Abstract
Ventricular fibrillation (VF), a cause of sudden cardiac death (SCD) in the setting of acute myocardial infarction (MI), remains a major therapeutic challenge. In humans, VF may occur within minutes or hours after the onset of chest pain, so its precise timing in relation to the onset of ischaemia is variable. Moreover, because VF usually occurs unobserved, out of hospital, and is usually lethal in the absence of intervention, its precise timing of onset is actually unknown in most patients. In animal models, the timing of susceptibility to VF is much better characterised. It occurs in two distinct phases. Early VF (defined as phase 1 VF, with possible subphases 1a and 1b in some animal species) occurs during the first 30 min of ischaemia when most myocardial injury is still reversible. Late VF, defined as phase 2 VF, occurs when myocardial necrosis is becoming established (after more than 90 min of ischaemia). Although much is known about the mechanisms and pharmacology of phase 1 VF, little is known about phase 2 VF. By reviewing a range of different types of data we have outlined the likely mechanisms and clinical relevance of phase 2 VF, and have evaluated possible future directions to help evolve a strategy for its suppression by drugs. The possibility that a proarrhythmic effect on phase 2 VF contributes to the adverse cardiac effects of certain cardiac and noncardiac drugs is also discussed in relation to the emerging field of safety pharmacology. It is concluded that suppression of phase 2 as well as phase 1 VF will almost certainly be necessary if drugs of the future are to achieve what drugs of the past and present have failed to achieve: full protection against SCD. Likewise, safety will require avoidance of exacerbation of phase 2 as well as phase 1 VF.
Keywords: Acute myocardial infarction, antiarrhythmic, arrhythmia, myocardial ischaemia, phase 2 ventricular fibrillation, proarrhythmia, safety pharmacology, sudden cardiac death
SCD, the problems posed by its clinical presentation, and its resistance to drug therapy
Sudden cardiac death (SCD) is a major cause of premature death in the U.K. (Wannamethee et al., 1995) and North America (Prystowsky, 2004). Lethal cardiac arrhythmias, especially VF, are accepted as a major cause of SCD (Campbell, 1983; 1987). Importantly, ventricular fibrillation (VF)-related mortality is not declining despite an overall decline in the prevalence of coronary artery disease (Zheng et al., 2001).
In the human population, susceptibility to VF appears to be highest during the first few hours after a coronary event, persisting for several hours before waning, although the precise temporal pattern of susceptibility is poorly characterised (Campbell et al., 1981; Adgey et al., 1982). In animal models of SCD in which local (regional) ischaemia and infarction are elicited by coronary artery ligation, it has been clearly established that VF can occur in two distinct and separate phases, the first associated with the period of reversible injury (the condition where reperfusion of ischaemic tissue leads to cell recovery) and the second associated with the period of infarct evolution (Johnston et al., 1983a, 1983b; Curtis, 1998). In humans, equivalent information on the timing of VF is difficult to obtain, but a susceptibility similar to that seen in animal models, occurring during both reversible and irreversible injury, can be inferred from data showing that approximately 50% of patients successfully resuscitated from VF have evidence of infarction while the other 50% do not (De Vreede-Swagemakers et al., 1998). Therefore, although difficult to prove beyond doubt, it would appear that separate phases of VF, as defined from animal studies, may occur in humans and that each may contribute to SCD. For reasons that will become apparent, this has important therapeutic implications.
When VF arises in man, it usually does so without warning and therefore, usually, before the victim has been admitted to hospital, and survival is less than 5% (Prystowsky, 2004). As a consequence, the only feasible means of evaluating possible new therapy in humans is a large controlled clinical trial of prophylactic intervention, with SCD as the primary end point (Pratt et al., 1998). This approach has proven problematic, however, and there is no accepted methodology for assigning patients to treatment according to a stratification of their SCD risk (Huikuri et al., 2003). For reasons of practicality, in the past, surrogate end points for VF have been used clinically as a guide to therapy, with the likelihood of benefit with a drug estimated on the basis of its ability to suppress spontaneously occurring ventricular premature beats (VPBs) or more severe arrhythmias induced by programmed electrical stimulation (e.g., Bourke et al., 1995). However, it is now established that these end points do not accurately predict the effectiveness of drugs in the prevention of SCD (Bourke et al., 1995; Goldstein et al., 1995); there are no surrogate end points for testing new drugs for VF / SCD.
Moving even further away from VF itself in an attempt to anticipate is occurrence, there are biochemical substances including troponin I, C-reactive protein (CRP) and lipoprotein-associated phospholipase A2 (Lp-PLA2) that have been used to predict the risk of adverse coronary events in humans (Biasucci, 2004; Oei et al., 2005). Whether these may also serve as ‘markers' of risk of VF is unknown, and whether they may be applied to selection of therapeutic intervention is even more uncertain. Although recent clinical evidence suggests that reduction of plasma CRP levels with statin therapy can reduce the risk of death (Muhlestein et al., 2004; Ridker et al., 2005), it is unclear whether this relates to a direct effect on VF. The possibility that these and other substances have direct effects on VF susceptibility is discussed later in this review.
It is not surprising, in view of these considerations, that several large SCD trials of antiarrhythmic drugs in post-MI patients, in which the choice of drugs has been guided in part by surrogate endpoint data, have had outcomes that have been disappointing. For some drugs the outcome has been catastrophic, with survival rate decreased by the drug (Pratt et al., 1998). This has had a major negative impact on drug development, and few pharmaceutical companies are now prepared to countenance the notion of investing in the development of new antiarrhythmic drugs for VF / SCD.
Of the SCD trials completed, the survival with oral D-sotalol (SWORD) trial, in which post-MI mortality was doubled in the treatment group, remains an enigma since there is no proven explanation for the failure of the drug (Cobbe, 1996; Pratt et al., 1998). The cardiac arrhythmia suppression trial (CAST) study, which also revealed a treatment-related increase in death (Goldstein et al., 1995), has been subjected to great scrutiny, from which it has been suggested that the test drugs exacerbated VF, either during the early phase of ischaemia or later, during the infarct evolution phase (Greenberg et al., 1995; Hallstrom et al., 1995). Importantly, it has been suggested from these and other clinical studies that a drug that suppresses one phase of VF (‘early' or ‘late') may fail to protect against, or may even facilitate, susceptibility to another phase of VF (Campbell, 1987; Greenberg et al., 1995). If the effects of drugs on VF in the human are phase-dependent and the underlying mechanisms of VF correspondingly phase-dependent, this has major relevance to the implementation of any strategy for development of effective new therapeutic interventions.
As the SWORD and CAST trials also show that the circumstances in humans in which antiarrhythmic drugs may be proarrhythmic are poorly characterised, this leads on to an issue separate from the main focus of this article (VF suppression), that of the possible relationship between VF occurring during infarct evolution and the safety of noncardiac drugs. Many non-cardiac drugs can evoke arrhythmias under circumstances that are generally well-recognised, including hypokalaemia and conditions associated with a long QT interval (Malik & Camm, 2001). However, the issue of noncardiac drug-induced proarrhythmia remains an important and controversial area in the emerging science of safety pharmacology, especially with regard to the identification of risk factors that may make an individual more susceptible (Kinter et al., 2004; Pugsley, 2004). It is therefore intriguing to note that there have been few, if any, attempts to equate apparent drug-induced proarrhythmia with possible exacerbation of infarct-related VF.
In view of these issues, there is a need to focus on the different phases of VF, to define and distinguish component mechanisms and mediators, and to explore protective strategies, and possible links with drug-induced proarrhythmia, using animal models. Despite the well-documented existence of different phases of VF in animal models of SCD (see Curtis, 1998), we find that there is no definition of the phases that is formally agreed. We offer the following: with one-stage maintained coronary obstruction, the early phase of VF occurring during the first 30 min of ischaemia is defined as phase 1, and the later phase, beginning approximately 90 min after the start of ischaemia, during infarct evolution, is defined as phase 2. For reasons explained below (which are largely to do with its neglect), phase 2 VF is the focus of the present article.
Animal models of ischaemia and infarction and their use in phase 2 VF study
Different models, utilising in vitro isolated buffer-perfused hearts (Bricknell & Opie, 1978; Daugherty et al., 1986; Hearse et al., 1999) and intact anaesthetised (Janse et al., 1979; Clark et al., 1980; Coker, 1989) and conscious animals (Curtis et al., 1984; Schwartz et al., 1984), have been employed to investigate the pathophysiological phenomena associated with ischaemia and infarction, and their modulation by drugs. Some of these models are suitable for the study of arrhythmias and others are not. With regard to the main focus of this article few, unfortunately, have been characterised for possible use in the study of phase 2 VF.
Isolated cardiac myocytes have been used for the study of the effects of ischaemia on cellular electrophysiology (Cerbai et al., 1991; Entman et al., 1992; Wu & Corr, 1994; Vanden Hoek et al., 1997). However, ischaemia must be simulated. Investigators seek to achieve this by superfusion with hypoxic solution (Buerke et al., 1994; Silverman et al., 1997; Nakano et al., 1998), by metabolic inhibition of ATP synthesis using inhibitors of oxidative phosphorylation (Williams et al., 2001; Rodrigo et al., 2002), or by superfusion with a solution mimicking the extracellular space in ischaemic myocardium (i.e., glucose-free, acidic solution containing high concentrations of lactate and K+, sometimes with added inhibitors of glycolysis) (Vanden Hoek et al., 1997; Wilders et al., 1999; Levraut et al., 2003). However, none of these approaches truly mimics whole heart ischaemia, and none have been designed to mimic the milieu associated with phase 2 VF. Moreover, isolated cardiac myocytes are not suitable for the study of VF (whether it be phase 1 or 2) or its suppression, a priori, since re-entry, the mechanism of VF and other arrhythmia propagation through the myocardial syncytium (Janse, 1991) cannot occur or be modelled in single-cells. Furthermore, the temporal variation of interstitial accumulation of substances leaving the ischaemic cell (whether associated with phase 1 or phase 2 VF) cannot easily be replicated in single-cell superfusion studies, making it difficult to assess the role of these substances in mediating the relevant electrophysiological dysfunction. These considerations indicate that whole hearts (in vivo and / or in vitro) with regional ischaemia are preferred for modelling VF / SCD, and essential if VF itself is the desired study variable.
Global ischaemia (affecting the whole heart) is modelled only in vitro (Bricknell & Opie, 1978; Kleber, 1983; Fukunami & Hearse, 1985). It is not known whether infarct-related phase 2 VF occurs in globally ischaemic hearts as it has not been studied. Globally ischaemic hearts develop asystole (no VF) within a few minutes of cessation of coronary flow, and asystole is sustained for the duration of ischaemia and the experiment, typically 30 min (Ridley et al., 1992). It may be the case that globally ischaemic hearts reanimate electrophysiologically after more sustained ischaemia and fibrillate, but this has not been tested in the laboratory (at least, not under normothermic and pharmacologically unadulterated conditions). The global ischaemia models can be modified by partially reducing coronary perfusion so that a residual flow (usually 10% of control flow) is retained. This is termed low-flow ischaemia, and can be achieved most conveniently, in vitro, by reducing the delivery pressure of the solution perfusing the heart or by controlling coronary flow using a pump (Culling et al., 1984; Cave et al., 1997; De Jonge & De Jong, 1999; Gogelein et al., 2001). Again, it is not known whether phase 2 VF occurs in these models.
Regional ischaemia, that involving a limited part of the heart, may be studied in vitro (Daugherty et al., 1986; Coker, 1989; Curtis et al., 1993a, 1993b; D'alonzo et al., 1994) and in vivo (Clark et al., 1980; Billman, 1994). Regional ischaemia models have provided the majority of the emerging data on phase 2 arrhythmias and their suppression by drugs.
Characteristics of phase 2 versus phase 1 VF in regional ischaemia models in vivo
Regional ischaemia is usually achieved by tying a ligature around a left coronary artery, leaving perfusion of the remainder of the arterial bed intact (Harris, 1950; Johns & Olson, 1954; Rushmer et al., 1963; Johnston et al., 1983a, 1983b; Curtis, 1998). Regional ischaemia is highly arrhythmogenic in vivo and in vitro, with the majority of control hearts experiencing VF at some point during the experiment in some species (Curtis, 1998). In animal models, there is a well-characterised transition period during which reversible injury changes to irreversible injury, whereupon reperfusion becomes incapable of salvaging the tissue. The process of transition requires a minimum of 20 min of continuous and severe ischaemia to begin, and a minimum of 60 min of continuous ischaemia to be complete (Garcia-Dorado et al., 1987). A bell-shaped relationship has been found to exist between the size of the ischaemic zone and susceptibility to phase 1 VF, with maximum susceptibility occurring when ischaemic zone size is between 30 and 50% of total ventricular weight, and this has important implications about arrhythmogenic mechanisms (Curtis & Hearse, 1989a, 1989b; Ridley et al., 1992). Unfortunately, the relationship between the size of the infarcting zone and susceptibility to phase 2 VF is unknown.
During the last 50 years, the regional ischaemia models most widely used for preclinical SCD drug research have been adapted from those of Harris (1950), Kenedi & Losonci (1973), Schwartz et al. (1984) and Lucchesi (Patterson et al., 1982). There are other, older models (Tillmanns et al., 1983), but they have not greatly contributed to the study of VF / SCD. Of those cited above, in their chronological order, Harris (1948) initially developed a one-stage complete coronary artery ligation model in the anaesthetised dog and found a high susceptibility to lethal VF during the first 10 min of ischaemia, but opted in subsequent studies to focus on arrhythmogenic mechanisms associated with sustained ischaemia. To achieve this, Harris (1950) developed the ‘two-stage ligation model', described in detail by Hashimoto et al. (1982). However, the model is not suitable for studies on VF because the first stage of coronary ligation, occurring at time zero, is intended to only partially occlude a coronary artery, thus generating low-flow regional ischaemia with minimal electrophysiological dysfunction and few arrhythmias. Consequently, early ischaemia-induced VF (that which is now referred to as phase 1 VF) is deliberately absent. VF may occur later, after a subsequent, second-stage, complete coronary ligation, but it is unclear whether this is a manifestation of delayed phase 1 VF or is true phase 2 VF (as characterised in other models). Moreover, the late-occurring VF is itself rare in this model (Hashimoto et al., 1982), so it appears that the two-stage ligation process itself delays or suppresses VF, making it as hard to study in the model as it would be to classify. As a consequence, the main end points measured in the Harris model have been the less severe arrhythmias, VPBs and ventricular tachycardia (VT). Despite this, the model has been used extensively (Hashimoto et al., 1982). The model has, however, been adapted to allow VF to be studied by the use of programmed electrical stimulation of the ventricles (Aidonidis et al., 1995) under the supposition that electrically evoked VF may serve as a surrogate end point for coronary ligation-induced spontaneous VF. Unfortunately, this can now be seen as an unwise approach, since spontaneous VPBs and VT and electrically induced VF are now known to have only limited predictive value when used as surrogates for VF in clinical studies designed to select drug therapy on an individual patient basis for prevention of SCD and to predict the effect of a drug on long-term survival (Bourke et al., 1995; Goldstein et al., 1995), as mentioned earlier.
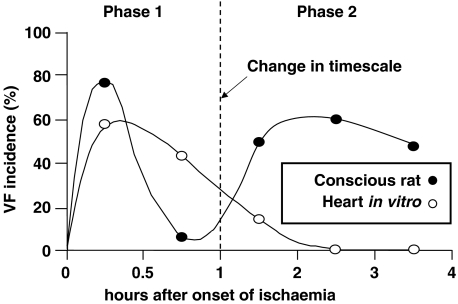
The Kenedi & Losonci (1973) model involves one-stage coronary artery ligation, and was described first as a method for use in rats. It would perhaps be better known as the Parratt / Szekeres / Walker model, as these were the investigators who independently adapted it, and used it extensively for the study of VF and its suppression (for reviews, see Curtis et al., 1987; Clements-Jewery & Curtis, 2003). The model was initially developed to downsize to small animals and simplify the first Harris (1948) model to allow convenient study of the arrhythmias occurring during a brief (30 min) period of ischaemia (e.g., Au et al., 1979), but was subsequently modified by simply extending the duration of the experiment. This seemingly trivial modification allowed unequivocal identification for the first time of a second phase of VF, occurring during infarct evolution (e.g., Clark et al., 1980). Characterisation studies have established that conscious rats surviving the first 30 min of ischaemia (which is associated with phase 1 VF and reversible myocardial injury) always experience a hiatus of normal sinus rhythm, which is then followed by the onset of a second phase of arrhythmias including VF (Figure 1). Phase 2 VF occurs spontaneously (i.e. without the need for electrical stimulation of the ventricle) in this model after about 2 h of sustained ischaemia, coinciding with the establishment of irreversible injury (Curtis et al., 1987). The onset of VF occurs much earlier and the prevalence of VF is much greater than that in the Harris two-stage ligation dog model (Harris, 1950), implying that the severity of ischaemia determines the likelihood and latency to the onset of phase 2 VF. The severity of ischaemia is minimised in the Harris dogs because the initial occlusion is partial, and also because most dog hearts (unlike rat hearts, in which phase 2 VF is best characterised) exhibit substantial collateral coronary blood flow (Meesmann, 1982; Curtis, 1998).
Figure 1.
Time course of onset of ventricular fibrillation in conscious rats (•) (n=18) and isolated Langendorff perfused rat hearts (○) (n=12) subjected to left coronary artery occlusion at the same site. The distinct phases of VF are termed phase 1 (occurring <2 h after coronary occlusion) and phase 2 (occurring >2 h after coronary occlusion). Reentry and the flow of ‘injury current' are the likely mechanisms responsible for initiation of phase 1 VF, while re-entry and abnormal automaticity are the likely corresponding mechanisms for phase 2 VF. Figure modified and adapted from Curtis (1993).
Presently, there is insufficient evidence to determine precisely the extent to which the timing and severity of phase 2 VF differ between species. Differences may be anticipated, since there are differences for phase 1 VF, with some species, including rats, exhibiting a single period of susceptibility and others, including the dog, exhibiting subcomponents known as phase 1a and phase 1b (Curtis, 1998). Despite this, there are similarities between species. For example, it is well established that rats have a much reduced susceptibility to arrhythmias once they have survived 24 h of continuous regional ischaemia (Clark et al., 1980; Curtis et al., 1987), and limited information appears to indicate the same to be the case in dogs (Kaumann & Aramendia, 1968). Although few attempts have been made to undertake characterisation in other species, phase 2 VF does occur where studied, in the anaesthetised pig (Pugsley et al., 1995) and possibly also the conscious baboon (Vatner et al., 1988b).
The Schwartz / Billman and Lucchesi canine models, which involve one-stage complete obstruction of a coronary artery in conjunction with exercise or with the superimposition of acute ischaemia in hearts with a previous infarction, have been used extensively to examine drug effects on phase 1 VF but, as a consequence of this focus, the duration of ischaemia has always been brief (<30 min) and so it is unknown whether in these models phase 2 VF would also occur were ischaemia to be maintained for longer.
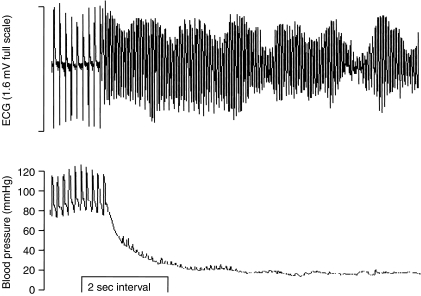
All in all, VF susceptibility following coronary obstruction in animals appears to have condition-dependent subcomponents that appear to be model-dependent and difficult to link with specific clinical counterparts but, nevertheless, two distinct main phases of susceptibility appear to exist. Although the relative importance of phase 2 versus phase 1 VF may be unclear and difficult to ascertain in humans (Campbell, 1987), phase 2 VF has been observed in vivo following permanent one-stage coronary ligation in all animal species examined to date, as noted above, so it would be no more wise to discount its possible clinical relevance than to discount the relevance of phase 1 VF. Indeed, although the finding does not reflect the majority of data derived from the model (Curtis et al., 1987), there is a study using the conscious rat that found the susceptibility to phase 2 VF to be much greater (17 / 21 animals) than susceptibility to phase 1 VF (4 / 23 animals) during permanent coronary occlusion (Opitz et al., 1995). An example trace of phase 2 VF with accompanying blood pressure recording is shown in Figure 2.
Figure 2.
Example of phase 2 VF in a pentobarbitone-anaesthetised rat. Accompanying changes in blood pressure are shown beneath the ECG trace.
Biochemical and electrophysiological events underlying phase 2 versus phase 1 VF
The distinct phases of VF in animal models, depicted in Figure 1, coincide with distinct biochemical and electrophysiological changes at the cellular and intercellular levels. Although their role in mediating VF is unproven (this remains the case even for phase 1 VF, according to strict criteria set out some years ago; Curtis et al., 1993b), these processes are well characterised (Curtis et al., 1993a, 1993b; Allessie et al., 1995; Cascio et al., 1995) and we describe them here only briefly. Specific metabolic dysfunction that occurs during the period of phase 1 VF includes depletion of ATP and accumulation of ADP and lactate as a result of anaerobic glycolysis (Bricknell & Opie, 1978). Electrophysiological changes during the phase 1 period include a depolarisation of the resting membrane potential, and an accumulation of extracellular K+ which is typically triphasic, with the initial rise and plateau phases occurring during the first 30 min period after coronary obstruction, commonly denoted as ‘acute ischaemia', coinciding well with the time when phase 1 arrhythmias occur (Hirche et al., 1980). The rise in membrane potential attenuates the amplitude and upstroke velocity of the action potential as a result of Na+ channel inactivation, and action potential duration typically lengthens and then shortens with prolonged ischaemia probably as a result of enhanced outward repolarising K+ currents (Cascio et al., 1995). The phase 1 period is also associated with the intercellular accumulation of many biochemicals, including catecholamines, K+, and amphiphiles such as lysophosphatidylcholine and platelet-activating factor, and although many have arrhythmogenic properties, no individual substance has yet been identified as being both sufficient and necessary for mediation of phase 1 VF (Curtis, 1993; Curtis et al., 1993b; Clements-Jewery & Curtis, 2003; Baker & Curtis, 2004).
Phase 2 VF coincides with the establishment of infarction (Ravingerova et al., 1995). In collateral-deficient hearts, the infarction process begins after approximately 15–20 min of sustained ischaemia (Hort & Dacanalis, 1965), and can be complete by approximately 1 h since reperfusion begun after this time will not salvage any myocardial tissue (Garcia-Dorado et al., 1987). However, the healing process may take between 24 and 48 h to complete (Bolli & Marban, 1999). This process of evolution of the infarct is accompanied by a number of metabolic, ionic and electrophysiological changes. Metabolically, this is characterised by low glycogen and high lactate intracellular contents, with virtual cessation of anaerobic glycolysis resulting in ATP and creatine phosphate levels below 10% and 2% of normal, respectively (Jennings et al., 1990). In addition, the adenine nucleotide pool consists chiefly of AMP and there are high intracellular contents of inosine and hypoxanthine as a result of adenosine deamination (Jennings et al., 1990). With respect to ionic changes, the extracellular K+ accumulation that begins during the phase 1 period of ischaemia proceeds monophasically during infarct evolution. In the in situ pig heart, the rise in extracellular K+ continues for at least the first hour of ischaemia (the limit of the cited study period), without sign of slowdown, and with extracellular K+ levels eventually exceeding 20 mM (Hill & Gettes, 1980). Intracellular K+ levels decline correspondingly, and a substantial reduction in intracellular K+ activity has been recorded in excised Purkinje fibres after 3 h of sustained ischaemia (Hanna et al., 1988). Infarction is also characterised by a high cardiac intracellular Na+ and Ca2+ content (Buja et al., 1975; Van Echteld et al., 1991). It remains uncertain which of these changes are sufficient and necessary for mediation of phase 2 VF.
Purkinje fibres, surviving but dysfunctional, have been proposed to be the main arrhythmogenic foci during the infarct evolution period (Friedman et al., 1973; Horowitz et al., 1976). After 1 h of ischaemia, Purkinje and surviving muscle fibres exhibit reduced resting potentials, reduced action potential amplitudes and reduced upstroke velocities (Fenoglio et al., 1979). The action potential duration, prolonged in muscle fibres, is shortened in Purkinje fibres, facilitating re-entry (Fenoglio et al., 1979).
In view of these considerations and the evident differences between reversible ischaemia and the evolving infarct in terms of the biochemical and electrophysiological milieu, it is unsurprising that differences appear to exist in the electrophysiological processes underlying phase 1 and phase 2 VF. Whereas re-entry and flow of ‘injury current' (Janse et al., 1980) coincide with the appearance of phase 1 VF (Janse, 1991), re-entry and abnormal automaticity (delayed after-depolarisations) are regarded as likely prevalent mechanisms during infarct development (Wit, 1989). On the other hand, there are no major differences in terms of ECG and haemodynamic characteristics in the few seconds preceding the occurrence of phase 2 versus phase 1 VF, according to data from our laboratory (Table 1). Table 1 shows that 30% of phase 1 and 30% of phase 2 VF are associated with preceding VPBs, bigeminy or salvos, and 60% of phase 1 VF and 50% of phase 2 VF occurred as a result of ‘degeneration' of VT. The only substantial differences between the antecedents of phase 1 and phase 2 VF are the magnitudes of the ECG's R and Q waves, which reflects the evolution of the infarct but does not delineate different underlying arrhythmogenic mechanisms. The future elucidation of underlying mechanisms is relevant, and perhaps essential, to rational drug targeting.
Table 1.
ECG and haemodynamic variables immediately prior to the onset of phase 1 or phase 2 VF
| Phase of VF | Blood pressure | ECG intervals | ECG magnitude | Proportion of VF thatwas preceded by VPBs / salvos / bigeminy (%) | Proportion of VFpreceded by degeneratingVT (%) | Occurrence ofspontaneousdefibrillation (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Systolic | Diastolic | RR | QT | PR | R wave | Q wave | ||||
| Phase 1 | 100±8 | 65±7 | 150±8 | 48±4 | 40±1 | 0.91±0.09 | 0.18±0.04 | 30 | 60 | 0 |
| Phase 2 | 110±6 | 76±4 | 140±5 | 54±1 | 41±1 | 0.63±0.06* | 0.47±0.05* | 30 | 50 | 10 |
In each case, data were taken from n=10 consecutive fibrillating anaesthetised rats (subjected to 4 h coronary occlusion). The 5 s period preceding the occurrence of VF was used to obtain the values above, taking note of whether VF occurred abruptly or was preceded by ectopy (VPBs, etc) or by a degenerating form of VT. Only one rat in the data set developed both phase 1 and phase 2 VF *Denotes P<0.05 versus Phase 1 VF, assuming that the data set represents a random sample of the population.
The effect of drugs on phase 2 VF, and limitations of available data
Actions of drugs on phase 1 VF is not a topic that will be dealt with here (it was our intention to refer readers to a review, but there are none suitable that incorporate recent data from preclinical studies – perhaps the time is right for an update). With regard to phase 2 VF, although the data are limited, there are a number of studies that have explored the effects of drugs. These studies have largely been conducted in rats in vivo, the findings of which are summarised in Table 2. Although there is some variation in the type of anaesthetic, age of animal and laboratory of study, the database is insufficiently large for detailed subanalysis. However, it is apparent that there is a clear lack of effectiveness of most drugs tested at ‘reasonable' dosage (i.e., dosage achieving intended molecular target selectivity). Perhaps the most important consideration of all is that many classes of potentially interesting drugs of relevance to ischaemic heart disease and arrhythmogenesis, such as angiotensin-converting enzyme inhibitors, sodium–proton exchange inhibitors, thrombin antagonists, antiplatelet drugs and platelet-activating factor (PAF) antagonists, have yet to be tested for their effects on phase 2 VF. Likewise, selective IKr blockers have not been examined for their potential to exacerbate phase 2 VF, a safety pharmacology issue revisited later in this article.
Table 2.
The effect of drugs on phase 1 and phase 2 VF in the same animals in vivo
| Drug | Species | Anaesthesia | Dose | Reduction of phase1 VF? | Reduction of phase2 VF? |
|---|---|---|---|---|---|
| Class I drugs | |||||
| Lidocaine1 | Rat | None | 2 mg kg− 1 i.v. | Yes | Yes |
| Lidocaine2 | Rat | Pentobarbitone | 10 mg kg− 1 + 5 mg kg− 1 h− 1 i.v. | Yes | No |
| Quinidine3 | Rat | None | 20 mg kg− 1 i.v. | Yes | No |
| Disopyramide3 | Rat | None | 10 mg kg− 1 i.v. | No | No |
| Mexiletine4 | Rat | None | 20 mg kg− 1 i.v. | Yes | No |
| Quinacainol5 | Rat | None | 4 mg kg− 1 i.v. | Yes | No |
| Class II drugs | |||||
| Labetalol6 | Rat | None | 5 mg kg− 1 i.v. | No | No |
| Oxprenolol7 | Rat | Pentobarbitone | 50 mg kg− 1 p.o. b.i.d | Yes | No |
| Pindolol1 | Rat | None | 15 μg kg− 1 i.v. | Yes | Yes |
| Propranolol6 | Rat | None | 0.2 mg kg− 1 + 0.1 μg kg− 1 min− 1 i.v. | No | No |
| Class III drugs | |||||
| Tedisamil8 | Rat | None | 2 mg kg− 1 i.v. | Yes | No |
| Class IV drugs | |||||
| Nifedipine9 | Rat | None | 0.5, 2 mg kg− 1 i.v. | Yes | No |
| Nifedipine9 | Rat | None | 10 mg kg− 1 i.v. | Yes | Yes |
| Verapamil10 | Rat | None | 20 mg kg− 1 i.v. | Yes | No |
| Felodipine11 | Rat | None | 4 mg kg− 1 i.v. | No | No |
| Anipamil12 | Pig | Pentobarbitone | 5 mg kg− 1 + 0.5 mg kg− 1 min− 1 | No | No |
| Miscellaneous interventions | |||||
| Blood K+ elevation13 | Rat | None | A range of KCl infusions | Yes | Yes |
| Nafazatrom14 | Rat | Ether | 100 mg kg− 1 b.i.d. | No | Yes |
| Aspirin15 | Rat | None | 100 mg kg− 1 i.v. | No | No |
Data are taken from: (1) (Lepran et al. (1983), (2) Clark et al. (1980), (3) Johnston et al. (1983a, 1983b), (4) Igwemezie et al. (1992), (5) Howard et al. (1992), (6) Botting et al. (1983), (7) Campbell et al. (1984), (8) Beatch et al. (1991), (9) Curtis & Walker (1988), (10) Curtis et al. (1984), (11) Curtis et al. (1985a). (12) Pugsley et al. (1995), (13) Saint et al. (1992), (14) Fiedler, (1983), (15) Johnston et al. (1983a, 1983b).
There are several problems with the data summarising drug effects on phase 2 VF, shown in Table 2. Most of the drugs were given as pretreatment and in most studies no check was made to ensure that blood levels of test drugs were adequate during the phase 2 period. Additionally, death due to phase 1 VF reduced group sizes in some studies (e.g., Curtis et al., 1984), giving rise to scope for type-2 statistical errors.
The reason why almost all the data on drug actions on phase 2 VF come from studies using rats is unclear, but it probably reflects the perceived favourable bioassay characteristics of rat permanent coronary ligation models that make them so convenient for phase 1 VF studies (Curtis et al., 1987), encouraging exploration of phase 2 VF – because it can be done, and easily. However, there are differences between rats that have recovered from preparative surgery and are subjected to coronary ligation while conscious versus rats that are prepared acutely and subjected to coronary ligation under anaesthesia which will inform model choice in any future research strategy. In anaesthetised rats, the control incidence of phase 2 VF can be variable and sometimes too low for detecting antiarrhythmic effects of drugs (Table 3), indicating that the favourable bioassay characteristics achieved for the study of phase 1 VF in the model are not always replicated for phase 2 VF. On the other hand, in conscious rats, although the control incidence of phase 2 VF is higher and more reproducible (Table 4), there are ethical and technical issues which make it more of a challenge to justify undertaking studies. Nevertheless, since differences do exist between the acutely prepared anaesthetised and the conscious settings, it can be argued that one or the other is providing potentially misleading data. Unfortunately, in the absence of a clinical template, it is not clear which, and more work is indicated, although the case for more conscious animal work would seem to be compelling.
Table 3.
Incidences of phase 2 VF in anaesthetised rats obtained by different investigators
| Incidence of phase 2 VF (%) | N | Reference |
|---|---|---|
| 46 | 24 | Clark et al. (1980) |
| 37 | 19 | Campbell et al. (1984) |
| 24 | 17 | Campbell et al. (1984) |
| 19 | 9 | Curtis et al. (1985a, 1985b, 1985c) |
Mean±s.d.: 32±12%.
Note that there are several other publications in which phase 1 and phase 2 VF incidence is recorded combined, meaning that it is not possible to identify the control incidence of phase 2 VF (e.g., Beatch & McNeill, 1988).
Table 4.
Incidences of phase 2 VF in conscious rats
| Incidence of phase 2 VF | Year |
|---|---|
| 90 | 1981 |
| 70 | 1981 |
| 50 | 1981 |
| 70 | 1983 |
| 55 | 1983 |
| 75 | 1984 |
| 60 | 1984 |
| 75 | 1985 |
| 75 | 1985 |
| 100 | 1986 |
| 89 | 1988 |
| 66 | 1992 |
| 81 | 1995 |
Mean±s.d.: 74±14%. Data are taken from Curtis et al. (1987); Opitz et al. (1995); Saint et al. (1992). For each year, the data represent the group mean incidence from groups of sizes ranging from 4 to 9 (variation due to variation in numbers of deaths occurring from phase 1 VF and cardiac output failure prior to the start of the phase 2 observation period).
As a further caveat, there has been a widespread preconception that the rat is an irrelevant species for the study of any form of ventricular arrhythmia whether it be induced by ischaemia (phase 1) or by infarction (phase 2). This is not because there is any evidence that arrhythmogenesis is atypical in the rat, nor because of evidence that the clinical effectiveness of drugs cannot be predicted in rats, but because the rat heart has characteristics that differ markedly from the human. These characteristics, along with those typical of hearts from dogs and humans, are displayed in Table 5. It is certainly true that the rat is unsuitable for evaluating the possible actions (protective or proarrhythmic) of delayed rectifying K+ channel-blocking drugs, since the relevant channels (HERG / minK) are not functional in rat ventricle (Tande et al., 1990). Although there is no a priori reason for concern regarding most other putative drug targets and the use of rat hearts, the issue of appropriate species choice for the study of phase 2 VF does remain unresolved (as it does for most therapeutic areas where there are few truly effective drugs – without a clinical template, it is impossible to judge the suitability of any model).
Table 5.
Typical characteristics of hearts from rats, dogs and humans
| Species | Typical heart weight: bodyweight ratio (g kg− 1) |
Typical body weight (Kg) |
Typical heart rate (beats min− 1) |
APD (msec) |
|---|---|---|---|---|
| Rat | 3.33a | 0.3a | 400 in vivod | 30–60e |
| 250–350 in vitrod | ||||
| Canine | ∼7b | 23b | 90–140 in vivob | 200–240f,g |
| Human | ∼5c | 70c | 70–90 | ∼300f,g |
Data are taken from:
The general paucity of data on the actions of drugs on phase 2 VF may be largely a historical quirk resulting from the fact that researchers (including ourselves) have tended to focus primarily on phase 1 VF and neglect subsequent events. The reasons for this are not necessarily scientifically justified. Thus, because phase 2 VF experiments take much longer to complete, and are therefore less appealing to perform than corresponding phase 1 VF experiments that allow the relatively quick generation of data, there is inevitably a disincentive to make the effort to study phase 2 VF. Additionally, if a heart fibrillates within 20 min of the onset of regional ischaemia (as it does in most animal models; see Curtis, 1998), an investigator may question the need to preserve the heart's rhythm to prolong survival in order to investigate later events. As a result of the influence of these factors, until recently (Clements-Jewery et al., 2002a, 2002b), there have been no publications on drug actions on phase 2 VF since 1995 (Pugsley et al., 1995) to the best of our knowledge. Perhaps the reasoning outlined below may provide compelling justification to end this hiatus.
Phase 2 VF models and the importance of continued preclinical investigation
There would need to be good reasons to justify further use of available phase 2 VF models (especially rat models) in the light of the considerations outlined. In fact there are several. From a practical viewpoint, study is possible. For example, with rats there are the advantages of their ability to survive phase 1 VF if resuscitation is attempted by the simple technique of ‘thump-version' (flicking the chest with a forefinger) with a success rate of up to 100% (Curtis & Walker, 1986; 1988) allowing study of phase 2 VF to proceed. Importantly, phase 2 VF occurs spontaneously following coronary ligation in rats (Curtis et al., 1987), permitting detection of drug actions without the need for programmed electrical stimulation. Furthermore, available rat drug data do not contradict available clinical data on SCD suppression, despite the intrinsic differences between rat and human hearts. Thus, clinically relevant doses of Class I and IV agents, which have no effect on SCD in humans (Antman et al., 1992), are ineffective against phase 2 VF in rats (Clark et al., 1980; Curtis et al., 1984; Igwemezie et al., 1992), while moderate blood K+ elevation, which is known to suppress SCD in man (Nordrehaug & von der Lippe, 1983), is protective against phase 2 (and phase 1) VF (Curtis et al., 1985b, 1985c; Saint et al., 1992). This accords encouragingly with the criteria of positive model reinforcement, postulated as an alternative approach to model justification in the absence of an established clinical template (Curtis, 1998).
This also leads on to another important justification for continued preclinical research on phase 2 VF and its suppression. Our premise is that the temporal changes in the biochemical and associated electrophysiological milieu that occur during the transition from acute ischaemia to infarction and were described above (Wit, 1989; Janse, 1991; Curtis et al., 1993a, 1993b) have pharmacological importance. It is interesting, therefore, that many of the drugs that are known to prevent phase 1 VF in rats at low dosage are ineffective (or are effective, but only at unacceptably high doses) against phase 2 VF in the same species / model (Curtis et al., 1984; Curtis & Walker, 1988; Beatch et al., 1991; Igwemezie et al., 1992). In other words, drugs appear to act differently on phase 2 versus phase 1 VF. This is perhaps the most important issue raised in the present article. From a clinical perspective, it means that if a reduction in overall susceptibility to SCD is ever to be achieved by a new antiarrhythmic drug or any indirect form of antiarrhythmic intervention, VF suppression will need to occur during infarct evolution as well as during acute ischaemia. Selective suppression of phase 1 VF will merely delay death for an hour or so. In the past, it was not seen as necessary that drugs be shown to suppress phase 2 as well as phase 1 VF in animals before being tested in humans. The outcome, a series of drugs that have failed to suppress SCD, is therefore unsurprising. Unfortunately, because the distinction between different phases of VF has often been overlooked, a misreading of the implications of the failure of individual models to predict drug effectiveness against SCD has generated a widespread lack of confidence in the value of animal models of VF (Allessie et al., 1995). It is true that an individual model of one aspect of the pathophysiological milieu may not be predictive, but, provided a drug is tested in a second appropriate model relevant to the remainder of the milieu, the individual models are likely to have value by together providing a predictive data set. The wise industrial drug development strategist will delay making a decision about whether to progress a putative new drug for prevention of SCD until data from a range of models are available.
The consequence of all these complex considerations is that, even though it may be difficult to ascertain the exact clinical relevance of phase 2 VF, there is sufficient evidence to warrant an interest in its mechanism and control. This has been a neglected area of study.
Future directions
The importance of avoiding reliance in the use of surrogate end points for VF, such as less severe but more easily-evoked arrhythmias (e.g., VPBs) or cellular electrophysiological variables, was explained earlier. However, this rather limits the scope for the study of the mechanisms and control of phase 2 VF. Mechanisms of different diseases are increasingly being explored by exploiting the possibilities afforded by transgenic mice. However, mouse hearts are notoriously resistant to developing VF in response to coronary ligation (there are no published studies in which VF was successfully elicited by coronary ligation in the mouse, to our knowledge). This means that it is necessary to utilise other species, all of which are unsuitable for routine transgenic manipulation. (A reliable mouse model of phase 2 (and phase 1) VF would be highly desirable.) It also means that study of the ability of selective pharmacological tools (i.e. carefully selected drugs at carefully selected concentrations) to prevent phase 2 VF using models in which phase 2 VF can be reliably evoked remains the best available approach. This approach has already provided a certain amount of information on phase 2 VF mechanisms, particularly concerning the involvement of blood-borne cells and substances, and the sympathetic nervous system. Additional clues have been provided by comparison of outcomes in different, but related models of phase 2 VF.
Comparison of outcomes in different rat models has revealed two possible mechanisms for phase 2 VF, one discussed here and the other discussed below, that might provide a basis for future drug discovery. The data are as follows. In the in vivo setting, more than 90% of control collateral-deficient dogs (Meesmann, 1982) or rats (Curtis & Walker, 1988) develop phase 1 VF, provided the ischaemic zone is sufficiently large. In rats (there are no equivalent dog data), 80–100% of defibrillated surviving control animals go on to develop phase 2 VF (Curtis & Walker, 1988). Likewise, in isolated buffer-perfused (blood-free) hearts, more than 80% develop phase 1 VF (Curtis, 1998). However, phase 2 VF is absent in buffer-perfused hearts (Curtis, 1998; Ravingerova et al., 1995; Clements-Jewery et al., 2002a). This is intriguing, all the more because microscopy has shown that in this experimental preparation sustained coronary artery ligation leads to severe structural damage to myocytes and coronary endothelium that is typical of infarcting myocardium (Ravingerova et al., 1995). This indicates that there is an absence of phase 2 VF in buffer-perfused hearts, and that this is not due to a lack of severe injury and infarction. Moreover, the marked susceptibility to phase 1 VF in this preparation (Curtis & Hearse, 1989a, 1989b; Clements-Jewery et al., 2002a) means that these hearts are not intrinsically resistant to developing VF. These observations indicate that the critical arrhythmogenic components necessary to cause phase 2 VF in vivo are absent in isolated buffer-perfused hearts. Although it is possible to offer an alternative explanation and argue that there is something provided by in vitro buffer perfusion that suppresses phase 2 VF, for example, the provision of tissue oedema (by the lack of colloid), or provision of a supranormal coronary flow in uninvolved tissue (caused by the lack of colloid and by the absence of red blood cells), this would seem to be unlikely in the absence of a rational hypothesis to account for the supposition. Moreover, the existence of a positive protective effect by these or other mechanisms is hard to reconcile with the total lack of equivalent suppression of phase 1 VF, which occurs in abundance in the same hearts (see Figure 1). Thus, an absence of an arrhythmogenic factor would seem to be the better explanation for the lack of phase 2 VF in buffer-perfused hearts. The identity of this arrhythmogenic component is intriguing, not least because it would appear to be unnecessary for the initiation of phase 1 VF.
Of the components that are absent in buffer-perfused hearts, blood is an obvious subject for consideration as a necessary mediator of phase 2 VF. Of all the blood components, there are reasons for considering neutrophils and other white blood cells and platelets as promising candidate mediators. It is well known that infarction generates an acute inflammatory response involving these cells (Frangogiannis et al., 2002). Furthermore, interpolation between published studies suggests that there is an apparent temporal concordance between myocardial accumulation of neutrophils from the blood (Sasaki et al., 1988; Entman et al., 1993) and the onset of phase 2 VF (Clark et al., 1980; Beatch et al., 1991). Additionally, the anti-inflammatory drug nafazatrom, which impedes neutrophil accumulation by inhibiting 5-lipoxygenase-dependent production of the chemoattractant, leukotriene B4, appears to afford some protection against phase 2 VF, although it could be argued that the published data are somewhat inconsistent and difficult to interpret (Fiedler, 1983; 1985). Evidence for a role for neutrophils and related cells in phase 2 VF is therefore intriguing but incomplete.
An attempt has been made to determine whether phase 2 VF can occur in neutrophil-containing isolated blood-perfused hearts, but the outcome was ambiguous owing to limitations of the model which included nonspecific activation of neutrophils and their loss from the perfusion circuit (Clements-Jewery et al., 2002a). Further work (using in vivo approaches) is indicated. It may be premature to contemplate possible mechanisms by which neutrophils may mediate phase 2 VF, but these cells are a source of numerous chemical substances that have the potential to adversely affect cardiac electrophysiology (Jordan et al., 1999).
In addition to neutrophils, there are other components of the acute inflammatory response that could play a role in determining susceptibility to phase 2 VF. Importantly, the effects of many of these components on cardiac electrophysiology, especially during the development of infarction, have yet to be characterised. For example, activated platelets are a source of serotonin, which can alter coronary blood flow and increase vascular permeability (Borgdorff et al., 1994), complement accumulation within infarcting tissue (Rossen et al., 1985; Crawford et al., 1988) can trigger mast cell degranulation and histamine release (Frangogiannis et al., 1998; 2002), mast cells are also a source of leukotrienes and prostaglandins (Gordon et al., 1990), and there are also a number of other chemoattractant substances, such as leukotriene B4 (Sasaki et al., 1988), PAF (Annable et al., 1985; Flores et al., 1994) and the interleukin IL-8 and IL-6 (Kukielka et al., 1995a, 1995b), which are present within the infarcting tissue and are putative mediators of phase 2 VF. It is also possible that inflammatory substances shown to predict risk of adverse coronary events, such as C-reactive protein and Lp-PLA2 (Biasucci, 2004; Oei et al., 2005), may have an arrhythmogenic propensity.
In addition to a role for neutrophils and other inflammatory cells, it is possible that phase 2 VF is initiated by an action of the autonomic nervous system. The absence of sympathetic innervation and of soluble blood components such as catecholamines is a possible explanation for the absence of phase 2 VF in isolated perfused hearts (Ravingerova et al., 1995). Moreover, it is possible to evoke VF in dogs with myocardial infarction by activating sympathetic drive to the heart (e.g., Schwartz et al., 1984). Unfortunately, although there are many studies that have focused on catecholamines and phase 1 VF (Bolli et al., 1984) or have utilised the Harris 2-stage ligation model with its inherent limitations to undertake equivalent studies (Scherlag et al., 1989), there are few studies that specifically address the role of locally released catecholamines in the initiation of phase 2 VF. If there is a role for catecholamines, their action is presumably mediated in the uninvolved or border zone region of the infarcting myocardium since, although catecholamine accumulation in the involved region increases during the first hour of ischaemia (Lameris et al., 2000), it has been shown that 1 h after coronary ligation β1-adrenoceptors (the likely molecular target of arrhythmogenic catecholamines) become uncoupled from adenylyl cyclase (Vatner et al., 1988a, 1988b). Clinically, β1 blockers can reduce mortality following myocardial infarction, but the reduction is limited and it is not clear whether this results from an effect on VF (Antman et al., 1992). In a recent study (Clements-Jewery et al., 2002a), catecholamine replenishment of isolated buffer-perfused rat hearts was found to be unable to restore the susceptibility to phase 2 VF seen in vivo. Thus, there may be a role for catecholamines, perhaps in conjunction with other substances, in mediating phase 2 VF, but it does not appear to be a necessary role.
The principal conclusion of these deliberations is that it is evident that further work is required to establish the exact role played by the host of putative (and indeed also the unheralded) intercellular and intracellular mediators of phase 2 VF.
Phase 2 VF and safety pharmacology
The battery of preclinical tests used in drug development to evaluate drug safety is evolving. The US Department of Health and Human Services has instigated an International Conference on Harmonization that has made recommendations on general aspects of safety pharmacology studies for human pharmaceuticals (FDA, 2001), but a guideline on how to deal with possible proarrhythmic effects of drugs, especially noncardiac drugs, remains in the draft stage (FDA, 2002). The reason for this is that it has proven difficult for a consensus to be reached on which core battery of tests should be undertaken in the laboratory in order to have reasonable assurance that a drug will not cause an unacceptable increase in the likelihood of cardiac arrhythmias.
The relevance of this to the present article is that, because individual models of specific types of arrhythmia (such as phase 1 VF) do not by themselves allow accurate prediction of drug effectiveness as antiarrhythmic protection against SCD, it is likely that an equivalent scenario exists for proarrhythmic effects of cardiac and noncardiac drugs. The logic underpinning a judgement of safety should be no different from that for judging effectiveness. Therefore, just as an antiarrhythmic drug must suppress VF in all settings likely to be encountered in ischaemic heart disease (i.e., ischaemia, infarction and perhaps also reperfusion) in order to be judged effective against SCD, it seems reasonable to propose that a judgement of safety from pro-arrhythmic activity requires demonstration of safety in the settings in which the drug will be used. Thus, it may be necessary to determine a drug's proarrhythmic activity during ischaemia and infarction (and perhaps also reperfusion, a topic beyond the scope of the present article). One may also conceive that yet further models could be deemed necessary to discount the possibility of drug-induced proarrhythmia, including models of heart failure and Na+ and K+ channelopathy (reviewed in Clancy & Kass, 2005) – for example, models with mutations in the SCN5A and KCNH2 genes that encode the cardiac Na+ channel, Nav1.5 and the HERG K+ ion channel, respectively. However, because the use of surrogate end points (such as IKr in isolated myocytes) has proven unsuccessful in predicting drug antiarrhythmic effectiveness against SCD (e.g., Waldo et al., 1996), the value of the use of surrogate end points in drug proarrhythmic safety evaluation may also be questioned. Thus, it may be wise to include in the evolving battery of ICH guidelines direct testing for exacerbation of phase 2 VF (as well as phase 1 VF and possibly reperfusion-induced VF) using animal models.
Experimental data on drug-induced proarrhythmia during the phase 2 VF-susceptible period are lacking. Even obvious experiments have yet to be undertaken. For example, it would be of interest to examine whether d-sotalol can exacerbate phase 2 VF as a possible explanation for its hitherto inexplicable exacerbation of SCD in the SWORD trial (Waldo et al., 1996).
Conclusion
Phase 2 VF occurs spontaneously in most animals surviving phase 1 VF. However, its mechanism differs from that of phase 1 VF and it is affected by drugs differently from phase 1 VF. Its suppression by antiarrhythmic drugs is likely to be necessary if these drugs are to be effective against SCD. Likewise, cardiac and noncardiac drugs may need to be shown to not exacerbate phase 2 VF in order to be judged safe.
Acknowledgments
The work done in our laboratory was funded by the British Heart Foundation (PG / 2000017 and PG03 / 054) and The British Pharmacological Society (AJ Clark studentship awarded to Dr Clements-Jewery). We thank Dr Jack H. Botting for encouraging our interest in the topic of phase 2 arrhythmias.
Abbreviations
- CAST
cardiac arrhythmia suppression trial
- MI
acute myocardial infarction
- SCD
sudden cardiac death
- SWORD
survival with oral D-sotalol
- VF
ventricular fibrillation
- VPB
ventricular premature beats
- VT
ventricular tachycardia
References
- ADGEY A.A., DEVLIN J.E., WEBB S.W., MULHOLLAND H.C. Initiation of ventricular fibrillation outside hospital in patients with acute ischaemic heart disease. Br. Heart J. 1982;47:55–61. doi: 10.1136/hrt.47.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AIDONIDIS I., BRACHMANN J., RIZOS I., ZACHAROULIS A., STAVRIDIS I., TOUTOUZAS P., KUBLER W. Electropharmacology of the bradycardic agents alinidine and zatebradine (UL-FS 49) in a conscious canine ventricular arrhythmia model of permanent coronary artery occlusion. Cardiovasc. Drugs Ther. 1995;9:555–563. doi: 10.1007/BF00878087. [DOI] [PubMed] [Google Scholar]
- ALLESSIE A., AVKIRAN M., BORGGREFE M., BRACHMANN J., BREITHARDT G., CAMM A.J., CARMELIET E., CINCA J., COBBE S.M., CURTIS M.J., DHEIN S., HAVERKAMP W., HINDRICKS G., JANSE M.J., KLEBER A.G., KOTTKAMP H., OPHTHOF T., PRIORI S.G., SACK S., SCHOLS W.J., SCHWARTZ P.J., VANOLI E., ZAA A. The role of basic arrhythmia research. The continued need for experiments in the intact heart and organism. Eur. Heart J. 1995;16:1469–1475. [PubMed] [Google Scholar]
- ANNABLE C.R., MCMANUS L.M., CAREY K.D., PINCKARD R.N. Isolation of platelet activating factor (PAF) from ischaemic baboon myocardium. Fed. Proc. 1985;44:1271. [Google Scholar]
- ANTMAN E.M., LAU J., KUPELNICK B., MOSTELLER F., CHALMERS T.C. A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts. Treatments for myocardial infarction. JAMA. 1992;268:240–248. [PubMed] [Google Scholar]
- AU T.L., COLLINS G.A., HARVIE C.J., WALKER M.J. The actions of prostaglandins I2 and E2 on arrhythmias produced by coronary occlusion in the rat and dog. Prostaglandins. 1979;18:707–720. doi: 10.1016/0090-6980(79)90091-1. [DOI] [PubMed] [Google Scholar]
- BAKER K.E., CURTIS M.J. Left regional cardiac perfusion in vitro with platelet-activating factor, norepinephrine and K+ reveals that ischaemic arrhythmias are caused by independent effects of endogenous ‘mediators' facilitated by interactions, and moderated by paradoxical antagonism. Br. J. Pharmacol. 2004;142:352–366. doi: 10.1038/sj.bjp.0705767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYON A., DELPACIO M.J.F., MONTES A.M., PANIZIO C.G. M-mode echocardiography study in growing Spanish mastiffs. J. Small Animal Practice. 1994;35:473–479. [Google Scholar]
- BEATCH G.N., ABRAHAM S., MACLEOD B.A., YOSHIDA N.R., WALKER M.J. Antiarrhythmic properties of tedisamil (KC8857), a putative transient outward K+ current blocker. Br. J. Pharmacol. 1991;102:13–18. doi: 10.1111/j.1476-5381.1991.tb12124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEATCH G.N., MCNEILL J.H. Ventricular arrhythmias following coronary artery occlusion in the streptozotocin diabetic rat. Can. J. Physiol. Pharmacol. 1988;66:312–317. doi: 10.1139/y88-053. [DOI] [PubMed] [Google Scholar]
- BIASUCCI L.M. CDC / AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: clinical use of inflammatory markers in patients with cardiovascular diseases: a background paper. Circulation. 2004;110:e560–e567. doi: 10.1161/01.CIR.0000148983.88334.80. [DOI] [PubMed] [Google Scholar]
- BILLMAN G. Effect of alpha 1-adrenergic receptor antagonists on susceptibility to malignant arrhythmias: protection from ventricular fibrillation. J. Cardiovasc. Pharmacol. 1994;24:394–402. doi: 10.1097/00005344-199409000-00007. [DOI] [PubMed] [Google Scholar]
- BOLLI R., FISHER D., TAYLOR A., YOUNG J., MILLER R. Effect of alpha-adrenergic blockade on arrhythmias induced by acute myocardial ischemia and reperfusion in the dog. J. Mol. Cell Cardiol. 1984;16:1101–1117. doi: 10.1016/s0022-2828(84)80037-1. [DOI] [PubMed] [Google Scholar]
- BOLLI R., MARBAN E. Molecular and cellular mechanisms of myocardial stunning. Physiol. Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- BORGDORFF P., KOK W.E., VIS M.A., VAN DEN BOS G.C. Vasodilation by shear-induced platelet aggregation in extracorporeal circuits. Am. J. Physiol. 1994;266:H891–H897. doi: 10.1152/ajpheart.1994.266.3.H891. [DOI] [PubMed] [Google Scholar]
- BOTTING J.H., JOHNSTON K.M., MACLEOD B.A., WALKER M.J. The effect of modification of sympathetic activity on responses to ligation of a coronary artery in the conscious rat. Br. J. Pharmacol. 1983;79:265–271. doi: 10.1111/j.1476-5381.1983.tb10520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOURKE J.P., RICHARDS D.A., ROSS D.L., MCGUIRE M.A., UTHER J.B. Does the induction of ventricular flutter or fibrillation at electrophysiologic testing after myocardial infarction have any prognostic significance. Am. J. Cardiol. 1995;75:431–435. doi: 10.1016/s0002-9149(99)80576-1. [DOI] [PubMed] [Google Scholar]
- BRICKNELL O.L., OPIE L.H. Effects of substrates on tissue metabolic changes in the isolated rat heart during underperfusion and on release of lactate dehydrogenase and arrhythmias during reperfusion. Circ. Res. 1978;43:102–115. doi: 10.1161/01.res.43.1.102. [DOI] [PubMed] [Google Scholar]
- BUERKE M., WEYRICH A.S., LEFER A.M. Isolated cardiac myocytes are sensitized by hypoxia-reoxygenation to neutrophil-released mediators. Am. J. Physiol. 1994;266:H128–H136. doi: 10.1152/ajpheart.1994.266.1.H128. [DOI] [PubMed] [Google Scholar]
- BUJA L.M., PARKEY R.W., DEES J.H., STOKELY E.M., HARRIS R.A., JR, BONTE F.J., WILLERSON J.T. Morphologic correlates of technetium-99 m stannous pyrophosphate imaging of acute myocardial infarcts in dogs. Circulation. 1975;52:596–607. doi: 10.1161/01.cir.52.4.596. [DOI] [PubMed] [Google Scholar]
- CAMPBELL C.A., PARRATT J.R., KANE K.A., BULLOCK G. Effects of prolonged administration of oxprenolol on severity of ischaemic arrhythmias, enzyme leakage, infarct size, and intracellular cardiac muscle action potentials. J. Cardiovasc. Pharmacol. 1984;6:369–377. doi: 10.1097/00005344-198405000-00001. [DOI] [PubMed] [Google Scholar]
- CAMPBELL R.W. Treatment and prophylaxis of ventricular arrhythmias in acute myocardial infarction. Am. J. Cardiol. 1983;52:55C–59C. doi: 10.1016/0002-9149(83)90633-1. [DOI] [PubMed] [Google Scholar]
- CAMPBELL R.W., MURRAY A., JULIAN D.G. Ventricular arrhythmias in first 12 hours of acute myocardial infarction. Natural history study. Br. Heart J. 1981;46:351–357. doi: 10.1136/hrt.46.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL R.W.F.Ventricular fibrillation: facts, fiction, and the future Life-threatening arrhythmias during ischemia and infarction 1987New York: Raven Press; 1–9.eds. Hearse, D.J., Manning, A.S. & Janse, M.J. pp [Google Scholar]
- CASCIO W.E., JOHNSON T.A., GETTES L.S. Electrophysiologic changes in ischemic ventricular myocardium: I. Influence of ionic, metabolic, and energetic changes. J. Cardiovasc. Electrophysiol. 1995;6:1039–1062. doi: 10.1111/j.1540-8167.1995.tb00381.x. [DOI] [PubMed] [Google Scholar]
- CAVE A.C., SILVERMAN A.S., APSTEIN C.S. Ischemic preconditioning does not protect against contractile dysfunction in the presence of residual flow: studies in the isolated, blood-perfused rat heart. Circulation. 1997;96:3087–3093. doi: 10.1161/01.cir.96.9.3087. [DOI] [PubMed] [Google Scholar]
- CERBAI E., AMBROSIO G., PORCIATTI F., CHIARIELLO M., GIOTTI A., MUGELLI A. Cellular electrophysiological basis for oxygen radical-induced arrhythmias. A patch-clamp study in guinea pig ventricular myocytes. Circulation. 1991;84:1773–1782. doi: 10.1161/01.cir.84.4.1773. [DOI] [PubMed] [Google Scholar]
- CLANCY C.E., KASS R.S. Inherited and acquired vulnerability to ventricular arrhythmias: cardiac Na+ and K+ channels. Physiol. Rev. 2005;85:33–47. doi: 10.1152/physrev.00005.2004. [DOI] [PubMed] [Google Scholar]
- CLARK C., FOREMAN M.I., KANE K.A., MCDONALD F.M., PARRATT J.R. Coronary artery ligation in anesthetized rats as a method for the production of experimental dysrhythmias and for the determination of infarct size. J. Pharmacol. Methods. 1980;3:357–368. doi: 10.1016/0160-5402(80)90077-7. [DOI] [PubMed] [Google Scholar]
- CLEMENTS-JEWERY H., CURTIS M.J.Biochemical mediators of ventricular arrhythmias in ischaemic heart disease Cardiac Drug Development Guide 2003Tottowa, NJ, U.S.A.: Humana Press; 203–226.ed. Pugsley M.K. pp [Google Scholar]
- CLEMENTS-JEWERY H., HEARSE D.J., CURTIS M.J. Independent contribution of catecholamines to arrhythmogenesis during evolving infarction in the isolated rat heart. Br. J. Pharmacol. 2002a;135:807–815. doi: 10.1038/sj.bjp.0704509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEMENTS-JEWERY H., HEARSE D.J., CURTIS M.J. The isolated blood-perfused rat heart: an inappropriate model for the study of ischaemia- and infarction-related ventricular fibrillation. Br. J. Pharmacol. 2002b;137:1089–1099. doi: 10.1038/sj.bjp.0704977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COBBE S.M. Class III Antiarrhythmics: put to the SWORD. Heart. 1996;75:111–113. doi: 10.1136/hrt.75.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COKER S.J. Anesthetized rabbit as a model for ischemia- and reperfusion-induced arrhythmias: effects of quinidine and bretylium. J. Pharmacol. Methods. 1989;21:263–279. doi: 10.1016/0160-5402(89)90064-8. [DOI] [PubMed] [Google Scholar]
- CRAWFORD M.H., GROVER F.L., KOLB W.P., MCMAHAN C.A., O'ROURKE R.A., MCMANUS L.M., PINCKARD R.N. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circulation. 1988;78:1449–1458. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- CULLING W., PENNY W.J., LEWIS M.J., MIDDLETON K., SHERIDAN D.J. Effects of myocardial catecholamine depletion on cellular electrophysiology and arrhythmias during ischaemia and reperfusion. Cardiovasc. Res. 1984;18:675–682. doi: 10.1093/cvr/18.11.675. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J. Characterisation, utilisation and clinical relevance of isolated perfused heart models of ischaemia-induced ventricular fibrillation. Cardiovasc. Res. 1998;39:194–215. doi: 10.1016/s0008-6363(98)00083-2. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J.Prevention of sudden cardiac death by immunopharmacological intervention Immunopharmacology of the Heart 1993London, U.K.: Academic Press; ed. Curtis, M.J. [Google Scholar]
- CURTIS M.J., GARLICK P.B., RIDLEY P.D. Anion manipulation, a novel antiarrhythmic approach: mechanism of action. J. Mol. Cell Cardiol. 1993a;25:417–436. doi: 10.1006/jmcc.1993.1048. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., HEARSE D.J. Ischaemia-induced and reperfusion-induced arrhythmias differ in their sensitivity to potassium: implications for mechanisms of initiation and maintenance of ventricular fibrillation. J. Mol. Cell Cardiol. 1989a;21:21–40. doi: 10.1016/0022-2828(89)91490-9. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., HEARSE D.J. Reperfusion-induced arrhythmias are critically dependent upon occluded zone size: relevance to the mechanism of arrhythmogenesis. J. Mol. Cell Cardiol. 1989b;21:625–637. doi: 10.1016/0022-2828(89)90828-6. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., JOHNSTON K.M., MACLEOD B.A., WALKER M.J. The actions of felodipine on arrhythmias and other responses to myocardial ischaemia in conscious rats. Eur. J. Pharmacol. 1985a;117:169–178. doi: 10.1016/0014-2999(85)90601-6. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., JOHNSTON K.M., WALKER M.J.A. Arrhythmias and serum potassium during myocardial ischaemia. ICRS Med. Sci. Res. 1985b;13:688–689. [Google Scholar]
- CURTIS M.J., MACLEOD B.A., WALKER M.J. Antiarrhythmic actions of verapamil against ischaemic arrhythmias in the rat. Br. J. Pharmacol. 1984;83:373–385. doi: 10.1111/j.1476-5381.1984.tb16497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS M.J., MACLEOD B.A., WALKER M.J. The effects of ablations in the central nervous system on arrhythmias induced by coronary occlusion in the rat. Br. J. Pharmacol. 1985c;86:663–670. doi: 10.1111/j.1476-5381.1985.tb08943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS M.J., MACLEOD B.A., WALKER M.J. Models for the study of arrhythmias in myocardial ischaemia and infarction: the use of the rat. J. Mol. Cell. Cardiol. 1987;19:399–419. doi: 10.1016/s0022-2828(87)80585-0. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., PUGSLEY M.K., WALKER M.J. Endogenous chemical mediators of ventricular arrhythmias in ischaemic heart disease. Cardiovasc. Res. 1993b;27:703–719. doi: 10.1093/cvr/27.5.703. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., WALKER M.J. The mechanism of action of calcium antagonists on arrhythmias in early myocardial ischaemia: studies with nifedipine and DHM9. Br. J. Pharmacol. 1988;94:1275–1286. doi: 10.1111/j.1476-5381.1988.tb11648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS M.J., WALKER M.J. The mechanism of action of the optical enantiomers of verapamil against ischaemia-induced arrhythmias in the conscious rat. Br. J. Pharmacol. 1986;89:137–147. doi: 10.1111/j.1476-5381.1986.tb11129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'alonzo A.J., DARBENZIO R.B., HESS T.A., SEWTER J.C., SLEPH P.G., GROVER G.J. Effect of potassium on the action of the KATP modulators cromakalim, pinacidil, or glibenclamide on arrhythmias in isolated perfused rat heart subjected to regional ischaemia. Cardiovasc. Res. 1994;28:881–887. doi: 10.1093/cvr/28.6.881. [DOI] [PubMed] [Google Scholar]
- DAUGHERTY A., FRAYN K., REDFERN W., WOODWARD B. The role of catecholamines in the production of ischaemia-induced ventricular arrhythmias in the rat in vivo and in vitro. Br. J. Pharmacol. 1986;87:265–277. doi: 10.1111/j.1476-5381.1986.tb10180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE JONGE R., DE JONG J.W. Ischemic preconditioning and glucose metabolism during low-flow ischemia: role of the adenosine A1 receptor. Cardiovasc. Res. 1999;43:909–918. doi: 10.1016/s0008-6363(99)00137-6. [DOI] [PubMed] [Google Scholar]
- DE VREEDE-SWAGEMAKERS J.J., GORGELS A.P., DUBOIS-ARBOUW W.I., DALSTRA J., DAEMEN M.J., VAN REE J.W., STIJNS R.E., WELLENS H.J. Circumstances and causes of out-of-hospital cardiac arrest in sudden death survivors. Heart. 1998;79:356–361. doi: 10.1136/hrt.79.4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENTMAN M.L., KUKIELKA G.L., BALLANTYNE C.M., SMITH C.W.The role of leukocytes in ischaemic heart disease Immunopharmacology of the Heart 1993U.K.: Academic Press; 55–74.ed. Curtis, M.J. pp [Google Scholar]
- ENTMAN M.L., YOUKER K., SHOJI T., KUKIELKA G., SHAPPELL S.B., TAYLOR A.A., SMITH C.W. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b / CD18-ICAM-1 adherence. J. Clin. Invest. 1992;90:1335–1345. doi: 10.1172/JCI115999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOOD AND DRUG ADMINISTRATION (FDA) ICH Harmonized Tripartite Guideline, S7A Safety Pharmacology Studies for Human Pharmaceuticals. US Department of Health and Human Services; 2001. [Google Scholar]
- FOOD AND DRUG ADMINISTRATION (FDA) ICH Draft Consensus Guideline, S7B Safety Pharmacology Studies for Assessing the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals. US Department of Health and Human Services; 2002. [Google Scholar]
- FENOGLIO J.J., JR, KARAGUEUZIAN H.S., FRIEDMAN P.L., ALBALA A., WIT A.L. Time course of infarct growth toward the endocardium after coronary occlusion. Am. J. Physiol. 1979;236:H356–H370. doi: 10.1152/ajpheart.1979.236.2.H356. [DOI] [PubMed] [Google Scholar]
- FIEDLER V.B. Failure of nafazatrom to reduce infarct size and arrhythmias in a porcine model of acute coronary occlusion. Eur. J. Pharmacol. 1985;114:189–195. doi: 10.1016/0014-2999(85)90627-2. [DOI] [PubMed] [Google Scholar]
- FIEDLER V.B. Reduction of myocardial infarction and dysrhythmic activity by nafazatrom in the conscious rat. Eur. J. Pharmacol. 1983;88:263–267. doi: 10.1016/0014-2999(83)90016-x. [DOI] [PubMed] [Google Scholar]
- FLORES N.A., GOULIELMOS N.V., SEGHATCHIAN M.J., SHERIDAN D.J. Myocardial ischaemia induces platelet activation with adverse electrophysiological and arrhythmogenic effects. Cardiovasc. Res. 1994;28:1662–1671. doi: 10.1093/cvr/28.11.1662. [DOI] [PubMed] [Google Scholar]
- FRANGOGIANNIS N.G., LINDSEY M.L., MICHAEL L.H., YOUKER K.A., BRESSLER R.B., MENDOZA L.H., SPENGLER R.N., SMITH C.W., ENTMAN M.L. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia / reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- FRANGOGIANNIS N.G., SMITH C.W., ENTMAN M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN P.L., STEWART J.R., FENOGLIO J.J., JR, WIT A.L. Survival of subendocardial Purkinje fibers after extensive myocardial infarction in dogs. Circ. Res. 1973;33:597–611. doi: 10.1161/01.res.33.5.597. [DOI] [PubMed] [Google Scholar]
- FUKUNAMI M., HEARSE D.J. Temperature-dependency of nifedipine as a protective agent during cardioplegia in the rat. Cardiovasc. Res. 1985;19:95–103. doi: 10.1093/cvr/19.2.95. [DOI] [PubMed] [Google Scholar]
- GARCIA-DORADO D., THEROUX P., ELIZAGA J., GALINANES M., SOLARES J., RIESGO M., GOMEZ M.J., GARCIA-DORADO A., FERNANDEZ AVILES F. Myocardial reperfusion in the pig heart model: infarct size and duration of coronary occlusion. Cardiovasc. Res. 1987;21:537–544. doi: 10.1093/cvr/21.7.537. [DOI] [PubMed] [Google Scholar]
- GOGELEIN H., RUETTEN H., ALBUS U., ENGLERT H.C., BUSCH A.E. Effects of the cardioselective KATP channel blocker HMR 1098 on cardiac function in isolated perfused working rat hearts and in anesthetized rats during ischemia and reperfusion. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;364:33–41. doi: 10.1007/s002100000391. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN S., BROOKS M.M., LEDINGHAM R., KENNEDY H.L., EPSTEIN A.E., PAWITAN Y., BIGGER J.T. Association between ease of suppression of ventricular arrhythmia and survival. Circulation. 1995;91:79–83. doi: 10.1161/01.cir.91.1.79. [DOI] [PubMed] [Google Scholar]
- GORDON J.R., BURD P.R., GALLI S.J. Mast cells as a source of multifunctional cytokines. Immunol. Today. 1990;11:458–464. doi: 10.1016/0167-5699(90)90176-a. [DOI] [PubMed] [Google Scholar]
- GREENBERG H.M., DWYER E.M.J., HOCHMAN J.S., STEINBERG J.S., ECHT D.S., PETERS R.W. Interaction of ischaemia and encainide / flecainide treatment: a proposed mechanism for the increased mortality in CAST I. Br. Heart J. 1995;74:631–635. doi: 10.1136/hrt.74.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLSTROM A.P., ANDERSON J.L., CARLSON M., DAVIES R., GREENE H.L., KAMMERLING J.M., ROMHILT D.W., DUFF H.J., HUTHER M. Time to arrhythmic, ischemic, and heart failure events: exploratory analyses to elucidate mechanisms of adverse drug effects in the Cardiac Arrhythmia Suppression Trial. Am. Heart J. 1995;130:71–79. doi: 10.1016/0002-8703(95)90238-4. [DOI] [PubMed] [Google Scholar]
- HANNA M.S., DRESDNER K.P., KLINE R.P., WIT A.L.Ischemia does not cause intracellular acidification of arrhythmogenic purkinje fibres in canine infarcts Circulation 198878II–461 [Google Scholar]
- HANZLICK R., RYDZEWSKI D. Heart weights of white men 20 to 39 years of age. An analysis of 218 autopsy cases. Am. J. Forensic. Med. Pathol. 1990;11:202–204. doi: 10.1097/00000433-199009000-00005. [DOI] [PubMed] [Google Scholar]
- HARRIS A.S. Delayed development of ventricular ectopic rhythms following experimental coronary occlusion. Circulation. 1950;1:1318–1328. doi: 10.1161/01.cir.1.6.1318. [DOI] [PubMed] [Google Scholar]
- HARRIS A.S. Terminal electrocardiographic patterns in experimental anoxia, coronary occlusion, and hemorrhagic shock. Am. Heart J. 1948;35:895–899. doi: 10.1016/0002-8703(48)90588-2. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO K., SATOH H., SHIBUYA T., IMAI S. Canine-effective plasma concentrations of antiarrhythmic drugs on the two-stage coronary ligation arrhythmia. J. Pharmacol. Exp. Ther. 1982;223:801–810. [PubMed] [Google Scholar]
- HAVERKAMP W., BREITHARDT G., CAMM A.J., JANSE M.J., ROSEN M.R., ANTZELEVITCH C., ESCANDE D., FRANZ M., MALIK M., MOSS A., SHAH R. The potential for QT prolongation and proarrhythmia by non-antiarrhythmic drugs: clinical and regulatory implications. Report on a policy conference of the European Society of Cardiology. Eur. Heart J. 2000;21:1216–1231. doi: 10.1053/euhj.2000.2249. [DOI] [PubMed] [Google Scholar]
- HEARSE D.J., FERRARI R., SUTHERLAND F.J. Cardioprotection: intermittent ventricular fibrillation and rapid pacing can induce preconditioning in the blood-perfused rat heart. J. Mol. Cell Cardiol. 1999;31:1961–1973. doi: 10.1006/jmcc.1999.1027. [DOI] [PubMed] [Google Scholar]
- HILL J.L., GETTES L.S. Effect of acute coronary artery occlusion on local myocardial extracellular K+ activity in swine. Circulation. 1980;61:768–778. doi: 10.1161/01.cir.61.4.768. [DOI] [PubMed] [Google Scholar]
- HIRCHE H., FRANZ C., BOS L., BISSIG R., LANG R., SCHRAMM M. Myocardial extracellular K+ and H+ increase and noradrenaline release as possible cause of early arrhythmias following acute coronary artery occlusion in pigs. J. Mol. Cell Cardiol. 1980;12:579–593. doi: 10.1016/0022-2828(80)90016-4. [DOI] [PubMed] [Google Scholar]
- HOROWITZ L.N., SPEAR J.F., MOORE E.N. Subendocardial origin of ventricular arrhythmias in 24-hour-old experimental myocardial infarction. Circulation. 1976;53:56–63. doi: 10.1161/01.cir.53.1.56. [DOI] [PubMed] [Google Scholar]
- HORT W., DACANALIS S. Studies on the rat heart with prolonged ligation of the left coronary artery with special reference to the infarct size. Virchows. Arch. Pathol. Anat. Physiol. Klin. Med. 1965;339:53–60. [PubMed] [Google Scholar]
- HOWARD P.G., MACLEOD B.A., WALKER M.J. Quinacainol, a new antiarrhythmic with class I antiarrhythmic actions in the rat. Eur. J. Pharmacol. 1992;219:1–8. doi: 10.1016/0014-2999(92)90572-l. [DOI] [PubMed] [Google Scholar]
- HUIKURI H.V., MAKIKALLIO T.H., RAATIKAINEN M.J., PERKIOMAKI J., CASTELLANOS A., MYERBURG R.J. Prediction of sudden cardiac death: appraisal of the studies and methods assessing the risk of sudden arrhythmic death. Circulation. 2003;108:110–115. doi: 10.1161/01.CIR.0000077519.18416.43. [DOI] [PubMed] [Google Scholar]
- IGWEMEZIE L.N., BEATCH G.N., MCERLANE K.M., WALKER M.J. Mexiletine's antifibrillatory actions are limited by the occurrence of convulsions in conscious animals. Eur. J. Pharmacol. 1992;210:271–277. doi: 10.1016/0014-2999(92)90415-z. [DOI] [PubMed] [Google Scholar]
- JANSE M.J.Re-entry rhythms The Heart and Cardiovascular System 1991New York: Raven press; 2055–2094.ed. Fozzard, H.A. pp [Google Scholar]
- JANSE M.J., CINCA J., MORENA H., FIOLET J.W., KLEBER A.G., DE VRIES G.P., BECKER A.E., DURRER D. The ‘border zone' in myocardial ischemia. An electrophysiological, metabolic, and histochemical correlation in the pig heart. Circ. Res. 1979;44:576–588. doi: 10.1161/01.res.44.4.576. [DOI] [PubMed] [Google Scholar]
- JANSE M.J., VAN CAPELLE F.J., MORSINK H., KLEBER A.G., WILMS-SCHOPMAN F., CARDINAL R., D'ALNONCOURT C.N., DURRER D. Flow of ‘injury' current and patterns of excitation during early ventricular arrhythmias in acute regional myocardial ischemia in isolated porcine and canine hearts. Evidence for two different arrhythmogenic mechanisms. Circ. Res. 1980;47:151–165. doi: 10.1161/01.res.47.2.151. [DOI] [PubMed] [Google Scholar]
- JENNINGS R.B., MURRY C.E., STEENBERGEN C., JR, REIMER K.A. Development of cell injury in sustained acute ischemia. Circulation. 1990;82:II2–II12. [PubMed] [Google Scholar]
- JOHNS T.N.P., OLSON B.J. Experimental myocardial infarction. 1. A method of coronary occlusion in small animals. Ann. Surg. 1954;140:675–682. doi: 10.1097/00000658-195411000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON K.M., MACLEOD B.A., WALKER M.J. Effects of aspirin and prostacyclin on arrhythmias resulting from coronary artery ligation and on infarct size. Br. J. Pharmacol. 1983a;78:29–37. doi: 10.1111/j.1476-5381.1983.tb09359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON K.M., MACLEOD B.A., WALKER M.J. Responses to ligation of a coronary artery in conscious rats and the actions of antiarrhythmics. Can. J. Physiol. Pharmacol. 1983b;61:1340–1353. doi: 10.1139/y83-193. [DOI] [PubMed] [Google Scholar]
- JORDAN J.E., ZHAO Z.Q., VINTEN-JOHANSEN J. The role of neutrophils in myocardial ischemia–reperfusion injury. Cardiovasc. Res. 1999;43:860–878. doi: 10.1016/s0008-6363(99)00187-x. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., ARAMENDIA P. Prevention of ventricular fibrillation induced by coronary ligation. J. Pharmacol. Exp. Therap. 1968;164:326–332. [PubMed] [Google Scholar]
- KENEDI I., LOSONCI A. Arrhythmia after left coronary ligation and the effect of isoproterenol in the rat. Acta. Physiol. Acad. Sci. Hung. 1973;43:133–141. [PubMed] [Google Scholar]
- KINTER L.B., SIEGL P.K., BASS A.S. New preclinical guidelines on drug effects on ventricular repolarization: safety pharmacology comes of age. J. Pharmacol. Toxicol. Methods. 2004;49:153–158. doi: 10.1016/j.vascn.2004.03.007. [DOI] [PubMed] [Google Scholar]
- KLEBER A.G. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ. Res. 1983;52:442–450. doi: 10.1161/01.res.52.4.442. [DOI] [PubMed] [Google Scholar]
- KUKIELKA G.L., SMITH C.W., LAROSA G.J., MANNING A.M., MENDOZA L.H., DALY T.J., HUGHES B.J., YOUKER K.A., HAWKINS H.K., MICHAEL L.H. Interleukin-8 gene induction in the myocardium after ischemia and reperfusion in vivo. J. Clin. Invest. 1995a;95:89–103. doi: 10.1172/JCI117680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUKIELKA G.L., SMITH C.W., MANNING A.M., YOUKER K.A., MICHAEL L.H., ENTMAN M.L. Induction of interleukin-6 synthesis in the myocardium. Potential role in postreperfusion inflammatory injury. Circulation. 1995b;92:1866–1875. doi: 10.1161/01.cir.92.7.1866. [DOI] [PubMed] [Google Scholar]
- LAMERIS T.W., DE ZEEUW S., ALBERTS G., BOOMSMA F., DUNCKER D.J., VERDOUW P.D., VELD A.J., VAN DEN MEIRACKER A.H. Time course and mechanism of myocardial catecholamine release during transient ischemia in vivo. Circulation. 2000;101:2645–2650. doi: 10.1161/01.cir.101.22.2645. [DOI] [PubMed] [Google Scholar]
- LEPRAN I., KOLTAI M., SIEGMUND W., SZEKERES L. Coronary artery ligation, early arrhythmias, and determination of the ischemic area in conscious rats. J. Pharmacol. Methods. 1983;9:219–230. doi: 10.1016/0160-5402(83)90041-4. [DOI] [PubMed] [Google Scholar]
- LEVRAUT J., IWASE H., SHAO Z.H., VANDEN HOEK T.L., SCHUMACKER P.T. Cell death during ischemia: relationship to mitochondrial depolarization and ROS generation. Am. J. Physiol. 2003;284:H549–H558. doi: 10.1152/ajpheart.00708.2002. [DOI] [PubMed] [Google Scholar]
- MALIK M., CAMM A.J. Evaluation of drug-induced QT interval prolongation: implications for drug approval and labelling. Drug Saf. 2001;24:323–351. doi: 10.2165/00002018-200124050-00001. [DOI] [PubMed] [Google Scholar]
- MEESMANN W.Early arrhythmias and primary ventricular fibrillation after acute myocardial ischaemia in relation to pre-existing coronary collaterals Early Arrhythmias Resulting from Myocardial Ischaemia 1982London, U.K.: McMillan; 93–112.ed. Parrat, J.R. pp [Google Scholar]
- MUHLESTEIN J.B., ANDERSON J.L., HORNE B.D., CARLQUIST J.F., BAIR T.L., BUNCH T.J., PEARSON R.R. Early effects of statins in patients with coronary artery disease and high C-reactive protein. Am. J. Cardiol. 2004;94:1107–1112. doi: 10.1016/j.amjcard.2004.07.074. [DOI] [PubMed] [Google Scholar]
- NAKANO M., KNOWLTON A.A., DIBBS Z., MANN D.L. Tumor necrosis factor-alpha confers resistance to hypoxic injury in the adult mammalian cardiac myocyte. Circulation. 1998;97:1392–1400. doi: 10.1161/01.cir.97.14.1392. [DOI] [PubMed] [Google Scholar]
- NORDREHAUG J.E., VON DER LIPPE G. Hypokalaemia and ventricular fibrillation in acute myocardial infarction. Br. Heart J. 1983;50:525–529. doi: 10.1136/hrt.50.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OEI H.H., VAN DER MEER I.M., HOFMAN A., KOUDSTAAL P.J., STIJNEN T., BRETELER M.M., WITTEMAN J.C. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation. 2005;111:570–575. doi: 10.1161/01.CIR.0000154553.12214.CD. [DOI] [PubMed] [Google Scholar]
- OPITZ C.F., MITCHELL G.F., PFEFFER M.A., PFEFFER J.M. Arrhythmias and death after coronary artery occlusion in the rat. Continuous telemetric ECG monitoring in conscious, untethered rats. Circulation. 1995;92:253–261. doi: 10.1161/01.cir.92.2.253. [DOI] [PubMed] [Google Scholar]
- PATTERSON E., HOLLAND K., ELLER B.T., LUCCHESI B.R. Ventricular fibrillation resulting from ischemia at a site remote from previous myocardial infarction. A conscious canine model of sudden coronary death. Am. J. Cardiol. 1982;50:1414–1423. doi: 10.1016/0002-9149(82)90484-2. [DOI] [PubMed] [Google Scholar]
- PRATT C.M., CAMM A.J., COOPER W., FRIEDMAN P.L., MACNEIL D.J., MOULTON K.M., PITT B., SCHWARTZ P.J., VELTRI E.P., WALDO A.L. Mortality in the survival with ORal D-sotalol (SWORD) trial: why did patients die. Am. J. Cardiol. 1998;81:869–876. doi: 10.1016/s0002-9149(98)00006-x. [DOI] [PubMed] [Google Scholar]
- PRYSTOWSKY E.N. Primary prevention of sudden cardiac death: the time of your life. Circulation. 2004;109:1073–1075. doi: 10.1161/01.CIR.0000121314.13795.F1. [DOI] [PubMed] [Google Scholar]
- PUGSLEY M.K. Safety pharmacology matures into a unique pharmacological discipline. J. Pharmacol. Toxicol. Methods. 2004;49:137–139. doi: 10.1016/j.vascn.2004.03.011. [DOI] [PubMed] [Google Scholar]
- PUGSLEY M.K., RIES C.R., GUPPY L.J., HARVIE C.J., WALKER M.J. Effects of anipamil, a long acting analog of verapamil, in pigs subjected to myocardial ischemia. Life Sci. 1995;57:1219–1231. doi: 10.1016/0024-3205(95)02070-y. [DOI] [PubMed] [Google Scholar]
- RAVINGEROVA T., TRIBULOVA N., SLEZAK J., CURTIS M.J. Brief, intermediate and prolonged ischemia in the isolated crystalloid perfused rat heart: relationship between susceptibility to arrhythmias and degree of ultrastructural injury. J. Mol. Cell Cardiol. 1995;27:1937–1951. doi: 10.1016/0022-2828(95)90016-0. [DOI] [PubMed] [Google Scholar]
- RIDKER P.M., CANNON C.P., MORROW D., RIFAI N., ROSE L.M., MCCABE C.H., PFEFFER M.A., BRAUNWALD E. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- RIDLEY P.D., YACOUB M.H., CURTIS M.J. A modified model of global ischaemia: application to the study of syncytial mechanisms of arrhythmogenesis. Cardiovasc. Res. 1992;26:309–315. doi: 10.1093/cvr/26.4.309. [DOI] [PubMed] [Google Scholar]
- RODRIGO G.C., LAWRENCE C.L., STANDEN N.B. Dinitrophenol pretreatment of rat ventricular myocytes protects against damage by metabolic inhibition and reperfusion. J. Mol. Cell Cardiol. 2002;34:555–569. doi: 10.1006/jmcc.2002.1536. [DOI] [PubMed] [Google Scholar]
- ROSSEN R.D., SWAIN J.L., MICHAEL L.H., WEAKLEY S., GIANNINI E., ENTMAN M.L. Selective accumulation of the first component of complement and leukocytes in ischemic canine heart muscle. A possible initiator of an extra myocardial mechanism of ischemic injury. Circ. Res. 1985;57:119–130. doi: 10.1161/01.res.57.1.119. [DOI] [PubMed] [Google Scholar]
- RUSHMER R.F., WATSON N., HARDING D., BAKER D. Effects of acute coronary occlusion on performance of right and left ventricles in intact unanesthetized dogs. Am. Heart J. 1963;66:522–531. doi: 10.1016/0002-8703(63)90385-5. [DOI] [PubMed] [Google Scholar]
- SAINT K.M., ABRAHAM S., MACLEOD B.A., MCGOUGH J., YOSHIDA N., WALKER M.J. Ischemic but not reperfusion arrhythmias depend upon serum potassium concentration. J. Mol. Cell Cardiol. 1992;24:701–709. doi: 10.1016/0022-2828(92)93384-v. [DOI] [PubMed] [Google Scholar]
- SASAKI K., UENO A., KATORI M., KIKAWADA R. Detection of leukotriene B4 in cardiac tissue and its role in infarct extension through leucocyte migration. Cardiovasc. Res. 1988;22:142–148. doi: 10.1093/cvr/22.2.142. [DOI] [PubMed] [Google Scholar]
- SCHERLAG B.J., PATTERSON E.S., BERBARI E.J., LAZZARA R.Experimental simulation of sudden cardiac death in humans: electrophysiological mechanisms and role of adrenergic influences The Adrenergic System and Ventricular Arrhythmias in Myocardial Infarction 1989Heidelberg, Germany: Springer-Verlag; 299–312.eds. Brachmann, J. & Schömig, A. pp [Google Scholar]
- SCHWARTZ P.J., BILLMAN G.E., STONE H.L. Autonomic mechanisms in ventricular fibrillation induced by myocardial ischemia during exercise in dogs with healed myocardial infarction. An experimental preparation for sudden cardiac death. Circulation. 1984;69:790–800. doi: 10.1161/01.cir.69.4.790. [DOI] [PubMed] [Google Scholar]
- SILVERMAN H.S., WEI S., HAIGNEY M.C., OCAMPO C.J., STERN M.D. Myocyte adaptation to chronic hypoxia and development of tolerance to subsequent acute severe hypoxia. Circ. Res. 1997;80:699–707. doi: 10.1161/01.res.80.5.699. [DOI] [PubMed] [Google Scholar]
- TANDE P.M., BJORNSTAD H., YANG T., REFSUM H. Rate-dependent class III antiarrhythmic action, negative chronotropy, and positive inotropy of a novel IK blocking drug, UK-68,798: potent in guinea pig but no effect in rat myocardium. J. Cardiovasc. Pharmacol. 1990;16:401–410. doi: 10.1097/00005344-199009000-00008. [DOI] [PubMed] [Google Scholar]
- TILLMANNS H., IKEDA S., BING R.J. Production of myocardial infarcts in animal experiments. Handbuch. Exp. Pharm. 1983;16:117–130. [Google Scholar]
- VAN ECHTELD C.J., KIRKELS J.H., EIJGELSHOVEN M.H., VAN DER MEER P., RUIGROK T.J. Intracellular sodium during ischemia and calcium-free perfusion: a 23Na NMR study. J. Mol. Cell Cardiol. 1991;23:297–307. doi: 10.1016/0022-2828(91)90066-u. [DOI] [PubMed] [Google Scholar]
- VANDEN HOEK T.L., LI C., SHAO Z., SCHUMACKER P.T., BECKER L.B. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J. Mol. Cell Cardiol. 1997;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- VATNER D.E., KNIGHT D.R., SHEN Y.T., THOMAS J.X.J., HOMCY C.J., VATNER S.F. One hour of myocardial ischemia in conscious dogs increases beta-adrenergic receptors, but decreases adenylate cyclase activity. J. Mol. Cell Cardiol. 1988a;20:75–82. doi: 10.1016/s0022-2828(88)80180-9. [DOI] [PubMed] [Google Scholar]
- VATNER S.F., PATRICK T.A., KNIGHT D.R., MANDERS W.T., FALLON J.T. Effects of calcium channel blocker on responses of blood flow, function, arrhythmias, and extent of infarction following reperfusion in conscious baboons. Circ. Res. 1988b;62:105–115. doi: 10.1161/01.res.62.1.105. [DOI] [PubMed] [Google Scholar]
- WALDO A.L., CAMM A.J., DERUYTER H., FRIEDMAN P.L., MACNEIL D.J., PAULS J.F., PITT B., PRATT C.M., SCHWARTZ P.J., VELTRI E.P. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival with oral d-sotalol. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- WANNAMETHEE G., SHAPER A.G., MACFARLANE P.W., WALKER M. Risk factors for sudden cardiac death in middle-aged British men. Circulation. 1995;91:1749–1756. doi: 10.1161/01.cir.91.6.1749. [DOI] [PubMed] [Google Scholar]
- WILDERS R., VERHEIJCK E.E., JOYNER R.W., GOLOD D.A., KUMAR R., VAN GINNEKEN A.C., BOUMAN L.N., JONGSMA H.J. Effects of ischemia on discontinuous action potential conduction in hybrid pairs of ventricular cells. Circulation. 1999;99:1623–1629. doi: 10.1161/01.cir.99.12.1623. [DOI] [PubMed] [Google Scholar]
- WILLIAMS H., KING N., GRIFFITHS E.J., SULEIMAN M.S. Glutamate-loading stimulates metabolic flux and improves cell recovery following chemical hypoxia in isolated cardiomyocytes. J. Mol. Cell Cardiol. 2001;33:2109–2119. doi: 10.1006/jmcc.2000.1474. [DOI] [PubMed] [Google Scholar]
- WIT A.L.The time course of occurrence of ventricular arrhythmias after experimental myocardial ischemia Adrenergic System and Ventricular Arrhythmias in Myocardial Infarction 1989Heidelberg, Germany: Springer-Verlag; 275–296.ed. Brachmann, J. & Schomig, A. pp [Google Scholar]
- WU J., CORR P.B. Palmitoyl carnitine modifies sodium currents and induces transient inward current in ventricular myocytes. Am. J. Physiol. 1994;266:H1034–H1046. doi: 10.1152/ajpheart.1994.266.3.H1034. [DOI] [PubMed] [Google Scholar]
- ZHENG Z.J., CROFT J.B., GILES W.H., MENSAH G.A. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]