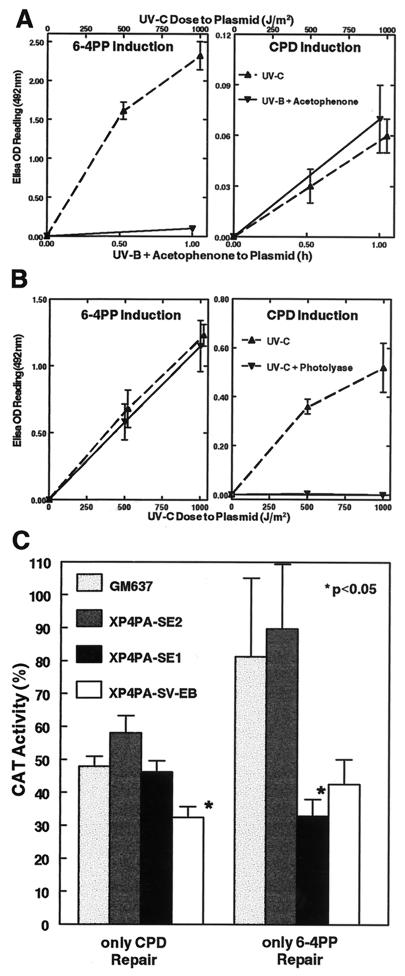
Figure 3.
Characterization of the photoproduct repair capacity in the two stably corrected XPC clones. Host cell reactivation of a reporter gene plasmid (pRSVcat) was used to assess the 6-4PP or CPD repair capability of the fully and partially corrected transfectants. (A) To induce only CPD lesions on the plasmid, pRSVcat was treated with acetophenone (10−2 M) + 313 nm UVB light (see Methods). 6-4PP and CPD lesions in the double-stranded DNA plasmid after treatment were measured with specific mAbs (as in Fig. 2) and compared with UVC-treated plasmid. The UVB + acetophenone treatment generated minimally detectable amounts of 6-4PP (Left), but induced about the same numbers of CPD as did a 1,000 J/m2 UVC treatment (Right). (B) To induce only 6-4PP lesions on the plasmid, pRSVcat was irradiated with UVC and subsequently photoreactivated with E. coli photolyase and 405 nm blue light (29). After this treatment, the number of UVC-induced 6-4PP was unchanged (Left), but all CPD lesions were removed from the plasmid (Right) as assessed with the ELISA assay. Data points are means of four determinations performed in quadruplicate (±SEM). (C) Either UVB + acetophenone-treated pRSVcat plasmid (only CPD Repair) or 1,000 J/m2 UVC + photolyase-treated pRSVcat plasmid (only 6-4PP Repair) was transfected into normal cells (GM637), the fully corrected XPC clone (XP4PA-SE2), the partially corrected XPC clone (XP4PA-SE1), or XPC cells (XP4PA-SV-EB). Relative CAT activity was measured 48 h after transfection compared with the corresponding untreated control plasmids. Bars represent means of at least triplicate experiments (±SEM). Specific activity with the untreated plasmids ranged from 1.59 to 22.38 nmol per min per mg protein. The partially corrected XP4PA-SE1 clone showed normal CPD repair but did not repair 6-4PP.