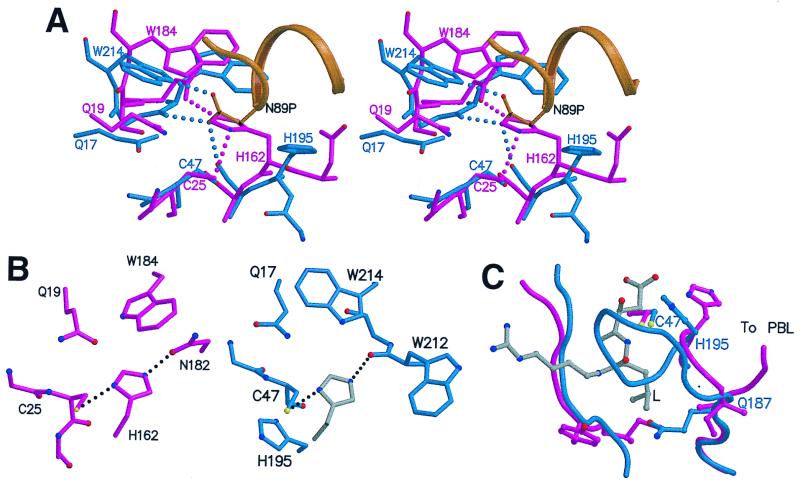
Figure 4.
Active site region of SpeB, compared with actinidin. (A) Stereo view of zSpeB (blue and gold) superimposed on actinidin (magenta). In zSpeB, the insertion of Asn-89p from the prosegment (gold) displaces His-195 from the catalytically competent position of His-162 in actinidin. Hydrogen bonds made by Asn-89p in SpeB and His-162 in actinidin are shown by dotted lines. (B) The Cys-His-Asn triad in actinidin (Left) compared with the putative Cys-His-(O=C) triad in mSpeB (Right). For mSpeB we assume that His-195 rotates from its position in the zymogen to a putative catalytic position shown in gray. (C) The S2 binding pocket. The leucyl side chain (L) of an inhibitor E64 (gray) is shown as it binds in actinidin (43). In zSpeB (blue) the loop 187–195 blocks the pocket; in actinidin (magenta) the chain goes in a different direction to form the PBL and the pocket is open.