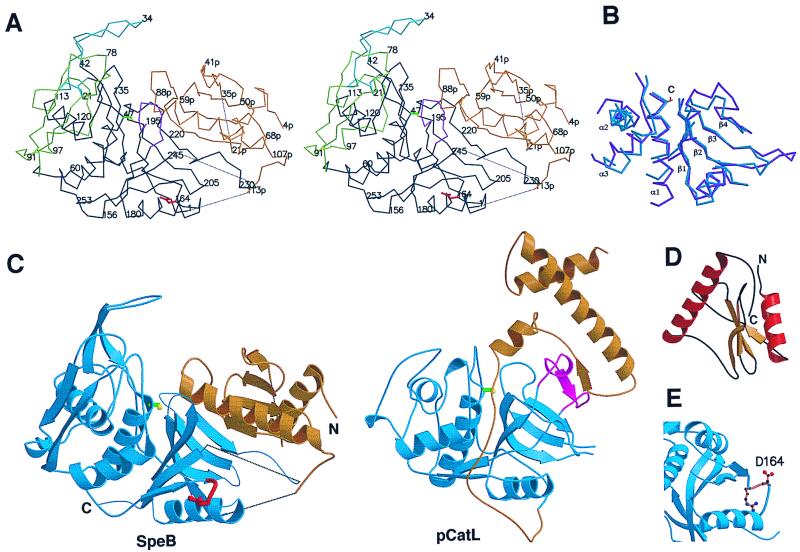
Figure 2.
Structure of SpeB and comparison with other papain-family proteins. (A) Stereo Cα plot of SpeB. The prosegment is colored gold and the protease domains black, except for the two N-domain insertions 19–42 (the finger loop, blue) and 66–113 (green) and the truncated β1-β2 C-domain loop 187–195 (magenta). Asp-164 of the RGD loop is red and the Ser-47 side chain yellow. Dotted lines indicate flexible regions. (B) Superposition of the core secondary structures of mSpeB (blue) and actinidin (magenta). The location of the catalytic cysteine (C) is shown. (C) Ribbon diagrams of SpeB (Left) and procathepsin L (Right), showing their similar protease domains (blue) and different prosegments (gold). The RGD loop of SpeB is red. The PBL, present in other papain-family enzymes but not SpeB, is colored magenta in procathepsin L. (D) Folding of the prosegment. (E). RGD loop (gold) of SpeB, showing the exposed Asp-164. These and other figures were produced with molscript (33) and raster3d (34).