Abstract
Mineralocorticoid receptor (MR) antagonism with spironolactone reduces mortality in heart failure on top of ACE inhibition. To investigate the underlying mechanism, we compared the actions of both aldosterone and spironolactone to those of angiotensin (Ang) II in the rat heart.
Hearts of male Wistar rats were perfused according to Langendorff. Ang II and aldosterone increased left ventricular pressure (LVP) by maximally 11±4 and 9±2%, and decreased coronary flow (CF) by maximally 36±7 and 20±4%, respectively. Spironolactone did not significantly affect LVP or CF.
In hearts that were exposed to a 45-min coronary artery occlusion and 3 h of reperfusion, a 15-min exposure to spironolactone prior to occlusion reduced infarct size (% of risk area) from 68±2 to 45±3%, similar to the reduction (34±2%) observed following ‘preconditioning' (15 min occlusion followed by 10 min reperfusion) prior to the 45-min occlusion. Aldosterone exposure did not affect infarct size (71±5%).
In cardiomyocytes, aldosterone decreased [3H]thymidine incorporation maximally by 73±3%, whereas in cardiac fibroblasts it decreased [3H]proline incorporation by 33±7%. Spironolactone inhibited both effects. Ang II increased DNA and collagen synthesis, and these effects were reversed by aldosterone.
In conclusion, aldosterone induces positive inotropic and vasoconstrictor effects in a nongenomic manner, and these effects are comparable to those of Ang II. Aldosterone reduces DNA and collagen synthesis via MR activation, and counteracts the Ang II-induced increases in these parameters. MR blockade reduces infarct size and increases LVP recovery following coronary artery occlusion. The MR-related phenomena may underlie, at least in part, the beneficial actions of spironolactone in heart failure.
Keywords: Aldosterone, angiotensin, cardiomyocyte, collagen, DNA synthesis, ischaemia, Langendorff heart, mineralocorticoid receptor, spironolactone
Introduction
Plasma aldosterone levels are elevated in patients with congestive heart failure, even when long-term ACE inhibitor therapy results in complete inhibition of membrane-bound ACE (Jorde et al., 2002). This elevation is not due to angiotensin (Ang) II generation by non-ACE enzymes such as chymase (MaassenVanDenBrink et al., 1999; Jorde et al., 2002; Tom et al., 2003), and thus factors other than Ang II (e.g., potassium, corticotropin and catecholamines) are responsible for the increased aldosterone production in heart failure during ACE inhibition (Weber, 2001).
Aldosterone excess has deleterious effects on cardiac function (Delcayre & Silvestre, 1999; Beggah et al., 2002; Sun et al., 2002; Ahokas et al., 2005), and treatment of patients experiencing severe heart failure with the aldosterone receptor antagonist spironolactone improves the morbidity and mortality on top of ACE inhibition (Pitt et al., 1999). The underlying mechanism of this beneficial effect is not entirely clear. Since the aldosterone receptor (mineralocorticoid receptor (MR)) occurs both in the kidney and extrarenal tissues like the heart and the vessel wall (Lombès et al., 1995; Mazak et al., 2004; Oberleithner et al., 2004), it is believed that spironolactone may exert its effects, at least in part, independently of the kidney. It has even been suggested that aldosterone, like Ang II (Danser et al., 1994; van Kats et al., 1998), is synthesized locally in the heart (Silvestre et al., 1998; 1999).
The MR-mediated effects of aldosterone are referred to as genomic effects. These effects involve binding of aldosterone to the intracellular MR (Kd≈1–2 nM) and the translocation of the steroid–MR complex to the nucleus, where it acts as a transcriptional regulator, inducing effects after several hours. In addition, rapid ‘nongenomic' effects of aldosterone (occurring within minutes) have been described (Lösel et al., 2002). These effects are likely to be transmitted via specific membrane receptors. They occur at subnanomolar levels of aldosterone, and involve, among others, inositol 1,4,5-triphosphate (IP3), protein kinase C and Ca2 + (Christ et al., 1993; 1995; Wehling et al., 1995). The identity of the receptor responsible for the nongenomic effects is currently not known.
The effects of aldosterone in the heart include inflammation, fibrosis, positive inotropy and coronary vasodilation (Campbell et al., 1993; Sun et al., 1993; Moreau et al., 1996; Barbato et al., 2002; Sun et al., 2002). Not all studies confirm these findings, however, nor has consensus been obtained on the receptor(s) mediating these effects. This is due to the fact that in some cases the results were obtained using only one concentration of aldosterone with or without spironolactone (Brilla et al., 1994; Moreau et al., 1996; Barbato et al., 2002), whereas in others the study involved in vivo treatment with spironolactone, which does not allow conclusions on the local cardiac effects of this drug (Rochetaing et al., 2003).
In the present study, we set out to distinguish the genomic and nongenomic effects of aldosterone in the heart. Using the isolated perfused rat Langendorff heart preparation, we evaluated the effects of aldosterone on left ventricular pressure (LVP) and coronary flow (CF), as well as its effects on infarct size and recovery of LVP and CF following ischaemia and reperfusion. In cultured neonatal rat cardiomyocytes and fibroblasts, we determined the effects of aldosterone on DNA and collagen synthesis. These effects were compared to the effects of aldosterone on DNA synthesis in aortic vascular smooth muscle cells (VSMCs). Genomic and nongenomic effects were distinguished using the MR receptor antagonist spironolactone. We also investigated the effects of Ang II, alone and in combination with aldosterone, because recent studies suggest that aldosterone exerts its effects, at least in part, via Ang II and / ;or Ang II type 1 (AT1) receptors (Mazak et al., 2004; Michel et al., 2004; Xiao et al., 2004).
Methods
Drugs
Ang II, aldosterone, spironolactone and trypan blue were purchased from Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands. Stock solutions of aldosterone (10 mM) and spironolactone (1 mM) were prepared in ethanol. All other chemicals were dissolved in distilled water.
Ethical approval
All experiments were performed under the regulations of the Animal Care Committee of the Erasmus MC, in accordance with the ‘Guiding Principles in the Care and Use of Laboratory Animals' as approved by the American Physiological Society.
Experiments in Langendorff hearts
Male Wistar rats (n=66, weight 300–340 g), obtained from Harlan, Zeist, The Netherlands, were anaesthetised with sodium pentobarbital (60 mg kg− 1, i.p.). Hearts were rapidly excised and cooled in ice-cold Krebs–Henseleit solution (composition in mM: NaCl 125, KCl 4.7, NaHCO3 20, NaH2PO4 0.43, MgCl2 1.0, CaCl2 1.3 and D-glucose 9.1; pH 7.4) until contractions stopped, and prepared for Langendorff perfusion. Continuously carbogen-gassed (95% O2 / ;5% CO2) Krebs–Henseleit solution at 37°C was perfused immediately after cannulation of the aorta, at a constant perfusion pressure of 80 mmHg. A water-filled latex balloon was placed in the left ventricle via the left atrium to measure LVP. The volume of the balloon was adjusted to achieve a stable left ventricular end-diastolic pressure (LVEDP) of 5 mmHg during initial equilibration, and this volume was maintained throughout the experiment. Hearts were paced at 350 beats min− 1. CF was measured by an inline flow probe (Transonic Systems, Ithaca, NY, U.S.A.).
After a stabilization period of 15 min, 100 μl bolus injections were applied to construct dose–response curves to Ang II, vehicle (ethanol), aldosterone and / ;or spironolactone. In a second series of experiments, we evaluated the effects of aldosterone and spironolactone during ischaemia and reperfusion. Hearts were subjected to 45 min left anterior descending coronary artery occlusion, followed by 3 h of reperfusion. Occlusion was preceded by either no treatment (control), preconditioning (15 min of occlusion followed by 10 min of reperfusion), or a 15-min exposure to vehicle (0.01% ethanol, final concentration in perfusion buffer), aldosterone (100 nM) or spironolactone (100 nM). Vehicle, aldosterone and spironolactone remained present in the perfusion buffer throughout the remainder of the experiment. After the 3-h reperfusion period, area at risk and infarct size were determined as described before (Schoemaker & van Heijningen, 2000).
Experiments in cells
Primary cultures of neonatal Wistar rat (Harlan) ventricular cardiomyocytes and fibroblasts were prepared as described before (van Kesteren et al., 1997; Saris et al., 2002). Briefly, ventricles from newborn 1–3-day-old Wister rats were minced and cells of ventricles were isolated by eight subsequent trypsinization steps. Nonmyocytes were separated from myocytes by differential preplating. Myocytes were seeded in 24-well plates at 1.5–1.75 × 105 cells cm− 2, giving a confluent monolayer of spontaneously beating cells after 24 h. The preplated cells (fibroblast fraction) were passaged after 4 days to 24-well plates at 0.75 × 105 cells cm− 2. Myocytes and fibroblasts were maintained at 37°C and 5% CO2–95% air in DMEM and Medium 199 (4 : 1), supplemented with 5% foetal calf serum (FCS), 5% horse serum, 100 U penicillin ml− 1 and 100 μg streptomycin ml− 1.
VSMCs were isolated from Sprague–Dawley rat thoracic aortas by enzymatic digestion as described previously (Haller et al., 1994). Cells were grown in SmGM-2 (Clonetics, Verviers, Belgium) supplemented with 5% FCS, and were passaged by harvesting with 0.05% trypsin / ;0.53 mM EDTA and seeding into 75 cm2 flasks. For experiments, cells between passages 3 and 8 were seeded in 24-well plates and grown to confluency at 37°C and 5% CO2–95% air.
Experiments were performed after the cells had been serum-deprived for at least 1 day. Before the start of each experiment, cells were rinsed three times with 1 ml warm (37°C) serum-free medium. Next, the cells were incubated for 24 h at 37°C with 400 μl serum-free medium (supplemented with 1% bovine serum albumin (BSA) in the case of myocytes and fibroblasts), and containing Ang II, vehicle (0.1% ethanol), aldosterone and / ;or spironolactone. DNA and collagen synthesis rates were determined in duplicate by quantifying [3H]thymidine and [3H]proline incorporation, respectively, during the last 6 h of the above 24-h incubation period. Total cellular protein and DNA were quantified after solubilization as described before (van Kesteren et al., 1997), using BSA and salmon sperm as standard, respectively.
Data analysis
Data are expressed as mean±s.e.m. and are shown either as percent change from baseline (LVP, CF) or relative to control values (DNA and collagen synthesis rates). Dose–response curves were analysed as described before (Tom et al., 2002) to obtain pEC50 (− 10log EC50) values. Statistical analysis was carried out by ANOVA, followed by post hoc evaluation according to Tukey. P-values <0.05 were considered significant.
Results
Experiments in Langendorff hearts
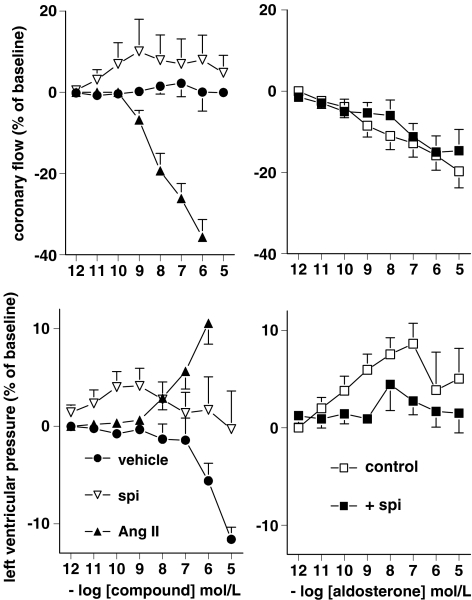
Baseline values of LVP and CF were 80±1.4 mmHg (n=60) and 12±0.3 ml min− 1, respectively. Ang II (n=6) and aldosterone (n=6) dose-dependently increased LVP (pEC50 7.4±0.3 and 9.8±0.4, respectively) and reduced CF (pEC50 8.0±0.7 and 8.7±0.6, respectively) (Figure 1). Maximum effects of Ang II occurred within 2–3 min and lasted 5–6 min, whereas the maximum effects of aldosterone occurred within 1–2 min and lasted <2 min. Spironolactone (n=6) did not significantly affect LVP or CF (Figure 1). Vehicle (n=6) also did not affect CF, and decreased LVP at the highest two concentrations that were evaluated (corresponding to 0.1 and 1% ethanol in the bolus injection fluid, respectively). Spironolactone (10 μM, n=6) reduced the effect of aldosterone on LVP, without changing its pEC50. Spironolactone did not alter the effects of aldosterone on CF.
Figure 1.
Left panels: Effect of Ang II, spironolactone (spi) and vehicle on CF and LVP. Right panels: Effect of aldosterone on CF and LVP in the absence (control) and presence of 10 μM spironolactone. Values (mean±s.e.m., n=6) are expressed as percentage change from baseline.
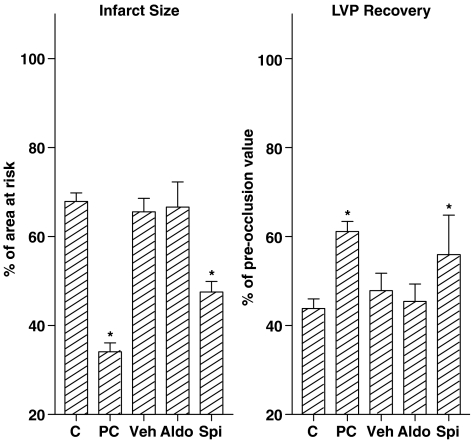
In six (three control hearts, two aldosterone-pretreated hearts and one vehicle-pretreated heart) of the 36 hearts that were exposed to ischaemia plus reperfusion, we could not determine LVP and CF during reperfusion because of severe ventricular arrhythmias. These hearts were therefore excluded from the analysis. In the remaining 30 hearts, occlusion of the left anterior descending coronary artery reduced LVP to ≈30 mmHg and decreased CF by approximately 50% (Table 1). In control hearts (n=6), LVP and CF increased rapidy during the reperfusion phase (Table 1), stabilizing after approximately 20 min at 44±3 and 86±13% of pre-ischaemia values (Figure 2). LVEDP increased to 23±5 mmHg. Pretreatment with aldosterone (n=6) or vehicle (n=6) did not affect LVP and CF recovery, nor did it prevent the rise in LVEDP. Preconditioning (n=6) as well as pretreatment with spironolactone (n=6) significantly enhanced recovery of LVP (P<0.05), but not of CF (Table 1 and Figure 2). Both procedures tended to reduce the rise in LVEDP (P>0.05). Ventricular fibrillation occurred within the first 10 min of reperfusion of all control hearts (Table 1). The incidence of fibrillation was reduced in hearts exposed to preconditioning or spironolactone, but not in hearts pretreated with aldosterone or vehicle. The area at risk was identical in all hearts (Table 1). Infarct size (expressed as a percentage of the area at risk) was 68±2% in control hearts (Figure 2). Preconditioning and spironolactone similarly reduced infarct size (P<0.05), whereas infarct sizes in aldosterone- and vehicle-treated hearts were not different from control.
Table 1.
Area at risk, fibrillation incidence, and haemodynamic parameters at baseline, after 45 min of coronary artery occlusion (‘ischaemia'), and during reperfusion
| Parameter | Pretreatment | ||||
|---|---|---|---|---|---|
| None | Preconditioning | Vehicle | Aldosterone | Spironolactone | |
| LVEDP (mmHg) | |||||
| Baseline | 5.2±0.2 | 5.1±0.2 | 5.2±0.2 | 5.3±0.3 | 5.2±0.2 |
| Ischaemia | 9.7±1.3 | 8.3±1.5 | 7.8±1.5 | 7.3±1.0 | 5.7±0.5 |
| Reperfusion | 23±5.3 | 14±2.0 | 33±10 | 24±7.2 | 11±13 |
| LVP (mmHg) | |||||
| Baseline | 75±6.9 | 82±2.3 | 82±3.1 | 81±3.7 | 81±2.7 |
| Ischaemia | 27±2.4 | 28±3.8 | 30±3.1 | 33±2.6 | 34±3.4 |
| Reperfusion | 31±3.2 | 50±1.8* | 39±3.5 | 38±4.9 | 52±4.9* |
| CF (ml min− 1) | |||||
| Baseline | 13±0.8 | 13±0.9 | 13±0.9 | 11±0.9 | 12±1.1 |
| Ischaemia | 7.7±0.8 | 6.2±1.5 | 6.1±0.8 | 4.0±0.3 | 6.7±0.7 |
| Reperfusion | 10±1.0 | 11±0.7 | 10±2.1 | 7.9±0.5 | 9.9±0.7 |
| Area at risk (%) | 50±3 | 52±3 | 52±3 | 52±3 | 53±3 |
| Fibrillation incidence | 6 / ;6 | 2 / ;6* | 5 / ;6 | 6 / ;6 | 2 / ;6* |
Data are mean±s.e.m. of six experiments.
P<0.05 vs no pretreatment. LVEDP, left ventricular end-diastolic pressure; LVP, left ventricular pressure; CF, coronary flow.
Figure 2.
Infarct size (left panel) and recovery of LVP (right panel) in hearts that were subjected to 45 min left anterior descending coronary artery occlusion, followed by 3 h of reperfusion, after either no pretreatment (C, control), preconditioning (PC, 15 min of occlusion followed by 10 min of reperfusion), or a 15-min exposure to vehicle (Veh), 100 nM aldosterone (Aldo), or 100 nM spironolactone (Spi). Values are mean±s.e.m. of six experiments. *P<0.05 vs control.
Experiments in cells
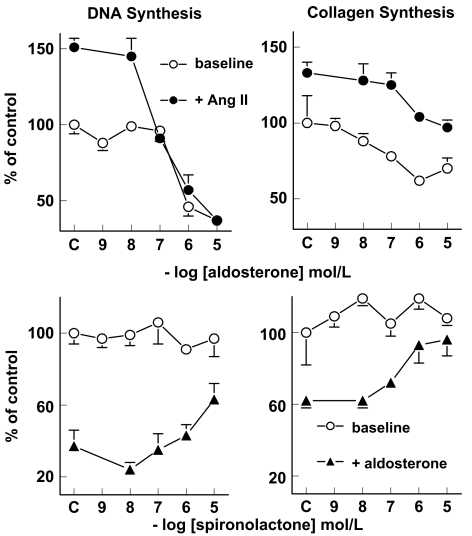
In cardiomyocytes, aldosterone decreased [3H]thymidine incorporation in a concentration-dependent manner (pEC50 6.6±0.4, n=9; Figure 3). Spironolactone (n=9) did not affect [3H]thymidine incorporation, and reversed the effects of 1 μmol l− 1 aldosterone in a concentration-dependent manner (pEC50 6.5±0.3, n=9). This indicates that the effects of aldosterone on DNA synthesis depend on MR activation. Ang II (100 nM; n=7) increased [3H]thymidine incorporation by 51±6% (P<0.05), and aldosterone concentration-dependently inhibited this effect (n=7). Vehicle did not affect [3H]thymidine incorporation (100±6%, n=9). Total protein (133±13 μg per well) and total DNA (5.3±0.5 μg per well) contents of myocytes were not affected by any of the (ant)agonists, their combination or vehicle (data not shown).
Figure 3.
Effect of aldosterone (top panels) and spironolactone (bottom panels) on [3H]thymidine incorporation (DNA synthesis) in myocytes and on [3H]proline incorporation (collagen synthesis) in fibroblasts, either at baseline or in the presence of 100 nM Ang II or 1 μM aldosterone. Values (mean±s.e.m., n=4–9) are expressed relative to control; C refers to the response in the absence of aldosterone or spironolactone. Ang II increased [3H]thymidine incorporation by 51±6% (P<0.05). Aldosterone decreased [3H]thymidine incorporation, both in the presence of Ang II (to 37±4% of control, P<0.001) and at baseline (to 37±3% of control, P<0.001). Ang II increased [3H]proline incorporation by 33±7% (P<0.05), and aldosterone fully reversed this effect, as evidenced by the absence of Ang II-induced effects on collagen synthesis (P=NS vs control) at aldosterone concentrations of 1 μM and higher. At baseline, aldosterone decreased [3H]proline incorporation to 62±5% (P<0.05) of control. Spironolactone did not exert effects at baseline, and, at a concentration of 10 μM, reversed the aldosterone-induced decreases in DNA and collagen synthesis to levels that were not significantly different from control.
In cardiac fibroblasts, aldosterone decreased [3H]proline incorporation in a concentration-dependent manner (pEC50 7.7±0.7, n=4; Figure 3). Spironolactone (n=4) did not affect [3H]proline incorporation, and reversed the effects of 1 μmol l− 1 aldosterone in a concentration-dependent manner (pEC50 6.6±0.7, n=4). This indicates that the effects of aldosterone on collagen synthesis depend on MR activation. Ang II (100 nM; n=4) increased [3H]proline incorporation by 33±7% (P<0.05), and aldosterone concentration-dependently inhibited this effect (n=4). Vehicle did not affect [3H]proline incorporation (98±2%, n=4). Total protein (64±14 μg per well) and total DNA (2.1±0.2 μg per well) contents of fibroblasts were not affected by any of the (ant)agonists, their combination or vehicle (data not shown).
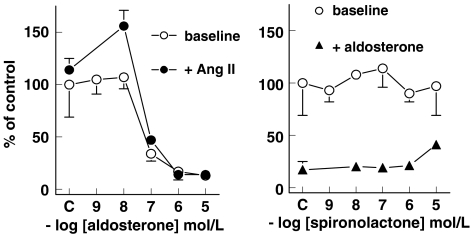
In aortic VSMCs, aldosterone decreased [3H]thymidine incorporation in a concentration-dependent manner (pEC50 7.4±0.4, n=7; Figure 4). Spironolactone (n=7) did not affect [3H]thymidine incorporation, and partially reversed the effects of 1 μmol l− 1 aldosterone. Ang II (100 nM; n=7) did not significantly affect [3H]thymidine incorporation. However, in the presence of 10 nM aldosterone, it increased [3H]thymidine incorporation by 56±15% (P<0.01). Higher concentrations of aldosterone decreased [3H]thymidine incorporation in the presence of Ang II, to the same degree as under baseline conditions (Figure 4). Vehicle did not affect [3H]thymidine incorporation (111±22%, n=7). Total protein (138±14 μg per well) and total DNA (4.1±0.4 μg per well) contents of VSMCs were not affected by any of the (ant)agonists, their combination or vehicle (data not shown).
Figure 4.
Effect of aldosterone (left panel) and spironolactone (right panel) on [3H]thymidine incorporation in vascular smooth muscle cells, either at baseline or in the presence of 100 nM Ang II or 1 μM aldosterone. Values (mean±s.e.m., n=7) are expressed relative to control; C refers to the response in the absence of aldosterone or spironolactone. Ang II did not significantly affect [3H]thymidine incorporation. On top of Ang II, aldosterone exerted a biphasic effect: at a concentration of 10 nM, it increased [3H]thymidine incorporation to 156±15% of control (P<0.01), and at higher concentrations it decreased [3H]thymidine incorporation to 14±5% of control (P<0.001). At baseline, aldosterone did not affect [3H]thymidine incorporation at concentrations up to 10 nM, whereas at higher concentrations it decreased [3H]thymidine incorporation to 13±6% of control (P<0.001). Spironolactone, at a concentration of 10 μM, partially blocked the effects of 1 μM aldosterone, as evidenced by the fact that the aldosterone-induced effects at this spironolactone concentration were still significantly different (P<0.05) from control.
Discussion
The present study distinguishes both MR (‘genomic')- and non-MR (‘nongenomic')-mediated effects of aldosterone in the rat heart. Genomic effects include inhibition of DNA synthesis in cardiomyocytes, inhibition of collagen synthesis in cardiac fibroblasts, and deleterious cardiac effects (including fibrillation) during ischaemia and reperfusion. Nongenomic effects include coronary vasoconstriction and, most likely, positive inotropy. The nongenomic effects of aldosterone on vasoconstriction and inotropy parallel the effects of Ang II on these parameters, whereas the genomic effects on growth and remodelling counteract those of Ang II.
Nongenomic effects
At first sight (Figure 1), it appears that spironolactone blocks the inotropic effects of aldosterone, thus suggesting that these effects are mediated via MR activation. However, in contrast with this conclusion, the inotropic effects occurred within minutes, whereas MR-induced effects usually occur after hours. Furthermore, the inotropic effects occurred at the same (subnanomolar) aldosterone concentration range as the nongenomic effects on CF, that is, at levels that were approximately 100 times lower than the levels required to induce MR-induced effects in cultured cardiac cells. Finally, spironolactone did not alter aldosterone potency; it only reduced aldosterone efficacy. With regard to the latter, it is important to note that both aldosterone and spironolactone were dissolved in ethanol. Ethanol, like methanol, decreases contractility in a concentration-dependent manner (Tom et al., 2001). The spironolactone concentration that we and others (Moreau et al., 1996) used (10 μM) corresponds with an ethanol concentration of 1% in the 100 μl bolus injection, and thus the ‘blocking' effects of spironolactone may in reality represent ethanol-induced physiological antagonism of aldosterone. Owing to the negative inotropic effect of ethanol, the positive inotropic effect of aldosterone in the present study is probably underestimated. Moreover, when correcting for the ethanol-induced negative inotropic effect, our study would reveal a positive inotropic action for spironolactone as well, thereby further arguing against the MR as the mediator of the inotropic effect of aldosterone. This conclusion is in full agreement with a previous report in the isolated working rat heart, where 10 nM spironolactone increased contractility on top of 10 nM aldosterone (Barbato et al., 2002). The ethanol concentration in the perfusion fluid in that study was maximally 0.036%. The ethanol concentration in the perfusion fluid in our experimental setup, at a CF of ≈12 ml min− 1, was most likely <1%, but >0.036%. Propanediol has also been used to dissolve aldosterone and spironolactone. This solvent, however, similarly reduces inotropy, and thus the ‘blocking' effect of 10 μM spironolactone (dissolved in propanediol) towards 10 nM aldosterone observed in an earlier study in the isolated rat heart (Moreau et al., 1996) may well be explained on the basis of the same physiological antagonism described above for ethanol. Taken together therefore, the inotropic effect of aldosterone is unlikely to be mediated via MR activation.
Positive inotropy favours coronary vasodilatation, and this may explain why Barbato et al. (2002), in the isolated working rat heart, observed vasodilation during a 45% increase in contractility in response to 10 nM aldosterone, as opposed to the aldosterone-induced vasoconstriction observed in the present study. In our Langendorff setup, the inotropic response to aldosterone was of modest proportion (maximally ≈10%) and this may not have been sufficient to result in a rise in CF. Rapid nongenomic vasoconstrictor effects of aldosterone have been described before (Schmidt et al., 2003), and hyperaldosteronism in hypertensive subjects results in impaired endothelium-dependent flow-mediated vasodilation (Nishizaka et al., 2004).
Ang II, like aldosterone, and in agreement with previous studies (de Lannoy et al., 1997; 1998; Libonati et al., 1997; Müller et al., 1998) caused coronary vasoconstriction and increased inotropy. The effects of Ang II were larger, occurred later and lasted longer than the effects of aldosterone on these parameters. Owing to this difference in time course (which has been noted before in cultured cells (Mazak et al., 2004)), we did not attempt to study the combined effects of Ang II and aldosterone on coronary vasoconstriction and inotropy. The difference in time course suggests that separate mechanisms underlie the contractile responses to Ang II and aldosterone, so that additive effects and / ;or synergy might be expected following combined application (Mazak et al., 2004; Michel et al., 2004).
Genomic effects
Spironolactone significantly improved the condition of the heart following ischaemia and reperfusion, as evidenced by a reduction in infarct size, incidence of fibrillation and LVEDP, and an increase in LVP recovery. The protective effects of spironolactone were comparable to those of preconditioning (Schoemaker & van Heijningen, 2000), and they cannot be contributed to ethanol, as in this part of the study we used a spironolactone concentration of 100 nM, that is, a concentration that is sufficiently low to avoid the ethanol-induced negative inotropic effects. Chronic in vivo treatment of rats with spironolactone similarly improved the condition of the heart when it was mounted in the Langendorff apparatus and exposed to low-flow ischaemia (Rochetaing et al., 2003). However, under those conditions it could not be concluded to what extent the effects of spironolactone were of cardiac origin. Our data now provide evidence that the beneficial effects of MR blockade during ischaemia indeed originate in the heart. There are several explanations for this finding. First, spironolactone may prevent adverse MR-mediated effects of locally synthesized aldosterone. Such effects include proarrhythmogenic actions (Tillmann et al., 2002) and increased synthesis of oxygen radicals (Mazak et al., 2004). Second, spironolactone might exert beneficial effects of its own, independently of aldosterone, for example, it acts antiarrhythmogenic through blockade of human Ether-a-Go-Go-Related gene (HERG) K+ channels (Caballero et al., 2003), and it inhibits calcium entry through calcium channels (Cargnelli et al., 2001). The lack of additional deleterious effects of aldosterone exposure, in addition to the evidence for aldosterone synthesis in the rat heart (Silvestre et al., 1998), particularly under ischemic conditions (Silvestre et al., 1999), favours the concept that spironolactone interferes with locally released aldosterone.
Aldosterone inhibited DNA and collagen synthesis in cultured cardiac cells, and spironolactone blocked these effects, thereby providing evidence for their MR-dependency. The aldosterone concentrations required to inhibit DNA and collagen synthesis were ≈100 times higher than the concentrations that induced vasoconstriction and positive inotropy. On the one hand, this suggests that the effects in cultured cells are indeed mediated via mechanisms / ;receptors different from the constrictor and inotropic effects in the intact heart, whereas, on the other hand, it is questionable whether such high concentrations actually exist at tissue sites in vivo. High aldosterone concentrations are required to activate cardiac MRs, because the levels of 11β-hydroxysteroid dehydrogenase 2 in the heart are relatively low (Lombès et al., 1995). This enzyme converts the MR-binding glucocorticoids cortisol and corticosterone (which circulate at concentrations that are several orders of magnitude higher than those of aldosterone) into the their non-MR-binding metabolites cortisone and 11-dehydrocorticosterone. Normally, glucocorticoid binding to MRs in cells maintained under serum-free conditions should not occur, unless the receptors are still occupied by glucocorticoids bound to the cells during their incubation with serum. Such cell binding also underlies the presence of renin–Ang system components in cardiomyocytes under serum-free conditions (van Kesteren et al., 1999).
The inhibitory effects of aldosterone on cardiac growth and remodelling contrast with in vivo studies showing cardiac fibrosis following long-term aldosterone exposure (Sun et al., 1993; 2002). However, fibrosis is also observed following conditional expression of an antisense mRNA of the MR in the mouse heart (Beggah et al., 2002). Thus, the effect of circulating aldosterone may differ from the effect of locally synthesised aldosterone, and myocardial fibrosis is unlikely to be a direct effect of aldosterone on cardiac fibroblasts. The effects of aldosterone on DNA and collagen synthesis oppose the effects of Ang II on these parameters, and this may explain why aldosterone, on top of Ang II, exerts a modulatory role in the development of cardiac hypertrophy in humans (Osterop et al., 1998; Deinum et al., 2001; Tsybouleva et al., 2004).
Finally, we verified the response of aldosterone on DNA synthesis in aortic VSMCs. When given at baseline, aldosterone reduced [3H]thymidine incorporation, and this effect was blocked, at least in part, by spironolactone. Thus, as in cardiomyocytes, aldosterone inhibited DNA synthesis in an MR-dependent manner. The fact that spironolactone did not completely block the effect of aldosterone suggests that other (nongenomic) pathways may also have played a role. Alternatively, the highest spironolactone concentration in this study (10 μM) may have been insufficient to fully prevent the almost complete inhibition of DNA synthesis in the presence of 1 μM aldosterone in VSMCs. In cardiomyocytes, the prevention at this concentration of spironolactone was more complete (allowing DNA synthesis to return to levels that were not significantly different from control), possibly because the effect of aldosterone in these cells was of more modest proportion.
In the presence of Ang II, aldosterone exerted a biphasic effect on DNA synthesis in VSMCs: at low concentrations (nM range) it enhanced the response to Ang II, and at high concentrations (μM range) it blocked the response to Ang II. Such a biphasic, Ang II-dependent, [3H]thymidine incorporation response to aldosterone has been noted before in rat VSMCs (Xiao et al., 2004). The mechanism mediating the enhanced response is not known, but may involve upregulation of AT1 receptors (Xiao et al., 2004). The fact that it occurred at nM concentrations of aldosterone is suggestive for a nongenomic phenomenon.
Conclusion
Aldosterone induces positive inotropic and vasoconstrictor effects in a nongenomic manner, and may thus potentiate the effects of Ang II on inotropy and vasoconstriction. Aldosterone reduces DNA and collagen synthesis via MR activation, and antagonises the Ang II-induced increases in these parameters. MR blockade reduces infarct size and restores cardiac function following a 45-min period of ischaemia to a similar degree as preconditioning. The cardiac MR-mediated effects may help to explain, at least in part, the beneficial actions of spironolactone and other MR antagonists on top of ACE inhibition in heart failure (Pitt et al., 1999; 2003).
Abbreviations
- Ang
angiotensin
- AT1 receptor
angiotensin II type 1 receptor
- CF
coronary flow
- LVEDP
left ventricular end-diastolic pressure
- LVP
left ventricular pressure
References
- AHOKAS R.A., SUN Y., BHATTACHARYA S.K., GERLING I.C., WEBER K.T. Aldosteronism and a proinflammatory vascular phenotype: role of Mg2 +, Ca2 +, and H2O2 in peripheral blood mononuclear cells. Circulation. 2005;111:51–57. doi: 10.1161/01.CIR.0000151516.84238.37. [DOI] [PubMed] [Google Scholar]
- BARBATO J.C., MULROW P.J., SHAPIRO J.I., FRANCO-SAENZ R. Rapid effects of aldosterone and spironolactone in the isolated working rat heart. Hypertension. 2002;40:130–135. doi: 10.1161/01.hyp.0000025879.29822.24. [DOI] [PubMed] [Google Scholar]
- BEGGAH A.T., ESCOUBET B., PUTTINI S., CAILMAIL S., DELAGE V., OUVRARD-PASCAUD A., BOCCHI B., PEUCHMAUR M., DELCAYRE C., FARMAN N., JAISSER F. Reversible cardiac fibrosis and heart failure induced by conditional expression of an antisense mRNA of the mineralocorticoid receptor in cardiomyocytes. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7160–7165. doi: 10.1073/pnas.102673599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRILLA C.G., ZHOU G., MATSUBARA L., WEBER K.T. Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J. Mol. Cell. Cardiol. 1994;26:809–820. doi: 10.1006/jmcc.1994.1098. [DOI] [PubMed] [Google Scholar]
- CABALLERO R., MORENO I., GONZALEZ T., ARIAS C., VALENZUELA C., DELPON E., TAMARGO J. Spironolactone and its main metabolite, canrenoic acid, block human ether-a-go-go-related gene channels. Circulation. 2003;107:889–895. doi: 10.1161/01.cir.0000048189.58449.f7. [DOI] [PubMed] [Google Scholar]
- CAMPBELL S.E., JANICKI J.S., MATSUBARA B.B., WEBER K.T. Myocardial fibrosis in the rat with mineralocorticoid excess. Prevention of scarring by amiloride. Am. J. Hypertens. 1993;6:487–495. doi: 10.1093/ajh/6.6.487. [DOI] [PubMed] [Google Scholar]
- CARGNELLI G., TREVISI L., DEBETTO P., LUCIANI S., BOVA S. Effects of canrenone on aorta and right ventricle of the rat. J. Cardiovasc. Pharmacol. 2001;37:540–547. doi: 10.1097/00005344-200105000-00006. [DOI] [PubMed] [Google Scholar]
- CHRIST M., EISEN C., AKTAS J., THEISEN K., WEHLING M. The inositol-1,4,5-trisphosphate system is involved in rapid effects of aldosterone in human mononuclear leukocytes. J. Clin. Endocrinol. Metab. 1993;77:1452–1457. doi: 10.1210/jcem.77.6.8263127. [DOI] [PubMed] [Google Scholar]
- CHRIST M., MEYER C., SIPPEL K., WEHLING M. Rapid aldosterone signaling in vascular smooth muscle cells: involvement of phospholipase C, diacylglycerol and protein kinase C alpha. Biochem. Biophys. Res. Commun. 1995;213:123–129. doi: 10.1006/bbrc.1995.2106. [DOI] [PubMed] [Google Scholar]
- DANSER A.H.J., VAN KATS J.P., ADMIRAAL P.J.J., DERKX F.H.M., LAMERS J.M.J., VERDOUW P.D., SAXENA P.R., SCHALEKAMP M.A.D.H. Cardiac renin and angiotensins. Uptake from plasma versusin situ synthesis. Hypertension. 1994;24:37–48. doi: 10.1161/01.hyp.24.1.37. [DOI] [PubMed] [Google Scholar]
- DE LANNOY L.M., DANSER A.H.J., BOUHUIZEN A.M.B., SAXENA P.R., SCHALEKAMP M.A.D.H. Localization and production of angiotensin II in the isolated perfused rat heart. Hypertension. 1998;31:1111–1117. doi: 10.1161/01.hyp.31.5.1111. [DOI] [PubMed] [Google Scholar]
- DE LANNOY L.M., DANSER A.H.J., VAN KATS J.P., SCHOEMAKER R.G., SAXENA P.R., SCHALEKAMP M.A.D.H. Renin–angiotensin system components in the interstitial fluid of the isolated perfused rat heart. Local production of angiotensin I. Hypertension. 1997;29:1240–1251. doi: 10.1161/01.hyp.29.6.1240. [DOI] [PubMed] [Google Scholar]
- DEINUM J., VAN GOOL J.M.G., KOFFLARD M.J.M., TEN CATE F.J., DANSER A.H.J. Angiotensin II type 2 receptors and cardiac hypertrophy in women with hypertrophic cardiomyopathy. Hypertension. 2001;38:1278–1281. doi: 10.1161/hy1101.096114. [DOI] [PubMed] [Google Scholar]
- DELCAYRE C., SILVESTRE J.S. Aldosterone and the heart: towards a physiological function. Cardiovasc. Res. 1999;43:7–12. doi: 10.1016/s0008-6363(99)00088-7. [DOI] [PubMed] [Google Scholar]
- HALLER H., QUASS P., LINDSCHAU C., LUFT F.C., DISTLER A. Platelet-derived growth factor and angiotensin II induce different spatial distribution of protein kinase C-alpha and -beta in vascular smooth muscle cells. Hypertension. 1994;23:848–852. doi: 10.1161/01.hyp.23.6.848. [DOI] [PubMed] [Google Scholar]
- JORDE U.P., VITTORIO T., KATZ S.D., COLOMBO P.C., LATIF F., LE JEMTEL T.H. Elevated plasma aldosterone levels despite complete inhibition of the vascular angiotensin-converting enzyme in chronic heart failure. Circulation. 2002;106:1055–1057. doi: 10.1161/01.cir.0000030935.89559.04. [DOI] [PubMed] [Google Scholar]
- LIBONATI J.R., EBERLI F.R., SESSELBERG H.W., APSTEIN C.S. Effects of low-flow ischemia on the positive inotropic action of angiotensin II in isolated rabbit and rat hearts. Cardiovasc. Res. 1997;33:71–81. doi: 10.1016/s0008-6363(96)00185-x. [DOI] [PubMed] [Google Scholar]
- LOMBÈS M., ALFAIDY N., EUGENE E., LESSANA A., FARMAN N., BONVALET J.P. Prerequisite for cardiac aldosterone action. Mineralocorticoid receptor and 11 beta-hydroxysteroid dehydrogenase in the human heart. Circulation. 1995;92:175–182. doi: 10.1161/01.cir.92.2.175. [DOI] [PubMed] [Google Scholar]
- LÖSEL R., FEURING M., WEHLING M. Non-genomic aldosterone action: from the cell membrane to human physiology. J. Steroid Biochem. Mol. Biol. 2002;83:167–171. doi: 10.1016/s0960-0760(02)00250-9. [DOI] [PubMed] [Google Scholar]
- MAASSENVANDENBRINK A., DE VRIES R., SAXENA P.R., SCHALEKAMP M.A.D.H., DANSER A.H.J. Vasoconstriction by in situ formed angiotensin II: role of ACE and chymase. Cardiovasc. Res. 1999;44:407–415. doi: 10.1016/s0008-6363(99)00249-7. [DOI] [PubMed] [Google Scholar]
- MAZAK I., FIEBELER A., MÜLLER D.N., PARK J.K., SHAGDARSUREN E., LINDSCHAU C., DECHEND R., VIEDT C., PILZ B., HALLER H., LUFT F.C. Aldosterone potentiates angiotensin II-induced signaling in vascular smooth muscle cells. Circulation. 2004;109:2792–2800. doi: 10.1161/01.CIR.0000131860.80444.AB. [DOI] [PubMed] [Google Scholar]
- MICHEL F., AMBROISINE M.L., DURIEZ M., DELCAYRE C., LEVY B.I., SILVESTRE J.S. Aldosterone enhances ischemia-induced neovascularization through angiotensin II-dependent pathway. Circulation. 2004;109:1933–1937. doi: 10.1161/01.CIR.0000127112.36796.9B. [DOI] [PubMed] [Google Scholar]
- MOREAU D., CHARDIGNY J.M., ROCHETTE L. Effects of aldosterone and spironolactone on the isolated perfused rat heart. Pharmacology. 1996;53:28–36. doi: 10.1159/000139412. [DOI] [PubMed] [Google Scholar]
- MÜLLER D.N., FISCHLI W., CLOZEL J.P., HILGERS K.F., BOHLENDER J., MÉNARD J., BUSJAHN A., GANTEN D., LUFT F.C. Local angiotensin II generation in the rat heart: role of renin uptake. Circ. Res. 1998;82:13–20. doi: 10.1161/01.res.82.1.13. [DOI] [PubMed] [Google Scholar]
- NISHIZAKA M.K., ZAMAN M.A., GREEN S.A., RENFROE K.Y., CALHOUN D.A. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109:2857–2861. doi: 10.1161/01.CIR.0000129307.26791.8E. [DOI] [PubMed] [Google Scholar]
- OBERLEITHNER H., LUDWIG T., RIETHMULLER C., HILLEBRAND U., ALBERMANN L., SCHAFER C., SHAHIN V., SCHILLERS H. Human endothelium: target for aldosterone. Hypertension. 2004;43:952–956. doi: 10.1161/01.HYP.0000123572.45556.a5. [DOI] [PubMed] [Google Scholar]
- OSTEROP A.P.R.M., KOFFLARD M.J.M., SANDKUIJL L.A., TEN CATE F.J., KRAMS R., SCHALEKAMP M.A.D.H., DANSER A.H.J. AT1 receptor A / ;C1166 polymorphism contributes to cardiac hypertrophy in subjects with hypertrophic cardiomyopathy. Hypertension. 1998;32:825–830. doi: 10.1161/01.hyp.32.5.825. [DOI] [PubMed] [Google Scholar]
- PITT B., REMME W., ZANNAD F., NEATON J., MARTINEZ F., RONIKER B., BITTMAN R., HURLEY S., KLEIMAN J., GATLIN M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- PITT B., ZANNAD F., REMME W.J., CODY R., CASTAIGNE A., PEREZ A., PALENSKY J., WITTES J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- ROCHETAING A., CHAPON C., MARESCAUX L., LE BOUIL A., FURBER A., KREHER P. Potential beneficial as well as detrimental effects of chronic treatment with lisinopril and (or) spironolactone on isolated hearts following low-flow ischemia in normal and infarcted rats. Can. J. Physiol. Pharmacol. 2003;81:864–872. doi: 10.1139/y03-081. [DOI] [PubMed] [Google Scholar]
- SARIS J.J., VAN DEN EIJNDEN M.M.E.D., LAMERS J.M.J., SAXENA P.R., SCHALEKAMP M.A.D.H., DANSER A.H.J. Prorenin-induced myocyte proliferation: no role for intracellular angiotensin II. Hypertension. 2002;39:573–577. doi: 10.1161/hy0202.103002. [DOI] [PubMed] [Google Scholar]
- SCHMIDT B.M.W., OEHMER S., DELLES C., BRATKE R., SCHNEIDER M.P., KLINGBEIL A., FLEISCHMANN E.H., SCHMIEDER R.E. Rapid nongenomic effects of aldosterone on human forearm vasculature. Hypertension. 2003;42:156–160. doi: 10.1161/01.HYP.0000083298.23119.16. [DOI] [PubMed] [Google Scholar]
- SCHOEMAKER R.G., VAN HEIJNINGEN C.L. Bradykinin mediates cardiac preconditioning at a distance. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1571–H1576. doi: 10.1152/ajpheart.2000.278.5.H1571. [DOI] [PubMed] [Google Scholar]
- SILVESTRE J.S., HEYMES C., OUBÉNAÏSSA A., ROBERT V., AUPETIT-FAISANT B., CARAYON A., SWYNGHEDAUW B., DELCAYRE C. Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation. 1999;99:2694–2701. doi: 10.1161/01.cir.99.20.2694. [DOI] [PubMed] [Google Scholar]
- SILVESTRE J.S., ROBERT V., HEYMES C., AUPETIT-FAISANT B., MOUAS C., MOALIC J.M., SWYNGHEDAUW B., DELCAYRE C. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J. Biol. Chem. 1998;273:4883–4891. doi: 10.1074/jbc.273.9.4883. [DOI] [PubMed] [Google Scholar]
- SUN Y., RATAJSKA A., ZHOU G., WEBER K.T. Angiotensin-converting enzyme and myocardial fibrosis in the rat receiving angiotensin II or aldosterone. J. Lab. Clin. Med. 1993;122:395–403. [PubMed] [Google Scholar]
- SUN Y., ZHANG J., LU L., CHEN S.S., QUINN M.T., WEBER K.T. Aldosterone-induced inflammation in the rat heart : role of oxidative stress. Am. J. Pathol. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILLMANN H.C., SCHUMACHER B., YASENYEV O., JUNKER M., CHRIST M., FEURING M., WEHLING M.Acute effects of aldosterone on intracardiac monophasic action potentials Int. J. Cardiol. 20028433–39.discussion 39–40 [DOI] [PubMed] [Google Scholar]
- TOM B., DE VRIES R., SAXENA P.R., DANSER A.H.J. Negative inotropic effect of bradykinin in porcine isolated atrial trabeculae: role of nitric oxide. J. Hypertens. 2001;19:1289–1293. doi: 10.1097/00004872-200107000-00014. [DOI] [PubMed] [Google Scholar]
- TOM B., DENDORFER A., DE VRIES R., SAXENA P.R., DANSER A.H.J. Bradykinin potentiation by ACE inhibitors: a matter of metabolism. Br. J. Pharmacol. 2002;137:276–284. doi: 10.1038/sj.bjp.0704862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOM B., GARRELDS I.M., SCALBERT E., STEGMANN A.P.A., BOOMSMA F., SAXENA P.R., DANSER A.H.J. ACE- versus chymase-dependent angiotensin II generation in human coronary arteries: a matter of efficiency. Arterioscler. Thromb. Vasc. Biol. 2003;23:251–256. doi: 10.1161/01.atv.0000051875.41849.25. [DOI] [PubMed] [Google Scholar]
- TSYBOULEVA N., ZHANG L., CHEN S., PATEL R., LUTUCUTA S., NEMOTO S., DEFREITAS G., ENTMAN M., CARABELLO B.A., ROBERTS R., MARIAN A.J. Aldosterone, through novel signaling proteins, is a fundamental molecular bridge between the genetic defect and the cardiac phenotype of hypertrophic cardiomyopathy. Circulation. 2004;109:1284–1291. doi: 10.1161/01.CIR.0000121426.43044.2B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN KATS J.P., DANSER A.H.J., VAN MEEGEN J.R., SASSEN L.M., VERDOUW P.D., SCHALEKAMP M.A.D.H. Angiotensin production by the heart: a quantitative study in pigs with the use of radiolabeled angiotensin infusions. Circulation. 1998;98:73–81. doi: 10.1161/01.cir.98.1.73. [DOI] [PubMed] [Google Scholar]
- VAN KESTEREN C.A.M., SARIS J.J., DEKKERS D.H.W., LAMERS J.M.J., SAXENA P.R., SCHALEKAMP M.A.D.H., DANSER A.H.J. Cultured neonatal rat cardiac myocytes and fibroblasts do not synthesize renin or angiotensinogen: evidence for stretch-induced cardiomyocyte hypertrophy independent of angiotensin II. Cardiovasc. Res. 1999;43:148–156. doi: 10.1016/s0008-6363(99)00057-7. [DOI] [PubMed] [Google Scholar]
- VAN KESTEREN C.A.M., VAN HEUGTEN H.A.A., LAMERS J.M.J., SAXENA P.R., SCHALEKAMP M.A.D.H., DANSER A.H.J. Angiotensin II-mediated growth and antigrowth effects in cultured neonatal rat cardiac myocytes and fibroblasts. J. Mol. Cell. Cardiol. 1997;29:2147–2157. doi: 10.1006/jmcc.1997.0448. [DOI] [PubMed] [Google Scholar]
- WEBER K.T. Aldosterone in congestive heart failure. N. Engl. J. Med. 2001;345:1689–1697. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- WEHLING M., NEYLON C.B., FULLERTON M., BOBIK A., FUNDER J.W. Nongenomic effects of aldosterone on intracellular Ca2 + in vascular smooth muscle cells. Circ. Res. 1995;76:973–979. doi: 10.1161/01.res.76.6.973. [DOI] [PubMed] [Google Scholar]
- XIAO F., PUDDEFOOT J.R., BARKER S., VINSON G.P. Mechanism for aldosterone potentiation of angiotensin II-stimulated rat arterial smooth muscle cell proliferation. Hypertension. 2004;44:340–345. doi: 10.1161/01.HYP.0000140771.21243.ed. [DOI] [PubMed] [Google Scholar]