Abstract
We studied 7-ethoxyresorufin deethylase as an index of cytochrome P4501A1 (CYP1A1) activity in liver microsomes from rats pretreated with 3-methylcholanthrene. The enzyme had complex kinetics compatible with a multisite model.
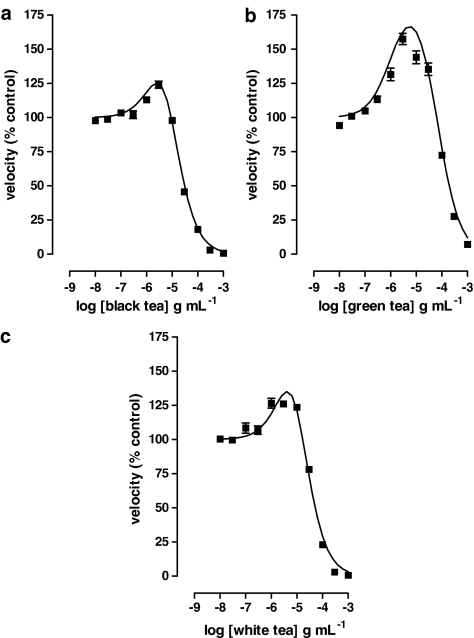
At 1 μM substrate, brewed black, green and white teas had complex effects on enzyme activity consisting of activation at low concentrations and inhibition at higher concentrations.
Data fit well to a two-site model that allowed us to determine maximal activation (% increase above control), pEC50 for activation (g ml−1) and pIC50 for inhibition (g ml−1). These parameters were 190±40, 5.9±0.1 and 4.51±0.09 for green tea, 350±40, 5.43±0.05 and 5.43±0.05 for black tea and 230±80, 5.3±0.3 and 4.7±0.2 for white tea, respectively.
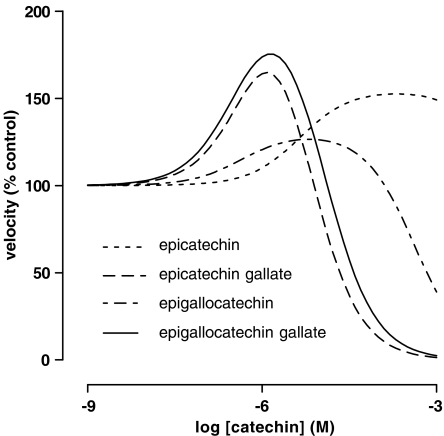
The effects of the brewed teas were mimicked to different degrees by the green tea polyphenols. Maximal activation, pEC50 (M) and pIC50 (M) were: (−)-epicatechin, 55±9, 5.4±0.3, 2±1; (−)-epicatechin gallate, 160±60, 6.2±0.3, 5.28±0.06; (−)-epigallocatechin 30±10, 6.5±0.5, 3.37±0.08; and (−)-epigallocatechin gallate 130±40, 6.7±0.3, 5.0±0.1. A crude extract of black tea polyphenols inhibited 7-ethoxyresorufin deethylase, but did not cause enzyme activation consistently.
Enzyme activation was dependent upon substrate concentration.
Heteroactivation of CYP1A1 may partially explain the lack of agreement between biological and epidemiological evidence of a role for tea in cancer prevention.
Keywords: CYP1A1, tea, polyphenols, EROD, catechins, activation, (−)-epigallocatechin gallate
Introduction
The pharmacology of tea (Camellia sinensis) is not restricted to its well-known stimulant effect. Significant evidence associates the consumption of beverages made from tea with a number of health benefits including the prevention of cancer, cardiovascular disease and osteoporosis (Yang & Landau, 2000; Liao et al., 2001; Higdon & Frei, 2003). However, the claim that tea consumption aids in cancer prevention is better supported by biological evidence that has been obtained from whole animal and in vitro experiments than it is by epidemiological evidence (Yang et al., 2002; Lambert & Yang, 2003). One possible mechanism of the chemoprotective effect of tea is its influence on the aryl hydrocarbon receptor (AhR)–cytochrome P4501A1 (CYP1A1) pathway.
CYP1A1 is a member of the multigene family of cytochromes P450, which are active in the biotransformation of both endogenous substances and xenobiotics. CYP1A1 is able to activate innocuous promutagens into their mutagenic and carcinogenic forms. For example, the polycyclic aromatic hydrocarbons 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), benzo[a]pyrene and 3-methylcholanthrene (3MC) are substrates for CYP1A1, which converts them to highly reactive epoxide intermediates that may bind potently to DNA and initiate carcinogenesis (Gonzalez & Gelboin, 1994). Through their action as AhR ligands, these compounds also induce the synthesis of more CYP1A1 protein (Denison & Whitlock, 1995; Whitlock, 1999) and consequently exacerbate their own toxicity. Thus, selective inhibition of CYP1A1 may protect against carcinogens that are activated by CYP1A1.
The biological activity of teas is generally attributed to the polyphenols that they contain (Mukhtar et al., 1992; Shiraki et al., 1994; Apostolides et al., 1997). Green, black and white teas differ in their polyphenolic content. Green tea, produced from steamed, unoxidized leaves, contains relatively simple compounds known collectively as catechins and exemplified by (−)-epigallocatechin (EGC). The predominant polyphenols in black tea, which is produced by fermentation of crushed leaves, are the more complex and poorly characterized thearubigens and the theaflavins (Yang et al., 2000). White tea, made from withered and dried leaves and buds, is claimed to contain higher concentrations of catechins than green tea (Dashwood et al., 2002).
Some green tea polyphenols, including (−)-epigallocatechin-3-gallate (EGCG), have been shown to inhibit the activity of both human and rat hepatic CYP1A1 (Wang et al., 1988; Muto et al., 2001). However, the effect of black tea polyphenols on CYP1A1 activity is not as well characterized. Feng et al. (2002) found that black tea polyphenols suppress CYP1A1 activity in human HepG2 cells and that this suppression is accompanied by a reduction in CYP1A1 protein and mRNA expression. But a direct effect of black tea polyphenols on enzyme activity – as opposed to expression – was not tested. We hypothesized that black tea would directly inhibit CYP1A1 in a similar manner to green tea, and set out to test this hypothesis.
We quantified CYP1A1 activity in 3MC-treated rat liver microsomes by measuring 7-ethoxyresorufin deethylase (EROD) activity (Moorthy, 2000). In preliminary experiments, we were surprised to find that black tea had a complex effect on CYP1A1 consisting of activation at low concentrations and inhibition at higher concentrations. In the present study, we have investigated this phenomenon and developed a method to quantify it. Furthermore, we show this heteroactivation of CYP1A1 to be a property of green tea and green tea polyphenols that has not been adequately recognized in previous work.
Methods
Rat liver microsomes
Female Wistar rats (Harlan) weighing 200–250 g were injected intraperitoneally with 3MC suspended in olive oil at a dose of 70 μmol kg−1 for four successive days. On the fifth day, the animals were killed by carbon dioxide asphyxiation and the livers were rapidly removed into ice-cold buffered sucrose solution. All subsequent steps were performed at 4°C unless stated otherwise. Livers were separated into individual lobes, cleared of fat, connective and vascular tissues and washed several times before being weighed and minced using a razor blade. Minces were added to buffered sucrose in a ratio of 1 g to 5 ml and homogenized with a Polytron P20 (Brinkman) for 15 s. Homogenates were centrifuged at 1400 × g for 10 min in a GPR centrifuge (Beckman) and supernatants were recentrifuged at 10,000 × g for 10 min in an L7 centrifuge (Beckman). Supernatants from this spin were pelleted by centrifugation at 87,000 × g for 30 min in the same instrument and resuspended in 0.2 M potassium phosphate buffer (pH 7.4) in a ratio of 5.7 ml per g wet weight of liver. The suspension was divided into aliquots and stored at −20°C until used. Protein concentration was determined by the method of Bradford (1976) adapted for a microplate using the Bio-Rad protein assay reagent and bovine serum albumin as standard, and was routinely 4–5 mg ml−1. Animals were cared for according to the guidelines of the Canadian Council on Animal Care and the protocols were approved by McMaster University's Animal Research Ethics Board.
EROD assays
EROD activity was determined by a kinetic fluorescence intensity assay using the general principles described by Crespi et al. (1997). Reactions were carried out at 37°C in black, 96-well plates (BD Falcon) in a final volume of 200 μl containing (final concentrations) potassium phosphate buffer pH 7.4 (0.15 M), NADP (250 μM), glucose-6-phosphate (816 μM), MgCl2 (825 μM), glucose-6-phosphate dehydrogenase (75 mU well−1), 7-ethoxyresorufin (ERES, variable), tea liquors or compounds (variable) and were started by the addition of microsomes (1–2 μg well−1). In some cases, 0.5% acetonitrile was used as vehicle, and was also present in appropriate control wells. The formation of resorufin was monitored every minute over a 10 min period by measuring emission at 590 nm after excitation at 535 nm using an HTS 7000 bioassay reader (Perkin-Elmer). Reaction velocities were calculated using the instrument's software (HTSoft 2.0). Fluorescence intensity was calibrated using authentic resorufin made up freshly in potassium phosphate buffer as a standard.
Teas
Black tea (Salada Orange pekoe) was a gift from the Tea Council of Canada, and generic green tea and white tea (Uncle Lee's tea) were obtained from local sources. In preliminary experiments, teas were brewed by adding 10 g of leaves to 250 ml of boiling distilled water, mixing thoroughly and allowing the suspension to infuse and cool at room temperature for 20 min. In subsequent experiments, the ratio was 4 g of leaves to 100 ml of water. A portion of the liquor was removed and centrifuged for 5 min at the fixed speed of an Eppendorf model 5413 microcentrifuge to sediment particulates. The supernatant was removed, diluted in distilled water as necessary and used immediately in the assay. The concentration of the undiluted liquor was considered to be 0.04 g ml−1.
Data analysis
Kinetic parameters for the enzyme reaction were obtained by fitting plots of the reaction velocity over 10 min versus the substrate concentration to different kinetic models by nonlinear least-squares regression. The simplest model was the Michaelis–Menten equation
where V is the reaction velocity at substrate concentration [S], Vmax is the maximal velocity of the reaction and Km is the Michaelis constant.
The same data were then applied to the Hill equation
where n is the Hill coefficient.
This was followed by fitting of the data to various forms of the two-site equation (3), adopted from Kenworthy et al. (2001) based on the principles defined by Segel (1975), which assumes that the substrate binds with equal affinity to two sites on the enzyme and allows for two different types of interaction between the two sites:
where Ks is the binding affinity of the substrate for the two sites, the parameter α governs the cooperativity between the two sites (when α=1, there is no cooperativity) and β reflects the degree to which the two occupied sites interact to alter the effective catalytic rate constant (when β=1, there is no change in the rate of product formation). The forms of the equation used were as follows: (3i) α=β=1, (3ii) α allowed to vary during the fitting process, β=1, (3iii) α=1, β allowed to vary, and (3iv) both α and β allowed to vary.
The applicability of increasingly complex models over simple models was assessed using the F-test (Motulsky & Ransnas, 1987) at P<0.01.
The effects of liquors or compounds were expressed as a percentage of control according to
where E is the percentage change with respect to control, Vc is the reaction velocity in the presence of compound or extract, B is the reaction velocity in the absence of microsomes and C is the reaction velocity in the absence of liquor or compound. For purposes of subsequent analysis, activity was normalized to 100% of that at the lowest no-effect concentration of liquor or compound on the rare occasions when the latter value was different from control.
Effects of liquors or compounds were initially analyzed by nonlinear least-squares fitting to the simple competitive equation
where [X] is the concentration of liquor or compound and pIC50 is the negative log of the concentration that inhibits enzyme activity by 50%.
The complex curves obtained in some cases suggested that compounds might first activate and then inhibit enzyme activity. Under these circumstances, activation (Ea) can be described by
where Emax is the maximum activation and pEC50 is the negative log of the concentration that activates the enzyme to 50% of Emax. Subsequent inhibition (Ei) is then given by
By applying the principles developed by Szabadi (1977) for compounds that simultaneously activate mutually antagonistic receptors, the resultant effect (Er) on enzyme activity is given by
Effects of liquors or compounds were analyzed by nonlinear least-squares fitting to equation (8), and the applicability of this complex model versus the simple model of equation (5) was assessed using the F-test (Motulsky & Ransnas, 1987) at P<0.01.
All assays were performed in quadruplicate and results are expressed as means±s.e.m. of n experiments.
The simulated curves shown in Figures 3 and 5 were obtained by reverse fitting to equation (8). Means of the location parameters Emax, pEC50 and pIC50 obtained from n experiments were entered into the equation and values of Er at a range of values of [X] spanning the experiment were calculated.
Figure 3.
Effects of green tea polyphenols on EROD activity in liver microsomes from 3MC-treated rats. The velocity of the deethylation of 1 μM ERES in the presence of 11 different concentrations of polyphenol was determined as described in Methods. Lines were obtained by reverse fitting of the mean data from Table 2 to equation (8) as described in Methods.
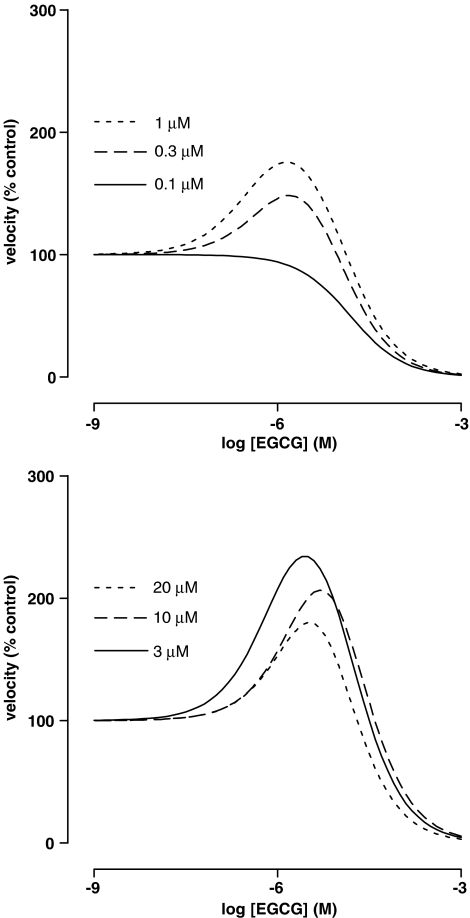
Figure 5.
Dependence of the effect of EGCG on EROD activity in liver microsomes from 3MC-treated rats on the concentration of ERES. The velocity of the deethylation of ERES in the presence of 11 different concentrations of EGCG was determined as described in Methods. Lines were obtained by reverse fitting of the mean data from Table 4 to equation (6) as described in Methods.
Materials
The buffered sucrose solution was composed of 250 mM sucrose and 20 mM morpholinopropane sulfonic acid with the pH adjusted to 7.4 with NaOH. All reagents were purchased from Sigma-Aldrich (Oakville, ON, Canada) except ERES (Molecular Probes, Eugene, OR, U.S.A.), NADP (Roche, Laval, QC, Canada) and acetonitrile, NaOH, potassium phosphates and sucrose, which were of the highest purity available from local sources.
Results
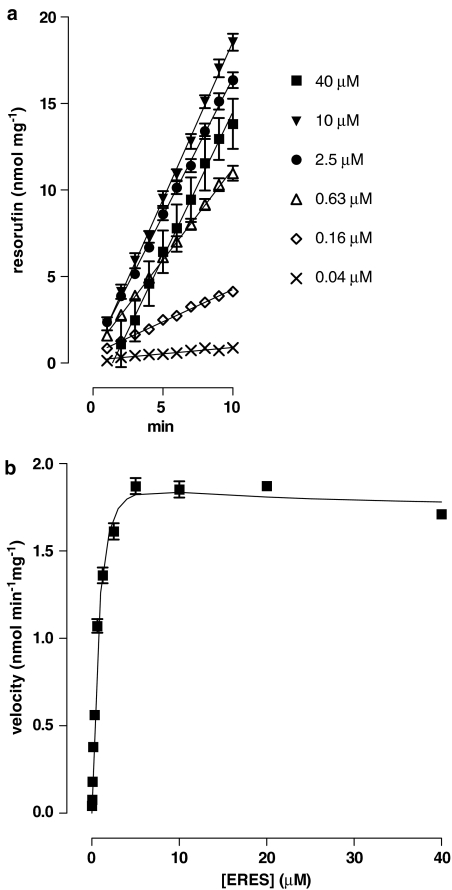
Under the conditions of our assays, EROD activity was linear over 10 min at concentrations of substrate up to 40 μM. Kinetic plots were complex and best fit by equation (3iii) (Figure 1). The mean Ks from five experiments was 3.4±0.5 μM, the Vmax was 4.1±0.3 nmol mg−1 min−1 and β was 0.29±0.03. One experiment in which half-maximal velocity was achieved at the lowest concentration of substrate (0.02 μM) was excluded from the analysis.
Figure 1.
Effect of the concentration of ERES on EROD activity in liver microsomes from 3MC-treated rats. (a) The time course of formation of resorufin from ERES was determined at 12 concentrations of substrate by determining the fluorescence intensity every minute for 10 min as described in Methods. For clarity, results from only six concentrations are shown. Lines are linear least-squares regressions. (b) Concentration/velocity plot using the full concentration range. The line shows the fit of the data to equation (3iii). The Ks for this experiment was 1.8 μM, Vmax was 4.5 nmol min−1 mg−1, β was 0.38 and α was fixed at 1. Each point represents the mean±s.e.m. of quadruplicate observations in a single experiment. Mean values from five such experiments using 10 or 12 concentrations of ERES are given in the text.
Black, green and white teas had complex effects upon EROD activity at 1 μM substrate, which consisted of an initial activation of the enzyme at low concentrations followed by inhibition at higher concentrations (Figure 2). All such data fit equation (8) significantly better than equation (5); this allowed us to calculate the parameters shown in Table 1.
Figure 2.
Effects of tea liquors on EROD activity in liver microsomes from 3MC-treated rats. The velocity of the deethylation of 1 μM ERES in the presence of 11 different concentrations of (a) black tea, (b) green tea and (c) white tea was determined as described in Methods. Lines show the fit of the data to equation (8). Data are from single experiments performed in quadruplicate. Parameters from multiple experiments of this type are given in Table 1.
Table 1.
Location parameters for the effect of teas on EROD activity in liver microsomes from 3MC-treated rats
| Emax (% above control) | pEC50 (g ml−1) | pIC50 (g ml−1) | n | |
|---|---|---|---|---|
| Black tea | 350±40 | 5.43±0.05 | 5.43±0.05 | 6 |
| Green tea | 190±40 | 5.9±0.1 | 4.51±0.09 | 6 |
| White tea | 230±80 | 5.3±0.3 | 4.7±0.2 | 4 |
Data were obtained from experiments like those illustrated in Figure 2 by nonlinear least-squares regression on equation (8).
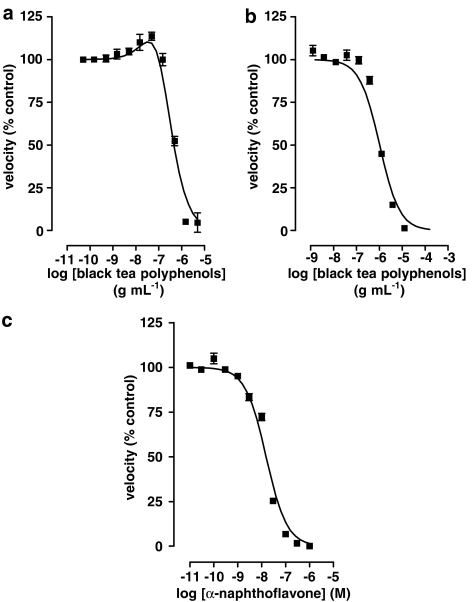
The green tea polyphenols (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), EGC and EGCG also had complex effects on EROD activity (Figure 3 and Table 2) and the shapes of the interaction curves were characteristic of the particular compound. Concentrations of green tea polyphenols producing maximum enzyme activation are given in Table 3. A commercial extract of black tea polyphenols, however, produced mixed results. Out of nine experiments, five were best fit by equation (5) and had a mean pIC50 of 5.87±0.03 g ml−1 while the other four were best fit by equation (8) and had activation (% control) 240±10, pEC50 (g ml−1) 6.7±0.2 and pIC50 (g ml−1) 6.7±0.2. As with black tea liquor, when the black tea polyphenol extract did cause significant activation, the pEC50 and pIC50 values were identical within a given experiment regardless of the starting values used for the nonlinear least-squares fit. α-Naphthoflavone, a polyphenol not found in significant amounts in tea, also failed to produce significant activation of the enzyme; the pIC50 (M) value was 8.36±0.07 (n=6) (Figure 4).
Table 2.
Location parameters for the effect of green tea polyphenols on EROD activity in liver microsomes from 3MC-treated rats
| Emax (% above control) | pEC50 (M) | pIC50 (M) | n | |
|---|---|---|---|---|
| EC | 55±9 | 5.4±0.3 | 2±1 | 4 |
| ECG | 160±60 | 6.2±0.3 | 3.37±0.08 | 4 |
| EGC | 30±10 | 6.3±0.1 | 4.9±0.2 | 4 |
| EGCG | 130±40 | 6.4±0.3 | 5.0±0.1 | 8 |
Data were obtained from experiments like those illustrated in Figure 2 by nonlinear least-squares regression on equation (8). All experiments were performed in quadruplicate.
Table 3.
Maximum activation of EROD activity in liver microsomes from 3MC-treated rats by green tea polyphenols
| Maximum activation (% control) | Concentration producing maximum activation (μM) | |
|---|---|---|
| EC | 152 | 200 |
| ECG | 164 | 1.2 |
| EGC | 126 | 6.5 |
| EGCG | 175 | 1.4 |
Data were obtained by interpolation of the curves shown in Figure 3.
Figure 4.
Effects of various polyphenols on EROD activity in liver microsomes from 3MC-treated rats. The velocity of the deethylation of 1 μM ERES in the presence of 11 different concentrations of polyphenol was determined as described in Methods. (a) A representative experiment with a commercial extract of black tea polyphenols that produced a best fit to equation (8). (b) A representative experiment with the same commercial extract of black tea polyphenols as in (a) that produced a best fit to equation (5). (c) A representative experiment with α-naphthoflavone which best fit to equation (5). The vehicle for this compound was 0.5% acetonitrile, which was also present in the appropriate controls and had no significant effect on enzyme activity (data not shown). Data are from single experiments performed in quadruplicate. Parameters from multiple experiments of this type are reported in the text.
Black, green and white teas inhibited EROD but did not produce significant activation when the substrate concentration was 0.1 μM. The pIC50 (g ml−1) values were 4.8±0.2 (n=4), 4.30±0.03 (n=3) and 4.4±0.1 (n=3), respectively. Experiments using EGCG revealed a complex dependence of the activation phenomenon upon substrate concentration. Although the pIC50 was relatively independent of the substrate concentration, Emax increased and pEC50 showed a biphasic dependence on increasing substrate concentrations, with a maximum sensitivity occurring at 1 μM (Table 4). The resultant effect of these changes, shown in Figure 5, was an increase in the peak activation at concentrations of ERES up to 3 μM, and a decrease thereafter.
Table 4.
Location parameters for the effect of EGCG on EROD activity in liver microsomes from 3MC-treated rats at different concentrations of substrate
| [ERES] (μM) | Emax (% above control) | pEC50 (M) | pIC50 (M) | n |
|---|---|---|---|---|
| 20 | 500±40 | 5.3±0.1 | 5.3±0.1 | 4 |
| 10 | 310±90 | 5.5±0.3 | 4.9±0.1 | 4 |
| 3 | 260±86 | 6.0±0.3 | 4.9±0.1 | 4 |
| 1 | 130±40 | 6.4±0.3 | 5.0±0.1 | 8 |
| 0.3 | 114±55 | 6.1±0.3 | 5.1±0.2 | 8 |
| 0.1 | — | — | 4.79±0.03 | 3 |
The velocity of the deethylation of ERES in the presence of 11 different concentrations of EGCG was determined as described in Methods and fit by nonlinear least-squares regression to equation (8), except for the results at 0.1 μM ERES, which had a superior fit to equation (5). All experiments were performed in quadruplicate.
Discussion
Green tea and green tea polyphenols have been shown to inhibit EROD (Wang et al., 1988; Obermeier et al., 1995), to prevent the activation of procarcinogens (Muto et al., 2001) and to suppress the induction of the cyp1A1 gene (Williams et al., 2000). On the basis of such experiments, Wang et al. (1988) suggested that extracts of green tea might have potential as anticarcinogens. In similar experiments, black tea polyphenols have been shown to suppress CYP1A1 in the human hepatoma cell line HepG2 (Feng et al., 2002) but it is unclear whether this suppression results from direct inhibition of the enzyme rather than – or in addition to – suppression of induction of the enzyme.
EROD activity in liver microsomes from 3MC-treated rats is well established as a functional measure of CYP1A1 activity (Moorthy, 2000). We used 3MC induction in these experiments because we were interested in the possible chemoprotective effects of teas. Our experimental conditions mimic the situation in which xenobiotics potentiate their own biotransformation through AhR activation and subsequent elevation of CYP1A1 activity (Denison & Whitlock, 1995). Treatment with 3MC may alter enzyme function in other – as yet unknown – ways, making caution advisable in extrapolating these results to the noninduced state.
The complex kinetics that were best fit by equation (3iii) without improvement when equation (3iv) was employed are compatible with substrate inhibition, a phenomenon that has been well described for a number of substrates of CYP3A4 (Houston & Galetin, 2003). Deviation from Michaelis–Menten kinetics is well established for CYP2C9 and CYP3A4 where multiple ligand binding models apply (Yoon et al., 2004), and our data suggest that this may be a common characteristic among the cytochrome P450 class.
We set out to test the simple hypothesis that black tea directly inhibits CYP1A1, and our demonstration that black tea and a commercial extract of black tea polyphenols inhibit EROD activity (Figures 2a, 4a and b) supports that hypothesis. However, our experiments also uncovered the phenomenon of heteroactivation by black tea and we subsequently showed heteroactivation to be a property also of green and white teas (Figure 2).
Although activation of CYP1A1 by green tea catechins has been observed previously (Obermeier et al., 1995), it has never been quantified and the possible consequences of this phenomenon have not been considered in subsequent work. Heteroactivation has been demonstrated for CYP1A2 (Ekins et al., 1998), CYP2C9 (Egnell et al., 2003) and CYP3A4 (Stresser et al., 2000; Kenworthy et al., 2001); so the present observations support the argument that the propensity to be activated by certain substrates is a phenomenon common to all cytochromes P450.
The data in Figure 3 and Table 2 support the contention that it is the catechin content of green and white teas that is responsible for activation of CYP1A1. However, although black tea polyphenols may be responsible for the inhibition of the enzyme by black tea, it is not abundantly clear that they are also responsible for the activation component. Black tea does contain catechins, albeit in lower concentrations than green tea, and it is possible that they are responsible for the activation seen. We consider this unlikely because the theoretical maximum activation is greater for black than either green or white teas (Table 1) and this cannot be explained by the catechin argument. In addition, when black tea polyphenols did activate the enzyme, they did so with the same potency as they inhibited it, which suggests a mechanistic difference between black tea on the one hand and green or white tea on the other. It is likely that the more active black tea polyphenols were not as well preserved in the commercial black tea polyphenol extract that we used as they were in our freshly brewed black tea liquor.
Heteroactivation of CYP3A4 has been shown to be dependent upon substrate concentration (Mäenpää et al., 1998; Galetin et al., 2002), and our demonstration that activation of CYP1A1 by EGCG is influenced by the ERES concentration (Table 4 and Figure 5) is consistent with this. The complexity of the changes in the various parameters (Table 4) should be helpful in attempts to model the enzymatic reaction (Ekins et al., 2003). That there is some degree of structural specificity to the activation phenomenon is supported by the differing potencies of the green tea catechins and the lack of activation by α-naphthoflavone, which is, interestingly, a potent activator of CYP3A4 (Stresser et al., 2000).
Along with others (Wang et al., 1988; Obermeier et al., 1995), we have assumed that green tea polyphenols exert their effects on EROD by binding at the active site of CYP1A1, and this is consistent with effects of these compounds on the P450 difference spectrum in the presence of carbon monoxide (Wang et al., 1988). The good fit of the complex effects of teas and tea polyphenols to equation (8) (Figures 2 and 4a) is consistent with a model in which the active components bind simultaneously to two sites. Binding at one site causes enzyme inhibition by competition with the substrate, while binding to the second site causes activation, presumably through an allosteric mechanism (Yoon et al., 2004).
Our analysis makes it possible to estimate the maximal amount of activation that would be seen if inhibition were absent. The model predicts that black tea can produce a greater activation than green tea even though this is not apparent from a cursory glance at the experimental data. This is because the difference in potency between activation and inhibition is much greater for green tea, and therefore activation actually manifests itself before it is masked by the inhibitory component. In pharmacological terms, black tea can be considered a low-potency, high-efficacy activator, while green and white teas are higher-potency, lower-efficacy activators. The quantification method will be useful in attempts to build a structure–activity relationship around the activation phenomenon. This approach has worked well in the past to quantify receptor-mediated effects (Szabadi, 1977; Popat & Crankshaw, 2001) although there is some concern over its ability to estimate accurately Emax when the potencies of the two components are similar (Popat & Crankshaw, 2001). However, the goodness of fit does not imply mechanistic correctness, and experience with other CYP enzymes would suggest that the true behavior of the enzyme is more complicated than our current model (Ekins et al., 2003), particularly in light of the fact that heteroactivation of CYP3A4 is dependent upon the substrate used (Stresser et al., 2000), as is inhibition of CYP1A1 (Schwarz & Roots, 2003).
Recent studies on the pharmacokinetics of the green tea polyphenols in humans show that peak plasma concentrations range from 0.08 μM for EGCG to 0.74 μM for EGC after the consumption of a single dose of 426 ml of green tea made from three tea bags (Henning et al., 2004). In our experiments at a substrate concentration of 1 μM (Figure 3), such concentrations fall on the early upward slope of the activation phase of the complex curve, with both EGCG and EGC producing enzyme activity at 119% of control. The value for EGCG is similar (116%) when the substrate concentration is 3 μM. Thus, if the effects of green tea catechins that we have demonstrated in 3MC-treated rat liver can be extrapolated to humans, moderate tea consumption will result in mild activation of CYP1A1 activity, not inhibition.
In the case of chronic ingestion of green tea polyphenols, best exemplified by experimental situations in which animals are given dilute tea to drink in place of water (Yang et al., 2003; Niwattisaiwong et al., 2004), the effects are more complex. This is because green tea polyphenols appear to be partial agonists of the AhR. Thus, green tea extract and EGCG inhibit TCDD-induced CYP1A1 mRNA and protein expression in HepG2 cells when the reagents are added simultaneously (Williams et al., 2000; 2003), and it may be through this mechanism that experimental chemoprevention is mediated. However, green tea extract alone increases the accumulation of CYP1A1 mRNA and protein in the same system. These observations probably explain why measures of CYP1A1 activity are elevated in the livers of both rats and mice after chronic tea consumption (Yang et al., 2003; Niwattisaiwong et al., 2004). In these circumstances, the overall consequences of heteroactivation will be dependent upon the pre-existing level of enzyme expression.
In summary, we have demonstrated complex effects of black, green and white teas and tea polyphenols on CYP1A1 from 3MC-treated rats and have developed a method for their quantification. Heteroactivation may be a phenomenon that is common to all CYP enzymes and complicates the prediction of drug/drug and food/drug interactions. In the specific case of CYP1A1, activation of the enzyme by teas may be part of the explanation for the lack of epidemiological support for cancer prevention in the face of biologically plausible mechanisms (Yang et al., 2002).
Acknowledgments
We thank Anne Mullen for preparing some of the microsomes used in this study, and Paul Cassar and Erin Rose for their participation in the initial pilot experiments. The work was supported in part by the Canadian Foundation for Women's Health and would not have been possible without the generous donation of the HTS 7000 plate reader by AstraZeneca R&D, Wilmington, DE.
Abbreviations
- AhR
aryl hydrocarbon receptor
- CYP
cytochrome P450
- EC
(−)-epicatechin
- ECG
(−)-epicatechin gallate
- EGC
(−)-epigallocatechin
- EGCG
(−)-epigallocatechin gallate
- ERES
7-ethoxyresorufin
- EROD
7-ethoxyresorufin deethylase
- 3MC
3-methylcholanthrene
- RFU
relative fluorescence units
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
References
- APOSTOLIDES Z., BALENTINE D.A., HARBOWY M.E., HARA Y., WEISBURGER J.H. Inhibition of PhIP mutagenicity by catechins, and by theaflavins and gallate esters. Mutat. Res. 1997;389:167–172. doi: 10.1016/s1383-5718(96)00143-x. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CRESPI C.L., MILLER V.P., PENMAN B.W. Microtiter plate assays for inhibition of human, drug-metabolizing cytochromes P450. Anal. Biochem. 1997;248:188–190. doi: 10.1006/abio.1997.2145. [DOI] [PubMed] [Google Scholar]
- DASHWOOD W.M., ORNER G.A., DASHWOOD R.H. Inhibition of beta-catenin/Tcf activity by white tea, green tea, and epigallocatechin-3-gallate (EGCG): minor contribution of H2O2 at physiologically relevant EGCG concentrations. Biochem. Biophys. Res. Commun. 2002;296:584–588. doi: 10.1016/s0006-291x(02)00914-2. [DOI] [PubMed] [Google Scholar]
- DENISON M.S., WHITLOCK J.P., Jr Xenobiotic-inducible transcription of cytochrome P450 genes. J. Biol. Chem. 1995;270:18175–18178. doi: 10.1074/jbc.270.31.18175. [DOI] [PubMed] [Google Scholar]
- EGNELL A.C., ERIKSSON C., ALBERTSON N., HOUSTON B., BOYER S. Generation and evaluation of a CYP2C9 heteroactivation pharmacophore. J. Pharmacol. Exp. Ther. 2003;307:878–887. doi: 10.1124/jpet.103.054999. [DOI] [PubMed] [Google Scholar]
- EKINS S., RING B.J., BINKLEY S.N., HALL S.D., WRIGHTON S.A. Autoactivation and activation of the cytochrome P450s. Int. J. Clin. Pharmacol. Ther. 1998;36:642–651. [PubMed] [Google Scholar]
- EKINS S., STRESSER D.M., WILIAMS J.A. In vitro and pharmacophore insights into CYP3A enzymes. Trends Pharmacol. Sci. 2003;24:161–166. doi: 10.1016/s0165-6147(03)00049-x. [DOI] [PubMed] [Google Scholar]
- FENG Q., TORII Y., UCHIDA K., NAKAMURA Y., HARA Y., OSAWA T. Black tea polyphenols, theaflavins, prevent cellular DNA damage by inhibiting oxidative stress and suppressing cytochrome P450 1A1 in cell cultures. J. Agric. Food Chem. 2002;50:213–220. doi: 10.1021/jf010875c. [DOI] [PubMed] [Google Scholar]
- GALETIN A., CLARKE S.E., HOUSTON J.B. Quinidine and haloperidol as modifiers of CYP3A4 activity: multisite kinetic model approach. Drug Metab. Dispos. 2002;30:1512–1522. doi: 10.1124/dmd.30.12.1512. [DOI] [PubMed] [Google Scholar]
- GONZALEZ F.J., GELBOIN H.V. Role of human cytochromes P450 in the metabolic activation of chemical carcinogens and toxins. Drug Metab. Dispos. 1994;26:165–183. doi: 10.3109/03602539409029789. [DOI] [PubMed] [Google Scholar]
- HENNING S.M., NIU Y., LEE N.H., THAMES G.D., MINUTTI R.R., WANG H., GO V.L., HEBER D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am. J. Clin. Nutr. 2004;80:1558–1564. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- HIGDON J.V., FREI B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- HOUSTON J.B., GALETIN A. Progress towards prediction of human pharmacokinetic parameters from in vitro technologies. Drug Metab. Rev. 2003;35:393–415. doi: 10.1081/dmr-120026870. [DOI] [PubMed] [Google Scholar]
- KENWORTHY K.E., CLARKE S.E., ANDREWS J., HOUSTON J.B. Multisite kinetic models for CYP3A4: simultaneous activation and inhibition of diazepam and testosterone metabolism. Drug Metab. Dispos. 2001;29:1644–1651. [PubMed] [Google Scholar]
- LAMBERT J.D., YANG C.S. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat. Res. 2003;523–524:201–208. doi: 10.1016/s0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- LIAO S., KAO Y.H., HIIPAKKA R.A. Green tea: biochemical and biological basis for health benefits. Vitam. Horm. 2001;62:1–94. doi: 10.1016/s0083-6729(01)62001-6. [DOI] [PubMed] [Google Scholar]
- MÄENPÄÄ J., HALL S.D., RING B.J., STROM S.C., WRIGHTON S.A. Human cytochrome P450 3A (CYP3A) mediated midazolam metabolism: the effect of assay conditions and regioselective stimulation by alpha-naphthoflavone, terfenadine and testosterone. Pharmacogenetics. 1998;8:137–155. [PubMed] [Google Scholar]
- MOORTHY B. Persistent expression of 3-methylcholanthrene-inducible cytochromes P4501A in rat hepatic and extrahepatic tissues. J. Pharmacol. Exp. Ther. 2000;294:313–322. [PubMed] [Google Scholar]
- MOTULSKY H.J., RANSNAS L.A. Fitting curves to data using nonlinear regression: a practical and nonmathematical review. FASEB J. 1987;1:365–374. [PubMed] [Google Scholar]
- MUKHTAR H., WANG Z.Y., KATIYAR S.K., AGARWAL R. Tea components: antimutagenic and anticarcinogenic effects. Prev. Med. 1992;21:351–360. doi: 10.1016/0091-7435(92)90042-g. [DOI] [PubMed] [Google Scholar]
- MUTO S., FUJITA K., YAMAZAKI Y., KAMATAKI T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat. Res. 2001;479:197–206. doi: 10.1016/s0027-5107(01)00204-4. [DOI] [PubMed] [Google Scholar]
- NIWATTISAIWONG N., LUO X.X., COVILLE P.F., WANWIMOLRUK S. Effects of Chinese, Japanese and Western tea on hepatic P450 enzyme activities in rats. Drug Metabol. Drug Interact. 2004;20:43–56. doi: 10.1515/dmdi.2004.20.1-2.43. [DOI] [PubMed] [Google Scholar]
- OBERMEIER M.T., WHITE R.E., YANG C.S. Effects of bioflavonoids on hepatic P450 activities. Xenobiotica. 1995;25:575–584. doi: 10.3109/00498259509061876. [DOI] [PubMed] [Google Scholar]
- POPAT A., CRANKSHAW D.J. Variable responses to prostaglandin E2 in human non-pregnant myometrium. Eur. J. Pharmacol. 2001;416:145–152. doi: 10.1016/s0014-2999(01)00852-4. [DOI] [PubMed] [Google Scholar]
- SCHWARZ D., ROOTS I. In vitro assessment of inhibition by natural polyphenols of metabolic activation of procarcinogens by human CYP1A1. Biochem. Biophys. Res. Commun. 2003;303:902–907. doi: 10.1016/s0006-291x(03)00435-2. [DOI] [PubMed] [Google Scholar]
- SEGEL I.H. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady State Enzyme Systems. New York: John Wiley & Sons; 1975. [Google Scholar]
- SHIRAKI M., HARA Y., OSAWA T., KUMON H., NAKAYAMA T., KAWAKISHI S. Antioxidative and antimutagenic effects of theaflavins from black tea. Mutat. Res. 1994;323:29–34. doi: 10.1016/0165-7992(94)90041-8. [DOI] [PubMed] [Google Scholar]
- STRESSER D.M., BLANCHARD A.P., TURNER S.D., ERVE J.C., DANDENEAU A.A., MILLER V.P., CRESPI C.L. Substrate-dependent modulation of CYP3A4 catalytic activity: analysis of 27 test compounds with four fluorometric substrates. Drug Metab. Dispos. 2000;28:1440–1448. [PubMed] [Google Scholar]
- SZABADI E. A model of two functionally antagonistic receptor populations activated by the same agonist. J. Theor. Biol. 1977;69:101–112. doi: 10.1016/0022-5193(77)90390-3. [DOI] [PubMed] [Google Scholar]
- WANG Z.Y., DAS M., BICKERS D.R., MUKHTAR H. Interaction of epicatechins derived from green tea with rat hepatic cytochrome P-450. Drug Metab. Dispos. 1988;16:98–103. [PubMed] [Google Scholar]
- WHITLOCK J.P., Jr Induction of cytochrome P4501A1. Annu. Rev. Pharmacol. Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- WILLIAMS S.N., PICKWELL G.V., QUATTROCHI L.C. A combination of tea (Camellia senensis) catechins is required for optimal inhibition of induced CYP1A expression by green tea extract. J. Agric. Food Chem. 2003;51:6627–6634. doi: 10.1021/jf030181z. [DOI] [PubMed] [Google Scholar]
- WILLIAMS S.N., SHIH H., GUENETTE D.K., BRACKNEY W., DENISON M.S., PICKWELL G.V., QUATTROCHI L.C. Comparative studies on the effects of green tea extracts and individual tea catechins on human CYP1A gene expression. Chem. Biol. Interact. 2000;128:211–229. doi: 10.1016/s0009-2797(00)00204-0. [DOI] [PubMed] [Google Scholar]
- YANG C.S., CHUNG J.Y., YANG G., CHHABRA S.K., LEE M.J. Tea and tea polyphenols in cancer prevention. J. Nutr. 2000;130:472S–478S. doi: 10.1093/jn/130.2.472S. [DOI] [PubMed] [Google Scholar]
- YANG C.S., LANDAU J.M. Effects of tea consumption on nutrition and health. J. Nutr. 2000;130:2409–2412. doi: 10.1093/jn/130.10.2409. [DOI] [PubMed] [Google Scholar]
- YANG C.S., MALIAKAL P., MENG X. Inhibition of carcinogenesis by tea. Annu. Rev. Pharmacol. Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- YANG M., YOSHIKAWA M., ARASHIDANI K., KAWAMOTO T. Effects of green tea on carcinogen-induced hepatic CYP1As in C57BL/6 mice. Eur. J. Cancer Prev. 2003;12:391–395. doi: 10.1097/00008469-200310000-00008. [DOI] [PubMed] [Google Scholar]
- YOON M.Y., CAMPBELL A.P., ATKINS W.M. ‘Allosterism' in the elementary steps of the cytochrome P450 reaction cycle. Drug Metab. Rev. 2004;36:219–230. doi: 10.1081/dmr-120033998. [DOI] [PubMed] [Google Scholar]