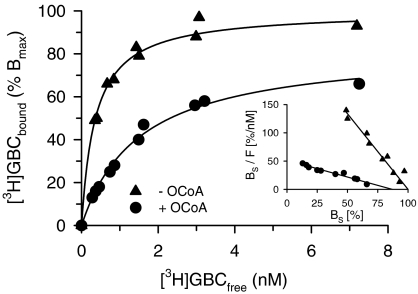
Figure 4.
Effect of OCoA (10 μM) on [3H]GBC binding to SUR1 in the absence of MgATP. Specific binding (BS), from two individual experiments, is shown as % BMAX. The fit of BS to the Law of Mass action (equation (3) in Methods) gave KD values of 0.36 (0.32, 0.41)/1.5 (1.2,1.8) nM and BMax values of 100 and 83±3% in the absence and presence of OCoA, respectively. 100% BMAX was 3.6 pmol (mg protein)−1. Nonspecific binding increased linearly with [3H]GBC concentration and was 20% of total binding at 7.5 nM [3H]GBC. Inset: Scatchard representation of the data (F denotes the free radioligand concentration, [3H]GBCfree). The control curve was fitted to a straight line with abscissa intercept 100 and gave a slope of −2.7±0.1 nM−1; the curve in the presence of OCoA gave a slope of −0.59±0.05 nM−1 and an abscissa intercept of 88±8%, which is slightly but significantly different from 100%.