41–40.9 μmol kg−1, i.v.) induced dose-dependent salivary secretion. To determine the effects of oxybutynin and solifenacin on these dose–response curves, secretion was measured at the time of maximum inhibition of pilocarpine-induced salivation, that is 0.5 h for oxybutynin and 2 h for solifenacin (Figure 2). Both oxybutynin (76.1 μmol kg−1) and solifenacin (62.4, 208 μmol kg−1) caused a rightward shift in the dose–response curve for pilocarpine-induced salivation, but the inhibitory effect of solifenacin, 62.4 μmol kg−1, was significantly weaker than that of oxybutynin (76.1 μmol kg−1). Thus, the pKB value for oxybutynin (5.53±0.03) was significantly greater (P<0.001) than that for solifenacin (4.85±0.05).
Figure 3.
Effects of oral administration of oxybutynin and solifenacin on the dose–response curve of pilocarpine-induced salivary secretion in mice. Mice received cumulative intravenous doses (0.41–40.9 μmol kg−1) of pilocarpine at 5-min intervals from 0.5 h after oral administration of oxybutynin (76.1 μmol kg−1) and 2 h after oral administration of solifenacin (62.4, 208 μmol kg−1), and then their total saliva was collected. Each point represents the mean±s.e.m. of four to nine mice. Daggers show a significant difference from each control value, †††P<0.001. Asterisks show a significant difference from each value of oxybutynin, **P<0.01, ***P<0.001.
Discussion
The usefulness of ex vivo and in vivo receptor binding assays in predicting the potency, organ selectivity and duration of action of drugs in relation to their pharmacokinetic and pharmacodynamic profiles is well documented (Beauchamp et al., 1995; Uchida et al., 1995; Ohkura et al., 1998; Yamada et al., 2001; 2002; 2003). In the present study, the in vivo muscarinic receptor binding of solifenacin was characterized and compared to that of oxybutynin by simultaneously measuring [3H]-NMS binding in various tissues, including the bladder and submaxillary gland. Also their plasma concentrations and effects on salivary secretion after oral administration were compared. The doses of solifenacin given (2.1–208 μmol kg−1) were similar to those shown to have an inhibitory effect in experimental models of bowel dysfunction in vivo (Kobayashi et al., 2001).
It is known that the heart and salivary gland contain predominantly M2 and M3 muscarinic subtypes, respectively (Giraldo et al., 1988; Caulfield, 1993), whereas the bladder contains both, with the M2 dominating over the M3 subtype (Wang et al., 1995). Furthermore, functional and binding studies have shown that, although M3-receptors are the main contributors, M1, M4 and M5 receptor subtypes may also have a role in muscarinic agonist-induced salivation in mice (Hammer et al., 1980; Yeomans et al., 2001; Takeuchi et al., 2002; Bymaster et al., 2003; Gautam et al., 2004; Nakamura et al., 2004). In the present in vitro experiments, both solifenacin and oxybutynin competed with [3H]-NMS for binding sites in the bladder, submaxillary gland and heart of mice in a concentration-dependent manner. The pKi values indicated that the muscarinic receptor binding affinity of solifenacin in these tissues was 2–3 times weaker than that of oxybutynin. Ikeda et al. (2002) have previously found that the pKi values of solifenacin for M1, M2 and M3 subtypes in binding assays were 7.6, 6.9 and 8.0, respectively. Thus, pKi values of this agent in the mouse heart and submaxillary gland (7.0 and 7.89; Table 1) agree well with the respective M2 and M3 values, while in the bladder the pKi (7.4) was intermediate between these two subtypes. A similar correlation in relative affinity between recombinant receptor subtypes and native tissues has been observed for oxybutynin (Ikeda et al., 2002).
After oral administration of solifenacin (62.4, 208 μmol kg−1) and oxybutynin (76.1 μmol kg−1), dose- and time-dependent increases in KD values for specific [3H]-NMS binding were seen in the bladder, prostate, submaxillary gland, heart, colon and lung of mice, with little effect on Bmax values. Given that an increase in KD values for radioligands in drug-pretreated tissues in this type of assay usually indicates competition between the agent and radioligand for the same binding sites (Ohkura et al., 1998; Yamada et al., 2003), these data strongly suggest that orally administered solifenacin and oxybutynin undergo significant binding to muscarinic receptors in these tissues. Differences were seen between the two drugs in the time course of their effects on the KD values for [3H]-NMS binding. With oxybutynin, the increase in KD in each tissue reached a maximum 0.5 h after oral administration, followed by a rapid decline. In contrast, the increase in KD with solifenacin in most tissues was greatest at 2 h and was maintained for up to 6 or 12 h, depending on dose; its effect was slower in onset and longer in duration compared to that of oxybutynin. This apparent difference between solifenacin and oxybutynin appears to depend largely on their rate of increase and disappearance in the plasma. The plasma concentration of oxybutynin reached a maximum 0.5 h after oral administration and then declined rapidly, whereas that of solifenacin peaked at 0.5 or 2 h and then declined more slowly. Based on the intensity and duration of the increases in KD values, the muscarinic receptor binding activity of solifenacin is greatest in the submaxillary gland and lowest in the heart and persistent in the bladder, prostate, submaxillary gland and colon but transient in the heart and lung. Recent data with M1–M5 subtype knockout mice show that the M3 subtype is expressed predominantly (70–80%) in the submaxillary gland and moderately in the prostate and bladder, whereas M2 is the main subtype present in the bladder, heart, lung and colon (Oki et al., unpublished observation). The tissue selectivity of oral solifenacin may therefore reflect the muscarinic subtype selectivity shown in the in vitro assay (Ikeda et al., 2002, Table 1). Consequently, it is possible that the higher affinity of solifenacin for the M3 compared to the M2 subtype accounts for its persistent binding to muscarinic receptors in the mouse bladder, prostate and submaxillary gland and transient binding in M2-predominant tissues. As mouse salivary gland has recently been shown to contain functional M1, M4 and M5-receptor subtypes, the partial binding of solifenacin to these non-M3-receptor subtypes cannot be ruled out. Moreover, it should be noted that NMS itself displays higher affinity for the M3 than M2 subtype (Waelbroeck et al., 1990). The persistent binding of solifenacin in the mouse colon remains to be clarified.
It is possible that solifenacin, similar to the long-lasting 1,4-dihydropyridine calcium channel antagonists, benidipine and amlodipine (Yamada et al., 2002) has slower receptor binding kinetics of association and dissociation. In our preliminary experiments in vitro, no significant difference was observed between solifenacin and oxybutynin in their association rates of muscarinic receptor binding in the submaxillary gland and heart. Solifenacin was more readily dissociated from muscarinic receptors in the submaxillary gland compared with the heart, while the dissociation rate of oxybutynin was similar in both tissues. These data do not appear to support the results obtained ex vivo after oral administration of these agents (Tables 2, 3 and 4). However, kinetic data obtained from in vitro studies may not necessarily reflect receptor binding kinetics under in vivo conditions, which could be greatly influenced by various pharmacokinetic factors, regional blood flow and intrinsic cholinergic neuronal activity in each tissue. A comparative kinetic analysis of drug concentration and muscarinic receptor binding in tissues of mice administered radiolabelled forms of solifenacin and oxybutynin might clarify any differences in the in vivo binding characteristics of these agents and also between tissues.
Recently, in contrast to our results, Nelson et al. (2004) found that oxybutynin selectively inhibits carbachol-stimulated phosphoinositide responses in slices of bladder from the guinea-pig when compared to the submandibular gland. Our finding, in mice, that oxybutynin binds more to receptors in the submaxillary gland than the bladder may be due to various factors present in in vivo experiments that could affect the concentration of drug reaching a tissue. These include a rapid rise of plasma drug concentration occurring after oral oxybutynin, the amount and rate of drug reaching each tissue depending on the organ blood flow and the formation of the active metabolite (DEOB). In fact, we have recently shown that i.v. injected DEOB, similar to oxybutynin, binds more extensively to muscarinic receptors in the submaxillary gland than in the bladder of rats (Oki et al., 2005).
It is possible that in our study the doses of solifenacin used exert anticholinergic effects in the bladder. In our preliminary experiments 93.2% of solifenacin bound to plasma protein and the free fractions present in plasma were estimated to be 60.2 nM (0.5 h), 37.1 nM (2 h) and 12.1 nM (6 h) at 62.4 μmol kg−1, and 93.9 nM (2 h), 54.7 nM (6 h) and 19.2 nM (12 h) at 208 μmol kg−1. As the in vitro Ki value of this agent in displacing bladder [3H]-NMS binding is 41.9 nM, it is possible that solifenacin at the oral doses used elicits significant blockade of muscarinic receptors in this tissue. In fact, we have shown that solifenacin at oral doses of 2.1–62.4 μmol kg−1 significantly attenuates the carbachol-induced increase in mouse intravesical pressure in a dose-dependent manner (Sato et al., unpublished observations).
In in situ experiments in anaesthetized rats, Ikeda et al. (2002) have shown that intravenous solifenacin is 2 and 6.5 times weaker than oxybutynin at inhibiting carbachol-induced bladder contractions and salivary secretion, respectively, indicating the selectivity of solifenacin for the bladder. Similarly, Ohtake et al. (2004) found that solifenacin had a greater selectivity for the urinary bladder compared to the salivary gland in rats. In the present study, pilocarpine-induced salivary secretion in mice was markedly reduced 0.5–6 h after oral solifenacin (62.4 and 208 μmol kg−1), but was completely abolished 0.5 h after oral administration of oxybutynin (76.1 μmol kg−1). The rapid cessation of salivary secretion induced by oral oxybutynin did not occur in mice receiving oral solifenacin and the pKB values showed that solifenacin was significantly (5 times) weaker than oxybutynin, at inhibiting pilocarpine-induced salivation. Thus, the intensity and time-course of the inhibitory effects of solifenacin and oxybutynin on salivary secretion after oral administration appear to accord well with those for muscarinic receptor binding in the submaxillary gland.
After oral administration of oxybutynin, similar concentrations of DEOB as those for oxybutynin were detected in the plasma of mice. The in vitro receptor binding affinity of DEOB in mouse tissues was 3–6 times higher than that of oxybutynin, with the highest affinity (6 times that of oxybutynin) occurring in the submaxillary gland. In man, Waldeck et al. (1997) showed a higher affinity of DEOB for muscarinic receptors in the parotid gland relative to the bladder. These data suggest that DEOB contributes more significantly to the blockade of muscarinic receptors in exocrine gland receptors than oxybutynin under in vivo conditions.
In conclusion, the results from the present study, show that oral solifenacin significantly binds to muscarinic receptors in various tissues of mice, including the bladder, and that this binding is persistent in tissues expressing the M3 subtype. Further, the inhibitory effect of solifenacin on pilocarpine-evoked salivary secretion was significantly weaker but more persistent than that of oxybutynin.
Acknowledgments
We thank Mr T. Kusaka for his excellent technical assistance. This work was supported in part by a Grant-in-Aid for Scientific Research (C) (2) (No. 15591703) from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations
- Bmax
maximum number of binding sites
- DEOB
N-desethyl-oxybutynin
- [3H]-NMS
[N-methyl-3H]-scopolamine methyl chloride
- KD
apparent dissociation constant
- Ki
inhibition constant
References
- ABRAMS P., CARDOZO L., FALL M., GRIFFITHS D., ROSIER P., ULMSTEN U., VAN KERREBROECK P., VICTOR A., WEIN A. Standardisation Sub-committee of the International Continence Society. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Committee Society. Neurourol. Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- ABRAMS P., FREEMAN R., ANDERSTROM C., MATTIASSON A. Tolterodine, a new antimuscarinic agent: as effective but better tolerated than oxybutynin in patients with an overactive bladder. Br. J. Pharmacol. 1998;81:801–810. doi: 10.1046/j.1464-410x.1998.00717.x. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.-E. Current concepts in the treatment of disorders of micturition. Drugs. 1988;35:477–494. doi: 10.2165/00003495-198835040-00006. [DOI] [PubMed] [Google Scholar]
- ANDERSON R.U., MOBLEY D., BLANK B., SALTZSTEIN D., SUSSET J., BROWN J.S. Once daily controlled versus immediate release oxybutynin chloride for urge urinary incontinence. J. Urol. 1999;161:1809–1812. [PubMed] [Google Scholar]
- BEAUCHAMP H.T., CHANG R.S.L., SIGEL P.K.S., GIBSON R.E. In vivo receptor occupancy of the angiotensin II receptor by nonpeptide antagonists: relationship to in vitro affinities and in vivo pharmacologic potency. J. Pharmacol. Exp. Ther. 1995;272:612–618. [PubMed] [Google Scholar]
- BULMER P., ABRAMS P. The overactive bladder. Rev. Contemp. Pharmacother. 2000;11:1–11. [Google Scholar]
- BYMASTER F.P., CARTER P.A., YAMADA M., GOMEZA J., WESS J., HAMILTON S.E., NATHANSON N.M., MCKINZIE D.L., FELDER C.C. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur. J. Neurosci. 2003;17:1403–1410. doi: 10.1046/j.1460-9568.2003.02588.x. [DOI] [PubMed] [Google Scholar]
- CHAPPLE C.R. Muscarinic receptor antagonists in the treatment of overactive bladder. Urology. 2000;55:33–46. doi: 10.1016/s0090-4295(99)00492-6. [DOI] [PubMed] [Google Scholar]
- CAULFIELD M.P. Muscarinic receptor: characterization, coupling and function. Pharmacol. Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M., REDDY H., WATSON N., CHALLIS R.A.J. Muscarinic acetylcholine receptors in smooth muscle. Trends Pharmacol. Sci. 1994;15:114–119. doi: 10.1016/0165-6147(94)90047-7. [DOI] [PubMed] [Google Scholar]
- EHLERT F.J., TRAN L.P. Regional distribution of M1, M2 and non-M1, non-M2 subtypes of muscarinic binding sites in rat brain. J. Pharmacol. Exp. Ther. 1990;255:1148–1157. [PubMed] [Google Scholar]
- GAUTAM D., HEARD T.S., CUI Y., MILLER G., BLOODWORTH L., WESS J. Cholinergic stimulation of salivary secretion studies with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol. Pharmacol. 2004;66:260–267. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]
- GIRALDO E., MARTOS F., GOMEZA A., GARCIA A., VIGANO M.A., LADINSKY H., SANCHEZ DE LA CUESTA F. Characterization of muscarinic receptor subtypes in human tissues. Life Sci. 1988;43:1507–1515. doi: 10.1016/0024-3205(88)90398-0. [DOI] [PubMed] [Google Scholar]
- GUPTA S.K., SATHYAN G. Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate-release oxybutynin. J. Clin. Pharmacol. 1999;39:289–296. [PubMed] [Google Scholar]
- HAMMER R., BERRIE C.P., BIRDSALL N.J.M., BURGEN A.S.V., HULME E.C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980;283:90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- HULME E.C., BIRDSALL N.J.M., BUCKLEY N.J. Muscarinic receptor subtypes. Annu. Rev. Pharmacol. Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- IKEDA K., KOBAYASHI S., SUZUKI M., MIYATA K., TAKEUCHI M., YAMADA T., HONDA K. M3 receptor antagonism by the novel antimuscarinic agent solifenacin in the urinary bladder and salivary gland. Naunyn Schmiedebergs Arch. Pharmacol. 2002;366:97–103. doi: 10.1007/s00210-002-0554-x. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI S., IKEDA K., MIYATA K. Comparison of in vitro selectivity profiles of solifenacin succinate (YM905) and current antimuscarinic drugs in bladder and salivary glands: a Ca2+ mobilization study in monkey cells. Life Sci. 2004;74:843–853. doi: 10.1016/j.lfs.2003.07.019. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI S., IKEDA K., SUZUKI M., YAMADA T., MIYATA K. Effects of YM905, a novel muscarinic M3-receptor antagonist, on experimental models of bowel dysfunction in vivo. Jpn. J. Pharmacol. 2001;86:281–288. doi: 10.1254/jjp.86.281. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- NAKAMURA T., MATSUI M., UCHIDA K., FUTATSUGI A., KUSAKAWA S., MATSUMOTO N., NAKAMURA K., MANABE T., TAKETO M.M., MIKOSHIBA K. M3 muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J. Physiol. 2004;558:561–575. doi: 10.1113/jphysiol.2004.064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON C.P., GUPTA P., NAPIER C.M., NAHORSKI S.R., CHALLISS R.A.J. Functional selectivity of muscarinic receptor antagonists for inhibition of M3-mediated phosphoinositide responses in guinea pig urinary bladder and submandibular salivary gland. J. Pharmacol. Exp. Ther. 2004;310:1255–1265. doi: 10.1124/jpet.104.067140. [DOI] [PubMed] [Google Scholar]
- NILVEBRANT L., ANDERSSON K.E., GILLBERG P.G., STAHL M., SPARF B. Tolterodine – a new bladder-selective antimuscarinic agent. Eur. J. Pharmacol. 1997;327:195–207. doi: 10.1016/s0014-2999(97)89661-6. [DOI] [PubMed] [Google Scholar]
- OHKURA T., YAMADA S., DEGUCHI Y., KIMURA R., MATSUSHIMA H., HIGUCHI S., INAGAKI O., HONDA K., TAKENAKA T. Ex vivo occupancy by tamslosin of α1-adrenoceptors in rat tissues in relation to the plasma concentration. Life Sci. 1998;63:2147–2155. doi: 10.1016/s0024-3205(98)00495-0. [DOI] [PubMed] [Google Scholar]
- OHTAKE A., UKAI M., HATANAKA T., KOBAYASHI S., IKEDA K., SATO S., MIYATA K., SASAMATA M. In vitro and in vivo tissue selectivity profile of solifenacin succinate (YM905) for urinary bladder over salivary gland in rats. Eur. J. Pharmacol. 2004;492:243–250. doi: 10.1016/j.ejphar.2004.03.044. [DOI] [PubMed] [Google Scholar]
- OKI T., KAWASHIMA A., UCHIDA M., YAMADA S.In vivo demonstration of muscarinic receptor binding activity of N-desethyl-oxybutynin, active metabolite of oxybutynin Life Sci. 2005(in press) [DOI] [PubMed]
- OKI T., KIMURA R., SAITO M., MIYAGAWA I., YAMADA S. Demonstration of bladder selective muscarinic receptor binding by intravesical oxybutynin to treat overactive bladder. J. Urol. 2004;172:2059–2064. doi: 10.1097/01.ju.0000138472.16876.8d. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI J., FULTON J., JIA Z., ABRAMOV-NEWERLY W., JAMOT L., SUD M., COWARD D., RALPH M., RODER J., YEOMANS J. Increased drinking in mutant mice with truncated M5 muscarinic receptor genes. Pharmacol. Biochem. Behav. 2002;72:117–123. doi: 10.1016/s0091-3057(01)00725-0. [DOI] [PubMed] [Google Scholar]
- UCHIDA S., YAMADA S., OHKURA T., HESHIKIRI M., YOSHIMI A., SHIRAHASE H., KIMURA R. The receptor occupation and plasma concentration of NKY-722, a water-soluble dihydropyridine-type calcium antagonist, in spontaneously hypertensive rats. Br. J. Pharmacol. 1995;114:217–223. doi: 10.1111/j.1476-5381.1995.tb14928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ROSSUM J.M., HURKMANS J.A.T.M., WOLTERS C.J.J. Cumulative dose–response curves II. Techniques for the making of dose–response curves in isolated organs and the evaluation of drug parameters. Arch. Int. Pharmacodyn. 1963;143:299–330. [PubMed] [Google Scholar]
- WAELBROECK M., TASTENOY M., CAMUS J., CHRISTOPHE J. Binding of selective antagonists to four muscarinic receptors (M1 to M4) in rat forebrain. Mol. Pharmacol. 1990;38:267–273. [PubMed] [Google Scholar]
- WALDECK K., LARSSON B., ANDERSSON K.-E. Comparison of oxybutynin and its metabolite, N-desethyl-oxybutynin, in the human detrusor and parotid gland. J. Urol. 1997;157:1093–1097. [PubMed] [Google Scholar]
- WANG P., LUTHIN G.R., RUGGIERI M.R. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J. Pharmacol. Exp. Ther. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]
- WEIN A.J. Pharmacologic treatment of incontinence. J. Am. Geriatr. Soc. 1990;38:317–325. doi: 10.1111/j.1532-5415.1990.tb03512.x. [DOI] [PubMed] [Google Scholar]
- YAMADA S., KUSAKA T., URAYAMA A., KIMURA R., WATANABE Y. In vitro and ex vivo effects of a selective nociceptin/orphanin FQ (N/OFQ) peptide receptor antagonist, CompB, on specific binding of [3H]N/OFQ and [35S]GTPγS in rat brain and spinal cord. Br. J. Pharmacol. 2003;139:1462–1468. doi: 10.1038/sj.bjp.0705371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMADA S., NAKAJIMA M., KUSAKA T., UCHIDA S., KIMURA R. In vivo receptor binding of benidipine and amlodipine in mesenteric arteries and other tissues of spontaneously hypertensive rats. Life Sci. 2002;70:1999–2011. doi: 10.1016/s0024-3205(01)01541-7. [DOI] [PubMed] [Google Scholar]
- YAMADA S., OKURA T., KIMURA R. In vivo demonstration of α1A-adrenoceptor subtype selectivity of KMD-3213 in rat tissues. J. Pharmacol. Exp. Ther. 2001;296:160–167. [PubMed] [Google Scholar]
- YAMADA S., YAMAMURA H.I., ROESKE W.R. Characterization of alpha-1 adrenergic receptors in the heart using [3H]WB4101: effect of 6-hydroxydomine treatment. J. Pharmacol. Exp. Ther. 1980;215:176–185. [PubMed] [Google Scholar]
- YARKER Y.E., GOA K.L., FITTON A. Oxybutynin. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in detrusor instability. Drugs Aging. 1995;6:243–262. doi: 10.2165/00002512-199506030-00007. [DOI] [PubMed] [Google Scholar]
- YEOMANS J., FORSTER G., BLAHA C. M5 muscarinic receptors are needed for slow activation of dopamine neurons and for rewarding brain stimulation. Life Sci. 2001;68:2449–2456. doi: 10.1016/s0024-3205(01)01038-4. [DOI] [PubMed] [Google Scholar]