Abstract
In the central nervous system (CNS), glutamate rapidly upregulates the activities of different excitatory amino-acid transporter subtypes (EAATs) in order to help protect neurons from excitotoxicity. Since human platelets display a specific sodium-dependent glutamate uptake activity, and express the three major glutamate transporters, which may be affected in neurological disorders, we investigated whether platelets are subject to substrate-induced modulation as described for CNS.
A time- and dose-dependent upregulation of [3H]-glutamate uptake (up to two-fold) was observed in platelets preincubated with glutamate. There was an increase in maximal velocity rate without affinity changes. Glutamate receptor agonists and antagonists did not modulate this upregulation and preincubation with glutamate analogues failed to mimic the glutamate effect. Only aspartate preincubation increased the uptake, albeit ∼35% less with respect to glutamate.
The effect of glutamate preincubation on the expression of the three major transporters was studied by Western blotting, showing an increase of ∼70% in EAAT1 immunoreactivity that was completely blocked by cycloheximide (CEM). However, L-serine-O-sulphate, at a concentration (200 μM) known to block EAAT1/3 selectively, did not completely inhibit the effect of glutamate stimulation, indicating the possible involvement of EAAT2.
In fact, glutamate stimulation was completely abolished only when, following CEM pre-incubation, the experiment was run in the presence of the selective EAAT2 inhibitor dihydrokainic acid. Since surface biotinylation experiments failed to show evidence of EAAT2 translocation, our results suggest the existence of a different way of regulating EAAT2 activity.
These findings indicate that human platelets display a substrate-dependent modulation of glutamate uptake mediated by different molecular mechanisms and confirm that ex vivo platelets are a reliable model to investigate the dysfunction of glutamate uptake regulation in patients affected by neurological disorders.
Keywords: Glutamate uptake, human platelets, substrate modulation, EAAT1
Introduction
In the central nervous system (CNS), glutamate transporters play a fundamental role in regulating the physiological clearance of neurotransmitter from the synaptic cleft (Tong & Jahr, 1994; Diamond & Jahr, 1997). Interestingly, under specific circumstances, glutamate transporters may also play a neuroprotective role (Rothstein et al., 1996; Tanaka et al., 1997; Gegelashvili et al., 2001), since high and prolonged exposure to glutamate in CNS is a critical event for the pathogenesis of numerous neurological disorders (Choi, 1994; Maragakis & Rothstein, 2001), either acute, such as stroke (Rossi et al., 2000) and epilepsy (Dutuit et al., 2002), or chronic, such as Alzheimer's disease (Masliah et al., 1996), Parkinson's disease (Plaitakis & Shashidharan, 2000), and amyotrophic lateral sclerosis (Rothstein et al., 1992). It is worth noting that glutamate uptake in the brain is tightly regulated via distinct cellular mechanisms. In particular, it has been shown that the activity of the excitatory amino-acid transporter-1 (EAAT1/GLAST) can be rapidly modulated by substrate interaction (Duan et al., 1999; Munir et al., 2000).
Therefore, in light of the important role played by glutamate transporters in the pathogenesis of several neurological disorders, the characterization of suitable peripheral models addressing both pathophysiological and diagnostic issues is of great interest. Human platelets appear to be a good target since they display functional similarities with neuronal elements such as the storing, releasing and uptaking of neurotransmitters such as glutamate, serotonin, GABA and dopamine, expressing their specific receptors and/or transporters (Da Prada et al., 1988). Regarding the glutamatergic system, N-methyl-D-aspartate (NMDA) receptors have recently been described in megakaryocytes (Genever et al., 1999; Skerry & Genever, 2001) and they appear to play a role in platelet activation and/or aggregation (Franconi et al., 1998). Moreover, a sodium- and energy-dependent glutamate transport system, similar to that described in synaptosomes, has been characterized previously (Mangano & Schwarcz, 1981). Finally, we have recently shown that platelets express both mRNA and protein for the three major glutamate transporters, namely EAAT1, EAAT2 and EAAT3 (Zoia et al., 2004). Further, recent studies support the hypothesis that platelets could be used as peripheral models for studying specific biochemical and pharmacological alterations in neurodegenerative diseases (Di Luca et al., 2000). Since glutamate uptake has been consistently shown to be dramatically affected in this peripheral model in different neurological conditions (Ferrarese et al., 1999; 2000; 2001), it would be interesting to investigate the mechanisms responsible for the modulation of the expression and activity of platelet glutamate transporters.
Therefore, the goal of this study was to investigate whether platelet glutamate transporter activity and/or expression is modulated by glutamate and whether this modulation shares the same pharmacological profile and regulatory pathways described in CNS (Gegelashvili et al., 1996; Gegelashvili & Schousboe, 1997; Swanson et al., 1997; Davis et al., 1998; Schlag et al., 1998). In fact, platelets might also be used as peripheral models to investigate the relevance of the same mechanisms in determining the dysfunction of glutamatergic homeostasis in patients affected by neurological diseases.
Methods
NMDA, dihydrokainic acid (DHK), L(−)-threo-3-hydroxyaspartic acid (THA), L-serine-O-sulphate potassium salt (SOS), (RS)-α-methyl-4-carboxyphenylglycine (MCPG) and 6-cyano-7-nitroquinoxaline 2,3 dione disodium salt (CNQX) were purchased from Tocris Coockson Inc. (Bristol, U.K.).
Sample preparation
For platelet separation, whole blood was collected from healthy donors by venipuncture, into 15% K2-EDTA. To minimize spurious platelet activation, blood withdrawal was performed using a 19-G needle and by releasing the tourniquet. Samples were centrifuged at 380 × g for 10 min at 4°C. The supernatant platelet-rich plasma (PRP) was transferred into ice-cold tubes and centrifuged at 6340 × g for 10 min at 4°C. The pellet was rinsed in 0.32 M sucrose, pH 7.4, and platelets were then resuspended in a volume of sucrose equal to one-fifth the original volume of PRP (Mangano & Schwarcz, 1981). Total protein concentration was estimated by spectrophotometer using Bradford's method.
Glutamate uptake assay
For glutamate preincubation experiments, platelets were treated with 100 μM glutamate (except where noted) in sodium-citrate buffer for 1 h at 37°C on a shaker. Control samples were preincubated in the same conditions, but without glutamate. Careful removal of the preincubation medium was performed by twice rinsing platelets in glutamate-free buffer followed by centrifugation at 6340 × g for 10 min, before resuspension of the platelets in the uptake medium. Platelet aliquots in sucrose buffer were settled on a shaker at 37°C for 5 min into tris-citrate buffer before starting the assay. For blank samples tris-citrate buffer had equimolar choline in place of sodium chloride and sodium citrate was replaced with equimolar potassium citrate.
The uptake assay was initiated by the addition of [3H]-glutamate (specific activity 42.9 Ci mm−1; NEN Life Science Products, Milan, Italy) at a final concentration of 60 μM. After 20 min, the uptake reaction was stopped by adding ice-cold tris-citrate buffer containing 10 mM cold glutamate and placing the tubes on ice for 1 min, followed by centrifugation for 10 min at 6340 × g. After rinsing, platelet pellets were dissolved overnight in formic acid–acetone (15 : 85), and then measured by β-counter. Net high-affinity glutamate uptake was calculated by subtracting sodium-free control measurements from uptake assays performed in the presence of sodium. Glutamate transport rate was expressed as pmol-glutamate mg-proteins−1 min−1.
Western blotting analysis
Following preincubation with or without glutamate, platelets were sonicated in phosphate buffer containing protease inhibitors. Samples were then separated by 8% SDS–PAGE electrophoresis, and subsequently transferred to Hybond nitrocellulose membranes (Amersham Pharmacia, Piscataway, NJ, U.S.A.). After blocking in PBS/5% nonfat dry milk/0.1% Tween® 20 (Sigma, Milan, Italy) for 1 h, the blots were incubated with antibodies against either EAAT1 (1 : 800, Santa Cruz, Santa Cruz, CA, U.S.A.), EAAT2 or EAAT3 (1 : 1000 Chemicon Int., Temecula, CA, U.S.A.) for 2 h at room temperature. Actin antibody (1 : 2000, Sigma) was also used to normalize for sample loading. Following washing in PBS/0.1% Tween® 20, the membranes were incubated for 1 h with the corresponding peroxidase-conjugated secondary antibody (1 : 1000 Chemicon Int.). Signals were detected using the ECL plus system (Amersham Pharmacia), and quantitative analysis was performed using a densitometer (Biorad).
Biotinylation procedure
Biotinylation of platelet surface proteins was performed as previously described by Qian et al. (1997), with minor modifications. Following glutamate preincubation, platelets were centrifuged at 3700 × g for 10 min at room temperature and then incubated with 1 ml biotin solution (sulpho-NHS-biotin, 1 mg/ml in PBS, Pierce, Rockford, IL, U.S.A.) for 20 min at 4°C with gentle shaking. Blocking of biotin solution was performed by adding 1 vol glycine solution (0.1 M) for 45 min. Then, pellets were washed with the same solution twice and finally stocked at −80°C overnight. Platelet pellets were then sonicated as described. Whole-cell fractions were incubated with 1 vol avidin-conjugated beads (Pierce) for 1 h and then centrifuged at 12,400 × g for 15 min. The supernatants, containing the intracellular fraction, were collected and stored for Western blot analysis and the pellets, containing the biotinylated cell-surface proteins, were rinsed before resuspension in Laemmli Buffer for 30 min at 4°C. These suspensions were centrifuged a final time at 12,400 × g for 10 min, the supernatants removed and the pellets (i.e., the biotinylated fraction) stored for Western blotting. The purity of both intracellular and membrane fractions was confirmed, respectively, by the presence or absence of beta-actin immunoreactivity.
High-performance liquid chromatography (HPLC) glutamate assay
To avoid glutamate degradation, 1 ml aliquots of plasma were immediately inactivated with 100 μl 4 M HClO4, and neutralized with 50 μl 7 M K2CO3, then filtered and stored at −80°C until analysis (Ferrarese et al., 1993). After precolumn derivatization with orthophthaldialdehyde (OPA), samples were run through a C18 reverse-phase column (Waters, 30 cm × 4.9 mm) by a multistep gradient of two solvents (A: 0.1 M Na-acetate buffer pH 7.2; B: methanol/tetrahydrofuran 97 : 3 vol vol−1), at a flow rate of 1.5 ml min−1. Glutamate peaks were easily detected in plasma as described previously (Ferrarese et al., 1993).
Statistical analysis
Data are expressed as mean±s.e.m. of at least four independent experiments. Either Student's t-test or analysis of variance (ANOVA) followed by Bonferroni post hoc analysis were used to evaluate the differences among treatments. The significance criteria are indicated in the figure legends.
Results
Glutamate preincubation enhances [3H]-glutamate uptake in human platelets
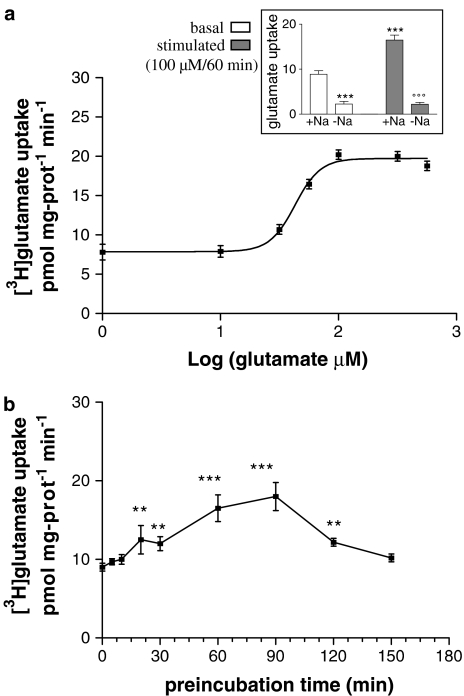
Following platelet preincubation with 100 μM glutamate for 60 min at 37°C, [3H]-glutamate uptake by the platelets increased about 90% compared to controls preincubated with glutamate-free medium (Figure 1a, inset). This increase was observed for sodium-dependent glutamate uptake, but not for the sodium-independent uptake (see Figure 1a, inset). The dose and preincubation time used were chosen because they were previously shown to induce a maximal stimulatory effect on glutamate uptake in primary murine astrocyte cultures (Duan et al., 1999).
Figure 1.
(a) Dose dependence of glutamate preincubation (60 min). EC50=42 μM; n=7. (Inset) Glutamate (100 μM) preincubation for 60 min (stimulated) induced ∼90% increase in sodium-dependent platelet glutamate uptake (+Na) with respect to glutamate-free medium preincubated samples (basal). Glutamate failed to stimulate glutamate uptake when incubated in Na-free medium (−Na). ***P<0.0005 vs +Na/basal, °°°P<0.0005 vs +Na/stimulated, Student's t-test; n=25. (b) Time course of glutamate preincubation (100 μM). A significant increase in glutamate uptake with respect to basal levels occurred after ∼30 min (**P<0.001, Bonferroni test) and reached the maximal value after ∼90 min (***P<0.0005, Bonferroni test); n=4.
A dose–response curve shows an EC50 of about 42 μM (Figure 1a). Analysis of this curve highlighted that glutamate preincubation stimulated platelet uptake only when glutamate concentration was at least 30 μM. This value, interestingly, is of the same order of magnitude of that assessed by HPLC in the corresponding plasma samples (plasma glutamate levels=27±2.3 μM; mean±s.e.m., n=7). Time dependence of glutamate stimulation was also analysed using 100 μM glutamate (Figure 1b). After about 30 min preincubation, there was already a significant stimulation (∼30%) of glutamate transport. This increase reached maximal values (about two-fold) at 60–90 min, and then decreased. After 150 min, glutamate uptake of pretreated platelets was comparable to control samples.
The saturation curves of [3H]-glutamate uptake in control- and glutamate-treated platelets were compared in order to determine if glutamate-induced uptake stimulation was due to either changes in the affinity (Km) or in the maximal velocity (Vmax) of glutamate internalization. Glutamate pretreatment (100 μM/60 min) induced about a 40% increase in Vmax (Figure 2a), while the Km value did not change, as shown by the Eadie–Hofstee plots (Figure 2b).
Figure 2.
(a) Saturation curves of glutamate transport in control (black squares) and glutamate-stimulated (100 μM/60 min; black triangles) platelets. (b) Eadie–Hofstee plots. Glutamate treatment induced a significant increase in Vmax (control: 11.7±0.5 vs stimulated: 16.2±0.9 pmol mg-proteins−1 min−1; P<0.01, Student's t-test; n=5) without changing the Km value (33.5±2.7 vs 35±4.7 μM, respectively, for control and stimulated platelets).
Characterization of glutamate stimulation of platelet glutamate uptake
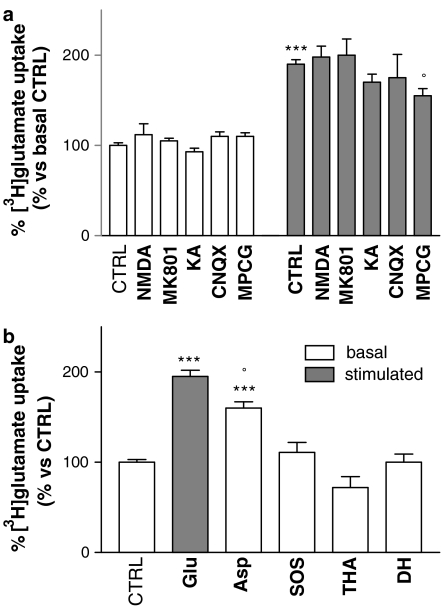
Since NMDA receptors sensitive to MK801 have been described in human platelets (Franconi et al., 1998), the possibility that these receptors mediate glutamate stimulation of [3H]-glutamate uptake was investigated. Preincubation with 100 μM NMDA or 100 μM MK801 had no effect on either basal or glutamate-stimulated uptake (Figure 3a). No other types of glutamate receptors have been described in platelets, but in any case, we preincubated platelets with a kainate-receptor antagonist (100 μM CNQX) and a metabotrobic receptor antagonist (200 μM MCPG). CNQX did not show any effect on basal- or glutamate-stimulated uptake, while MCPG did not alter basal transport, but was apparently able to induce a small, albeit significant, decrease in glutamate-stimulated uptake (Figure 3a).
Figure 3.
(a) Effects of preincubation with glutamate receptor agonists or antagonists (60 min, in bold) on platelet glutamate uptake. NMDA (100 μM), MK801 (100 μM), kainate (KA; 100 μM) or CNQX (100 μM) preincubation all failed to modulate basal and stimulated glutamate uptake. Only preincubation with the metabotropic receptor antagonist MCPG (200 μM) appeared to reduce glutamate-stimulated uptake. ***P<0.0001 vs CTRL/basal, °P<0.02 vs CTRL/stimulated, Bonferroni test; n=7. (b) Effects of preincubation with glutamate analogues (60 min) on glutamate uptake. Platelets preincubated with D-aspartate (100 μM, Asp) showed a significant increase in glutamate transport, although this was weaker than that elicited by preincubation with equimolar glutamate concentrations (Glu). ***P<0.0001 vs CTRL, °P<0.01 vs Glu, Bonferroni test; n=7. Preincubation with SOS (200 μM), THA (500 μM) or DHK (500 μM) failed to induce any increase in glutamate transport.
We also investigated the effect of preincubation with the following glutamate analogues (Figure 3b): 100 μM D-aspartic acid, SOS (200 μM), THA (500 μM) or the nontransportable DHK (500 μM). Only D-aspartic acid had any effect, resulting in a significant increase in glutamate transport (although weaker than that elicited by equimolar glutamate concentrations), while SOS and DHK had no effect on glutamate uptake.
Effects of glutamate preincubation on platelet expression of glutamate transporters
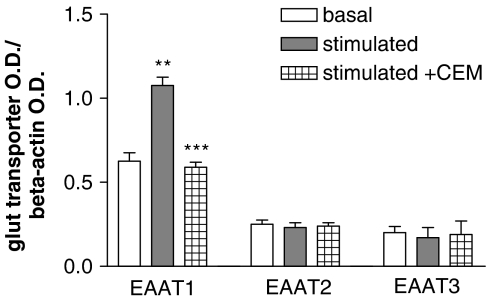
To investigate whether the substrate modulation of glutamate uptake in human platelets might be due to changes in the expression profile of specific transporters, we performed a Western blot analysis. As shown in Figure 4, a significant increase in EAAT1 was shown for glutamate-treated platelets with respect to controls. In contrast, preincubation with glutamate did not significantly affect the expression of either EAAT2 or EAAT3.
Figure 4.
Expression of glutamate transporters in human platelets. Glutamate treatment (100 μM/60 min) increased EAAT1 immunoreactivity, while coincubation with 10 μM cycloheximide (CEM) completely blocked the synthesis of new EAAT1 transporters. **P<0.003 vs EAAT1/basal, ***P<0.0005 vs EAAT1/stimulated, Student's t-test; n=5.
In order to clarify the molecular mechanisms responsible for the observed increase in EAAT1 immunoreactivity, the effect of CEM, an inhibitor of protein synthesis, was determined. Coincubation with CEM (10 μM/60 min) completely blocked the increase of EAAT1 mediated by glutamate, probably by inhibiting the synthesis of new transporters, without influencing the expression of the other two investigated transporter subtypes, EAAT2 and EAAT3 (Figure 4). Moreover, in functional experiments CEM preincubation failed to affect basal glutamate transport, while it clearly reduced glutamate-stimulated uptake (see Figure 5b, see CEM).
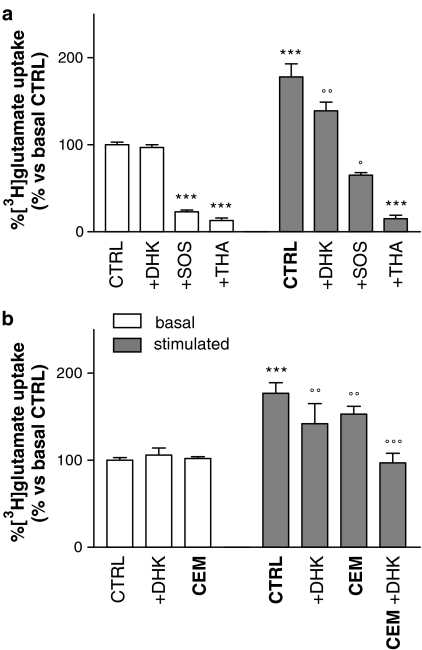
Figure 5.
(a) Pharmacological characterization of basal and glutamate-stimulated glutamate uptake in platelets. The presence of THA (500 μM, +THA) or SOS (200 μM, +SOS) strongly inhibited basal glutamate transport (basal), while DHK (500 μM, +DHK) failed to show any effect. Following glutamate preincubation (100 μM/60 min; stimulated), the same profiles of inhibition were shown in the THA-sensitive component, while the presence of DHK resulted in a significant decrease in glutamate-stimulated uptake. Moreover, SOS-mediated inhibition was lower in glutamate-stimulated platelets with respect to control, suggesting EAAT2 involvement. ***P<0.0001 vs CTRL/basal, °°P<0.001 vs CTRL/stimulated, °P<0.05 vs +SOS/basal, Bonferroni test; n=12. (b) Synergistic effect of CEM preincubation (10 μM/60 min, CEM) and DHK direct inhibition (+DHK) on stimulated platelet glutamate uptake. Neither preincubating with CEM nor running the assay in the presence of 500 μM DHK affected basal uptake (basal). Following glutamate preincubation (100 μM/60 min; stimulated), the effect was completely abolished only when both procedures were performed together.***P<0.0002 vs CTRL/basal, °°°P<0.0005, °°P<0.001 vs CTRL/stimulated, Bonferroni test; n=4.
In human platelets glutamate preincubation elicits a novel DHK-sensitive component of glutamate uptake that is not due to EAAT2 membrane translocation
To determine which classes of glutamate transporters are active in basal and in glutamate-induced transport, the sensitivity to specific inhibitors was also evaluated. [3H]-glutamate uptake in basal and glutamate-stimulated platelets was assayed in the presence of either 500 μM THA, 200 μM SOS, which preferentially inhibits EAAT1 and EAAT3 (Bridges et al., 1999), or 500 μM DHK, selective for EAAT2 (Arriza et al., 1994). Basal glutamate uptake was strongly inhibited in the presence of SOS and THA, but in contrast, DHK did not have any effect. Following glutamate preincubation, no increase in THA sensitivity was observed, but, surprisingly, the presence of DHK caused a reduction in glutamate-stimulated uptake (Figure 5a). Moreover, in line with this observation, SOS-mediated inhibition was lower in glutamate-stimulated platelets, further suggesting the specific involvement of EAAT2 (Figure 5a).
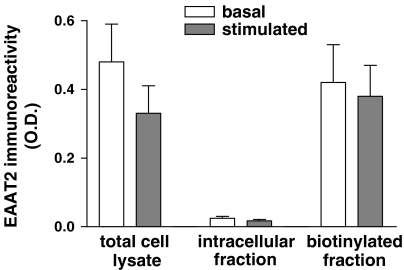
The glutamate-mediated effect on uptake was completely abolished only when protein synthesis was blocked by co-preincubation with CEM and the assay was then run in the presence of DHK (Figure 5b). Hence, the possibility that this DHK-sensitive component was due to increased expression of EAAT2 in plasma membrane was investigated. Biotinylation experiments showed that EAAT2-like immunoreactivity was present only in the biotinylated fraction (plasma membrane) both in control and in stimulated platelets, and no evidence for translocation was detected (Figure 6).
Figure 6.
Analysis of subcellular localization of EAAT2 transporters in human platelets. Glutamate preincubation failed to induce any rearrangement of EAAT2 on to the cell surface; n=5.
Discussion
Accumulating evidence has been reported that several neurotransmitters can rapidly upregulate the activity and the expression of the respective transporters in CNS (Qian et al., 1997; Davis et al., 1998; Bernstein & Quick, 1999; Duan et al., 1999). In particular, a substrate-induced modulation of glutamate uptake was observed both in astrocytes where the translocation of the glutamate transporter GLAST/EAAT1 has been reported (Duan et al., 1999), and in neurons where glutamate might rapidly stimulate the activity of glutamate transporters without causing redistribution on the plasma membrane (Munir et al., 2000). Possibly, both mechanisms could be involved in rapid and effective regulation of glutamate transporters in neurons and glial cells in order to control extracellular glutamate levels and prevent excitotoxicity.
In this study, we used human platelets as a model for studying the mechanisms involved in glutamate uptake regulation. These cells express the three major glutamate transporter subtypes and possess a high-affinity glutamate uptake activity similar to that described in CNS, which have been shown to be affected in neurodegenerative disorders (Ferrarese et al., 1999; 2000; 2001). Here, we report clear evidence that glutamate preincubation increases sodium-dependent L-[3H]-glutamate transport in human platelets. Moreover, glutamate treatment did not stimulate transport in sodium-free medium, indicating that the increase in glutamate transport was mediated by specific glutamate transporters.
The involvement of kainate and/or metabotropic receptors in this regulation was also excluded by preincubating with specific agonists and antagonists. The observed MCPG effects on glutamate-stimulated uptake might be due to a putative steric interaction or competition for the transporters. This hypothesis is partly sustained by the evidence that many glutamate metabotropic receptor agonists can also serve as substrates for glutamate transporters (Ye & Sontheimer, 1999), and that no evidence for the presence of metabotropic receptors has been shown in platelets up to now. To rule out the possibility that glutamate-mediated increase in uptake is related to the activation of NMDA-like receptors, known to be expressed in human platelets (Franconi et al., 1998; Berk et al., 2001), the effects of both NMDA and of the specific antagonist MK801 were tested. No changes in basal or stimulated uptake were shown, therefore excluding a role for NMDA receptors in mediating the effect of glutamate. Moreover, maximal stimulation of uptake was observed only for glutamate concentrations saturating the transport mechanism and these concentrations far exceed the known receptor affinity for glutamate.
Interestingly, a direct correlation between glutamate plasma concentrations and the starting point in the stimulation curve (Figure 1a) was shown, a phenomenon that we hypothesize is related to a physiologic role. In fact, platelets might be involved in keeping glutamate plasma levels within a specific range in order to modulate NK cell response, for example Kuo et al. (2001), or to regulate platelet aggregation through the activation of NMDA receptors (Franconi et al., 1998).
Analysing the time course of uptake following stimulation with 100 μM glutamate (Figure 1b) showed an increase after about 20 min, and the maximum effect was achieved at about 60–90 min, with a reduced effect after longer preincubation times. These data reproduce what has been previously reported in primary murine astrocyte cultures (Duan et al., 1999). This may possibly be interpreted as a mechanism of deactivation of EAAT1 and/or EAAT2, with the aim of avoiding large changes in the glutamatergic homeostasis, operating both in CNS and in platelets.
To understand if this glutamate-stimulated uptake was specific for glutamate itself, we analysed the effects of preincubation with glutamate analogues. D-Aspartate mimicked the glutamate effect as has already been reported in glial cells (Duan et al., 1999), although the rate of stimulation was lower with respect to equimolar L-glutamate concentrations. No other analogues were effective in stimulating glutamate uptake, suggesting that only highly specific substrates for glutamate transporters can enhance the uptake activity through this mechanism.
Several strategies were used to identify the mechanisms involved in this increase in transport activity and to test if different transporter subtypes play a role. Since glutamate stimulation resulted in a change in the rate of maximal velocity (Vmax), but not in the affinity to the substrate (Km), and this observation is in agreement with the assumption that there is an increase in the number of functional transporters present on the cell membrane, the immunoreactivity of EAAT1, EAAT2 and EAAT3 was measured in order to test the hypothesis that these transporters are newly synthesised. Our results showed that glutamate treatment induced an increase in EAAT1 total expression, while EAAT2 and EAAT3 were unaffected. Moreover, when CEM, a protein synthesis inhibitor, was coincubated with glutamate, no increase in EAAT1 expression was shown, sustaining the hypothesis that glutamate preincubation may elicit the synthesis of novel EAAT1 molecules. These data suggest that in platelets de novo synthesis might be a rapid way to respond to various stimuli. Interestingly, platelets lack a nuclear structure but do store mRNA, which is readily available for translation, and they are characterized by an extremely specialized functional profile necessary for a fast and effective response to tissue injury. It is noteworthy that the mRNA for the three major glutamate transporters has previously been described in platelets (Zoia et al., 2004). In accordance with this neosynthesis hypothesis, protracted glutamate exposure induced the expression of GLAST/EAAT1 in both rat brain and astrocyte primary cultures (Gegelashvili et al., 1996; Suarez et al., 2000). Moreover, an increase in EAAT1 expression was recently observed in brains of Alzheimer's disease patients (Scott et al., 2002), possibly representing an attempt to prevent the excitotoxic damage known to be operative in the brain of these patients.
In order to clarify whether the synthesis of new transporters is the only molecular mechanism involved, glutamate uptake was measured following CEM coincubation with the result that the effect of glutamate stimulation was reduced but not completely abolished. In fact, it is only in the presence of DHK (a nontransportable inhibitor of EAAT2 that has been demonstrated to display little if no activity on the other EAATs; Bridges et al., 1999) that glutamate and CEM coincubation completely fails to stimulate glutamate uptake. This indicates that a new DHK-sensitive functional component, not evidenced in basal uptake, becomes operative. These data were further supported by the finding that 200 μM SOS was less able to inhibit glutamate uptake following stimulation, if compared with basal conditions, since SOS at this concentration does not block EAAT2.
However, Western blot experiments showed that EAAT2 immunoreactivity was unchanged following glutamate preincubation, raising the possibility that EAAT2 might be transported to the membrane. To test this, we preformed biotinylation experiments that did not show any evidence of membrane translocation of EAAT2, suggesting that mechanisms other than redistribution of this transporter might be involved. A possibility might be the direct activity modulation of this transporter subtype, possibly through the activation of kinases (O'Shea, 2002). Interestingly, our hypothesis that at least two different mechanisms are involved in the glutamate-induced stimulation of the uptake rate is consistent with the apparent biphasic increase of the uptake caused by varying the preincubation time. In fact, as shown in Figure 1b, a first peak was shown at 20–30 min, while a second peak was evident at 60–90 min, indicating the possibility of two distinct phenomena operating with different velocities. It is conceivable that the synthesis of new transporters would take a longer time than direct activity modulation.
In conclusion, we demonstrated that glutamate can induce a rapid upregulation of glutamate uptake in human platelets in a manner which is similar to that previously described in glial or neuronal cells. Western blot analysis and pharmacological studies also provided evidence that not all transporter subtypes are equally involved in this phenomenon, which is mediated at least by two different mechanisms: new synthesis of EAAT1 transporters and a putative interaction of glutamate with EAAT2, possibly mediated by other associated proteins.
Since glutamate uptake plays a critical role in the prevention of excitotoxicity, future studies are extremely important in order to completely understand the complex mechanisms involved in its regulation and to clarify which are the associated signalling pathways. In this view, we propose that human platelets may represent an interesting and accessible model to study ex vivo glutamate transport in patients affected by neurological disorders. Theoretically, this line of investigation might eventually provide an understanding of the role of the glutamatergic dysfunction operative in each single patient, with possible important implications for optimizing the available diagnostic, prognostic and therapeutic strategies.
Acknowledgments
We thank all the donors and the staff of the Transfusion Center of the San Gerardo Hospital (Monza-Italy) for their help in collecting the blood samples used in this study.
Abbreviations
- CEM
cycloheximide
- CNQX
6-cyano-7-nitroquinoxaline 2,3 dione disodium salt
- DHK
dihydrokainic acid
- EAAT
excitatory amino-acid transporter
- HPLC
high-performance liquid chromatography
- MCPG
(RS)-α-methyl-4-carboxyphenylglycine
- NMDA
N-methyl-D-aspartate
- SOS
L-serine-O-sulphate potassium salt
- THA
L(−)-threo-3-hydroxyaspartic acid
References
- ARRIZA J.L., FAIRMAN W.A., WADICHE J.I., MURDOCH G.H., KAVANAUGH M.P., AMARA S.G. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERK M., PLEIN H., FERREIRA D. Platelet glutamate receptor supersensitivity in major depressive disorder. Clin. Neuropharmacol. 2001;24:129–132. doi: 10.1097/00002826-200105000-00002. [DOI] [PubMed] [Google Scholar]
- BERNSTEIN E.M., QUICK M.W. Regulation of gamma-aminobutyric acid (GABA) transporters by extracellular GABA. J. Biol. Chem. 1999;274:889–895. doi: 10.1074/jbc.274.2.889. [DOI] [PubMed] [Google Scholar]
- BRIDGES R.J., KAVANAUGH M.P., CHAMBERLIN A.R. A pharmacological review of competitive inhibitors and substrates of high-affinity, sodium-dependent glutamate transport in the central nervous system. Curr. Pharm. Des. 1999;5:363–379. [PubMed] [Google Scholar]
- CHOI D.W. Glutamate receptors and the induction of excitotoxic neuronal death. Prog. Brain Res. 1994;100:47–51. doi: 10.1016/s0079-6123(08)60767-0. [DOI] [PubMed] [Google Scholar]
- DA PRADA M., CESURA A.M., LAUNAY J.M., RICHARDS J.G. Platelets as a model for neurones. Experientia. 1988;44:115–126. doi: 10.1007/BF01952193. [DOI] [PubMed] [Google Scholar]
- DAVIS K.E., STRAFF D.J., WEINSTEIN E.A., BANNERMAN P.G., CORREALE D.M., ROTHSTEIN J.D., ROBINSON M.B. Multiple signalling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma. J. Neurosci. 1998;67:508–516. doi: 10.1523/JNEUROSCI.18-07-02475.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J.S., JAHR C.E. Transporters buffer synaptically released glutamate on a submillisecond time scale. J. Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI LUCA M., COLCIAGHI F., PASTORINO L., BORRONI B., PADOVANI A., CATTABENI F. Platelets as a peripheral district where to study pathogenetic mechanism of Alzheimer disease: the case of amyloid precursor protein. Eur. J. Pharmacol. 2000;405:277–283. doi: 10.1016/s0014-2999(00)00559-8. [DOI] [PubMed] [Google Scholar]
- DUAN S., ANDERSON C.M., STEIN B.A., SWANSON R.A. Glutamate induced rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J. Neurosci. 1999;19:10193–10200. doi: 10.1523/JNEUROSCI.19-23-10193.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUTUIT M., TOURET M., SZYMOCHA R., NEHLIG A., BELIN M.F., DIDIER-BAZES M. Decreased expression of glutamate transporters in genetic absence epilepsy rats before seizure occurrence. J. Neurochem. 2002;80:1029–1038. doi: 10.1046/j.0022-3042.2002.00768.x. [DOI] [PubMed] [Google Scholar]
- FERRARESE C., PECORA N., FRIGO M., APOLLONIO I., FRATTOLA L. Assessment of reliability and biological significance of glutamate levels in cerebrospinal fluid. Ann. Neurol. 1993;33:316–319. doi: 10.1002/ana.410330316. [DOI] [PubMed] [Google Scholar]
- FERRARESE C., ZOIA C., PECORA N., PIOLTI R., FRIGO M., BIANCHI G., SALA G., BEGNI B., RIVA R., FRATTOLA L. Reduced platelet glutamate uptake in Parkinson's disease. J. Neur. Transm. 1999;106:685–692. doi: 10.1007/s007020050189. [DOI] [PubMed] [Google Scholar]
- FERRARESE C., BEGNI B., CANEVARI C., ZOIA C., PIOLTI R., FRIGO M., FRATTOLA L. Glutamate uptake is decreased in platelets from Alzheimer's disease patients. Ann. Neurol. 2000;47:641–643. [PubMed] [Google Scholar]
- FERRARESE C., SALA G., RIVA R., BEGNI B., ZOIA C., TREMOLIZZO L., GALIMBERTI G., MILLUL A., BASTONE A., PENNINI T., BALZARINI C., FRATTOLA L., BEGHI E., THE ITALIAN ALS STUDY GROUP Decreased platelet glutamate uptake in patients with amyotrophic lateral sclerosis. Neurology. 2001;56:270–272. doi: 10.1212/wnl.56.2.270. [DOI] [PubMed] [Google Scholar]
- FRANCONI F., MICELI M., ALBERTI L., SEGHIERI G., DE MONTIS M.G., TAGLIAMONTE A. Futher insights into the anti-aggregating activity of NMDA in human platelets. Br. J. Pharmacol. 1998;124:35–40. doi: 10.1038/sj.bjp.0701790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEGELASHVILI G., CIVENNI G., RACAGNI G., DANBOLT N.C., SCHOUSBOE I., SCHOUSBOE A. Glutamate receptor agonist up-regulate glutamate transporter GLAST in astrocytes. Neuroreport. 1996;8:261–265. doi: 10.1097/00001756-199612200-00052. [DOI] [PubMed] [Google Scholar]
- GEGELASHVILI G., SCHOUSBOE A. High affinity glutamate transporters: regulation of expression and activity. Mol. Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- GEGELASHVILI G., ROBINSON M.B., TROTTI D., RAUEN T. Regulation of glutamate transporters in health and disease. Prog. Brain Res. 2001;132:267–286. doi: 10.1016/S0079-6123(01)32082-4. [DOI] [PubMed] [Google Scholar]
- GENEVER P.G., WILKINSON D.J.P., PATTON A.J., PEET N.M., HONG Y., MATHUR A., ERUSALIMSKY J.D., SKERRY T.M. Expression of a functional N-methyl-D-aspartate-type glutamate receptor by bone marrow megakaryocytes. Blood. 1999;23:2876–2883. [Google Scholar]
- KUO J.S., CHEN S.F., HUANG H.J., YANG C.S., TSAI P.J., HSUEH C.M. The involvement of glutamate in recall of the conditioned NK cell response. J. Neuroimmunol. 2001;118:245–255. doi: 10.1016/s0165-5728(01)00340-x. [DOI] [PubMed] [Google Scholar]
- MANGANO R.M., SCHWARCZ R. The human platelet as a model for the glutamatergic neuron: platelet uptake of L-glutamate. J. Neurochem. 1981;36:1067–1076. doi: 10.1111/j.1471-4159.1981.tb01701.x. [DOI] [PubMed] [Google Scholar]
- MARAGAKIS N.J., ROTHSTEIN J.D. Glutamate transporters in neurologic disease. Arch. Neurol. 2001;58:365–370. doi: 10.1001/archneur.58.3.365. [DOI] [PubMed] [Google Scholar]
- MASLIAH E., ALFORD M., DE TERESA R., MALLORY M., HANSEN L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann. Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- MUNIR M., CORREALE M.D., ROBINSON M.B. Substrate-induced up-regulation of Na+-dependent glutamate transport activity. Neurochem. Int. 2000;37:147–162. doi: 10.1016/s0197-0186(00)00018-8. [DOI] [PubMed] [Google Scholar]
- O'SHEA R.D. Roles and regulation of glutamate transporters in the central nervous system. Clin. Exp. Pharmacol. Physiol. 2002;29:1018–1023. doi: 10.1046/j.1440-1681.2002.03770.x. [DOI] [PubMed] [Google Scholar]
- PLAITAKIS A., SHASHIDHARAN P. Glutamate transport and metabolism in dopaminergic neurons of substantia nigra: implications for the pathogenesis of Parkinson's disease. J. Neurol. 2000;247 Suppl 2:II25–II35. doi: 10.1007/pl00007757. [DOI] [PubMed] [Google Scholar]
- QIAN Y., GALLI A., RAMAMOORTHY S., RISSO S., DEFELICE L.J., BLAKELY R.D. Protein kinasi C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J. Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSI D.J., OSHIMA T., ATTWELL D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN J.D., MARTIN L.J., KUNCL R.W. Decrease glutamate transport by the brain and spinal cord in Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 1992;326:1464–1469. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN J.D., DYKES-HOBERG M., PARDO C.A., BRISTOL L.A., JIN L., KUNCL R.W., KANAI Y., HEDIGER M.A., WANG Y., SCHIELKE J.P., WELTY D.F. Knockout of glutamate transporters reveals a major role for astroglial transport in excititoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- SCHLAG B.D., VONDRASEK J.R., MUNIR M., KALANDADZE A., ZELENAIA-TROITSKAYA O.A., ROTHSTEIN J.D., ROBINSON M.B. Regulation of the glial Na+-dependent glutamate transporters by cAMP analogs and neurons. Mol. Pharmacol. 1998;53:355–369. doi: 10.1124/mol.53.3.355. [DOI] [PubMed] [Google Scholar]
- SCOTT H.L., POW D.V., TANNENBERG A.E.G., DODD P.R. Aberrant expression of the glutamate transporter excitatory amino acid transporter 1 (EAAT1) in Alzheimer's disease. J. Neurosci. 2002;22:RC206. doi: 10.1523/JNEUROSCI.22-03-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKERRY T.M., GENEVER P.G. Glutamate signalling in non-neuronal tissues. Trends Pharmacol. Sci. 2001;22:174–181. doi: 10.1016/s0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- SUAREZ I., BODEGA G., FERNANDEZ B. Modulation of glutamate transporters (GLAST, GLT-1 and EAAC1) in the rat cerebellum following portocaval anastomosis. Brain Res. 2000;859:293–302. doi: 10.1016/s0006-8993(00)01993-4. [DOI] [PubMed] [Google Scholar]
- SWANSON R.A., LIU J., MILLER J.W., ROTHSTEIN J.D., FARREL K., STEIN B.A., LONGUEMARE M.C. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J. Neurosci. 1997;17:932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA K., WATASE K., MANABE T., YAMADA K., WATANABE M., TAKAHASHI K., IWAMA H., NISHIKAWA T., ICHIHARA N., HORI S., TAKIMOTO M., WADA K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- TONG G., JAHR C.E. Block of glutamate transporters potentiates postsynaptic excitation. Neuron. 1994;13:1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- YE Z.C., SONTHEIMER H. Metabotropic glutamate receptor agonists reduce glutamate release from cultured astrocytes. Glia. 1999;25:270–281. [PubMed] [Google Scholar]
- ZOIA C., COGLIATI T., TAGLIABUE E., CAVALETTI G., SALA G., GALIMBERTI G., RIVOLTA I., ROSSI V., FRATTOLA L., FERRARESE C. Glutamate transporters in platelets: EAAT1 decrease in aging and in Alzheimer's disease. Neurobiol. Aging. 2004;25:149–157. doi: 10.1016/s0197-4580(03)00085-x. [DOI] [PubMed] [Google Scholar]